Abstract
Pre-arming therapeutic cells with bispecific antibodies (BiAbs) before infusion can home the cells to specific tissue antigens in the body. With the development of nanotechnology, we developed a novel strategy, namely magnetic bispecific cell engager (MagBICE), that combines BiAbs with biodegradable iron nanoparticles. Compared to conventional BiAbs, the latter enables magnetic targeting and imaging. This editorial discusses current knowledge of BiAbs and their applications in targeting activated T cells to cancerous tissues or targeting bone marrow-derived stem cells to myocardial infarction. We will also discuss the fabrication of MagBICE and its application in treating rodents with myocardial infarction.
1. Introduction
Cell therapy is becoming a promising strategy for tissue regeneration or cancer eradication. For tissue regeneration, stem cells need to be targeted to the damaged tissue to initiate repair; for cancer therapy, cytotoxic T cells need to be redirected to the tumor cells to initiate eradication. For example, bispecific antibodies (BiAbs) that recognize both the antigens on the T cells and the tumor cells therefore can physically link the two together so that the T cells will lyse the tumor cells Citation[1]. BiAbs that conjoin the binding regions of two different monoclonal antibodies are emerging with numerous potential applications Citation[2].
The combination of BiAbs with nanoparticles has been a recent innovation in the field and applied to eradicate cancer or regenerate damaged tissue Citation[3]. Nanoparticles used in combination with BiAbs include therapeutic liposomes and iron oxide nanoparticles. The applications to date are primarily for imaging purposes, but research geared towards development of therapeutics has begun. This paper will focus on the ground breaking studies that combine BiAbs, stem cells, and nanoparticles for therapeutic applications, including: i) arming activated T cells with BiAbs to treat cancer; ii) arming bone marrow-derived stem cells with BiAbs to treat myocardial infarction; and iii) combining BiAbs with iron nanoparticles for both therapeutic stem cell targeting and imaging. We will also discuss future directions and challenges for designing and testing bispecific nanomedicines.
2. Bispecific T cell engager
BiAbs, antibody constructs composed of two variable regions with different specificities, were first described 25 years ago Citation[4]. Yet, the use of the BiAb to connect a cytotoxic T cell with its target is a recent development known as the bispecific T cell engager (BiTE). The BiTE including specificity for CD3-positive cytotoxic T cells has demonstrated efficacy in animal models of malignant disease and in human clinical trials Citation[4].
Manipulating a patient’s own immune system to attack tumor cells offers the potential for a cancer treatment that is less toxic to the patient than current chemotherapy drugs. The strategy of using specific cytotoxic T lymphocytes of patients for cancer therapy, however, is often limited by the numbers of T cells that can be produced in the body to combat large tumor burdens and the ability of tumors to suppress antitumor responses. Since the early immunotherapy trials using lymphokine-activated killer cells or tumor-derived infiltrating lymphocytes, it has been very difficult to unequivocally show an antitumor effect from cell therapies. The T cells are expanded in vitro to reach as many as 3 × 1011 cells before immunotherapy. The T cells and BiTE antibodies are introduced simultaneously into the patient. Ex vivo arming of immune effector cells with BiTEs prior to infusion could provide large numbers of targeted effector cells and minimize the infusion of free antibody Citation[5].
BiTE antibodies are designed to target cell-dependent activation of T cells and transiently engage activated T-cells for lysis of selected cancer cells () Citation[6]. BiTE antibodies have so far been constructed to target > 10 different tumor antigens, including CD19, EpCAM, Her2/neu, EGFR, CD66e (or CEA, CEACAM5), CD33, EphA2 and MCSP (or HMW-MAA) Citation[6]. In the study conducted by L.G. Lum and his colleagues, BiTE (anti-CD3 × anti-Her2/neu)-armed T cells could augment the anti-tumor immune response toward hormone-refractory prostate cancer and increase the secretion of TH1 cytokines, granulocyte-macrophage colony-stimulating factor, TNF-α and IFN-γ in vitro Citation[7]. In one clinical study, anti-CD19 × anti-CD3 BiTE demonstrated high clinical activity in B cell leukemia and lymphoma patients Citation[5]. Furthermore, T cells armed with anti-CD3 × anti-EGFR BiTE appeared to overcome some limitations associated with targeting the epidermal growth factor receptor when using the antitumor antibody cetuximab alone Citation[8].
Figure 1. (A) Bispecific T cell engager antibodies are designed to exclusively target T cells and transiently engage activated T-cells for lysis of selected cancerous cells. (B) Bispecific antibodies were armed with CD34+ stem cells prior to intravenous infusion. After infusion, armed CD34+ stem cells could target the injured myocardium, which expressed an injury biomarker MLC. (C) Anti-CD45 antibody was modified with Traut’s reagent and anti-MLC was modified with sulfosuccinimidyl 4-(N-maleimidomethyl) cyclohexane1-carboxylate (SulfoSMCC); mixture of the two and overnight crosslinking produced a BiAbs with anti-CD45 × anti-MLC specificities.
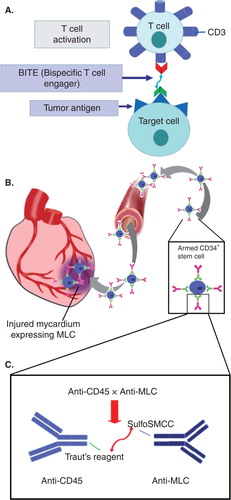
3. BiAbs for targeting stem cells
The therapeutic efficacy of stem cell transplantation for heart repair has been limited by the number of stem cells that migrate to, engraft in, and proliferate at sites of injured myocardium. To alleviate this limitation, Lum et al. Citation[9] studied whether hematopoietic stem cells (HSC) pre-armed with BiAbs could target specifically to injured myocardium. Definite therapeutic improvements were achieved after transplanting HSCs derived from bone marrow Citation[10] with purified CD34+ cells Citation[11]. Lum et al. Citation[9,12] also constructed BiAbs to selectively target CD45 and myosin light chain (MLC)-1, CD45 is expressed on CD34+ HSCs Citation[2]. MLC-1 is normally confined in the contractile apparatus of a myocyte but is exposed on the cell membrane after ischemic injury; thus MLC-1 can be used as a biomarker for myocardial injury. Anti-human CD45 antibody was modified with Traut’s reagent and anti-MLC was modified with sulfosuccinimidyl 4-(N-maleimidomethyl) cyclohexane1-carboxylate (SulfoSMCC); overnight crosslinking of the two produced a BiAb with anti-CD45 × anti-MLC specificities (). The BiAbs were incubated with CD34+ cells for 1 h at 4°C prior to intravenous infusion. The number of pre-armed HSCs accumulating in the injury zone increased significantly and augmented cardiac repair in a nude rat myocardial infarction model Citation[9]. Moreover, a study by Zhao et al. Citation[12] indicated that armed CD34+ HSC administered intravenously 48 h after myocardial infarction localized to the ischemic regions of the mouse heart. This localization was associated with improved ventricular function, enhanced angiogenesis and reduced fibrosis. Furthermore, administration of armed stem cells prevented cardiac remodeling. Arming CD34+ cells with the engineered BiAbs not only increased the number of CD34+ cells that homed to infarcted tissue, but also improved the function of the affected left ventricular tissue.
4. Magnetic bifunctional cell engager
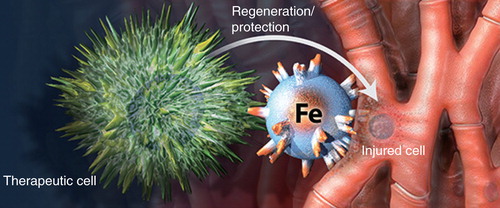
We pioneered the concept of magnetic bispecific cell engager (MagBICE) to achieve stem cell-mediated tissue repair, imaging and localized enrichment with magnetic targeting Citation[4]. MagBICE agents are iron nanoparticles conjugated with two types of antibodies (one against antigens on therapeutic cells and the other directed at injured cells) (). The antibodies link the therapeutic cells to the injured cells, whereas the iron core of MagBICE enables magnetic targeting and MRI.
Figure 2. Magnetic bispecific cell engager agents are iron nanoparticles conjugated with two types of antibodies, one against antigens on therapeutic cells and the other directed at injured cells.
This is certainly not the first use of magnetic particles for targeted drug delivery or imaging Citation[13]. In the design of MagBICE, we used Ferumoxytol (trade name: Feraheme® [FH]) as the iron nanoparticle Citation[3]. Its chemical formula is Fe5874O8752C11719H18682O9933Na414 with an apparent molecular weight of 750 kDa and colloidal particle diameters of 17 – 31 nm. Relative to other iron nanoparticles, Feraheme contains very little free iron, allowing large amounts to be administered intravenously to humans (510 mg of Feraheme has been administered safely in as little as 17 s for a rate of 30 mg/s). Feraheme was first used as an intravenous iron preparation for treatment of the anemia of chronic kidney disease. The use of an FDA-approved particle as the core for MagBICE may facilitate regulatory evaluation of the novel product. Feraheme not only are these magnetic particles in clinical trials as an MRI contrast agent, but they are also coated with a carboxylated dextran that can be used as a convenient surface for antibody attachment.
For the manufacture of MagBICE Citation[3], the carboxyl groups on the Feraheme nanoparticles are activated with water-soluble 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide (EDAC). The EDAC reacts with the carboxyl groups to create an active ester that can bind to the primary amines on the antibodies of interest.
The first MagBICE particle tested (MagBICE1) was generated by conjugating anti-CD45 and anti-MLC antibodies to the magnetic particles. Confocal imaging, flow cytometry and Prussian blue iron staining confirmed that MagBICE1 could bind efficiently to rat bone marrow stem cells (BMCs) and injured cardiomyocytes in vitro. Cardiac MRI revealed that intravenously delivered MagBICE1 efficiently targeted to the injured myocardium, creating a signal void in T2* weighted MRI images in a rat ischemia/reperfusion injury model. Meanwhile, MagBICE1 infusion did not exacerbate inflammation in post-myocardial infarction heart. After intravenous infusion of MagBICE1 together with labeled BMCs, BMC targeting to the heart increased and off-target cell loss into the lungs decreased. Furthermore, more cardiomyocytes were present in the peri-infarct area of MagBICE1-treated hearts, consistent with de novo cardiomyogenesis.
The iron core of the MagBICE is potentially useful not just for imaging but also for magnetic targeting, that is, physical enrichment via application of an external magnetic force Citation[14,15]. Magnetic targeting has the potential to enhance the therapeutic effects of stem cells through increased retention of transplanted cells. In order to address the possibility of MagBICE to enrich endogenous stem cells in the infarct area and the benefits from magnetic targeting, we created another MagBICE (MagBICE2) by conjugating anti-CD34 and anti-MLC antibodies to the magnetic nanoparticles. In the rat myocardial infarct model, MagBICE2 linked endogenous CD34+ stem cells to the injured myocardium and exerted functional benefits. The targeting effect was further strengthened by the application of a 1.3-T magnetic field Citation[3].
As a platform technology, the concept of MagBICE is generalizable to multiple diseases where a disease-specific antigen and a therapeutic cell antigen can be identified. Besides myocardial repair, the utility of MagBICE can be anticipated in other models of disease. For example, to treat peripheral vascular diseases (PVD) or stroke, MagBICE particles could target angiogenic CD34+ cells to ischemic blood vessels, with localization enhanced by magnetic attraction Citation[3,14,15]. Markers expressed by activated/injured endothelium include the leukocyte adhesion molecules intercellular adhesion molecule-1 and vascular cell adhesion molecule, which help to mediate leukocyte-endothelial interaction. In infectious diseases, MagBICE could be designed to engage macrophages and natural killer cells with the pathogen (e.g., bacterial) for targeted eradication. In treating cancer, the advantages of MagBICE over traditional BiTE could even extend beyond magnetic targeting and imaging; alternating current electromagnetic field could be applied to generate hyperthermia at MagBICE particles to enhance cancer cell death.
5. Expert opinion
The ultimate goal of the field of bispecific agents is to achieve the linkage between a therapeutic (e.g. drugs, cells) and a target (e.g. tumor, tissue injury). BiTE antibodies represent a more effective approach of immunotherapy. Amgen’s Blinatumomab has gained FDA approval in December 2014 as the first approved BiTE therapy for treating B-precursor acute lymphoblastic leukemia, a rapidly progressing cancer of the blood and bone marrow. More BiTE candidates are in clinical development, but critical hurdles remain before more BiTE therapies can successfully translate into the clinic. For example, a sufficient amount of effector T cells must be present for activity. ex vivo expansion of T cells together with in vitro pre-arming can mitigate this challenge, but the process is neither simple nor inexpensive. Moreover, side effects may come from a burst of cytokine release from activated T-cell (‘cytokine storm’). Also, doctors need to face the uncertainty in the biodistribution of reintroduced T cells. Targeting therapeutic stem cells to the injured tissue for repair is a new use of BiAbs outside of oncology field. Preclinical animal studies have been focusing on the in vitro and vivo safety and efficacy of this strategy. More evidence should be provided for accurate assessment of stem cells targeting rate and fate in vivo. Also, unlike T cells, stem cells are not specialized for invasion; therefore, further investigation should determine whether arming stem cells with targeting can alter normal cell migration, differentiation and/or function.
MagBICE tackles a central challenge in therapeutics: how to achieve selective, targeted cell-cell engagement and interaction, together with in vivo tracking capability with noninvasive imaging technologies. MagBICE and BiAbs (such as bispecific T-cell engager) are similar in that both employ conjoined antibodies to achieve cellular co-localization. MagBICE, differs fundamentally from BiAbs in that the iron core of MagBICE enables visualization of tissue localization by MRI and further enhancement of targeting efficacy by external magnetic fields. These features may overcome some of the limitations of conventional cell therapy and BiAbs, including elusive biodistribution and poor cell retention. Systemic delivery by intravenous infusion is minimally invasive, but suffers from insufficient homing to the target organ. As mentioned in Section 4, we tested two prototypes of MagBICEs to target systematically delivered stem cells or endogenous stem cells to injured heart tissue, generating improved therapeutic outcomes compared to those seen in preclinical models of direct BMCs transplantation. Compared with a previous report using bispecific antibody-armed BMCs, the MagBICE technology represents a significant technological advancement. First, the MagBICE reagent does not require culture, expansion and modification of the BMCs in vitro before cell infusion; instead, MagBICE can capture endogenous cells in vivo. Moreover, the iron nanocore enables detection by MRI and physical (magnetic) enrichment, which are impossible with traditional BiAbs.
However, before MagBICEs translate into the clinic, questions of the potential risks have to be answered, and the mechanisms underlying the restorative effects of MagBICE therapy should be further elucidated. The hypoenhancement created by the iron core of MagBICE may distort the quality of MRI during the initial period after intravenous infusion. However, the degradation and absorption of iron should solve this problem over time. Second, the therapeutic efficacy of MagBICE heavily relies on the abundance of (circulating) endogenous stem cells. Can adjunctive bone marrow-stimulating agents such as granulocyte-colony-stimulating factor enhance MagBICE efficacy? Such a conjectural therapy merits future investigation. Third, MagBICE was delivered immediately after reperfusion in rats. In many clinical scenarios, a delayed administration may be more realistic, but we have not yet studied the effects of delayed administration. However, as a biologics drug, MagBICE could be applied in an emergency setting such as myocardial infarction. Finally, definitive studies in large animals elucidating function and potential risk of MagBICE are essential before human trials.
In summary, magnetic bifunctional cell engager (MagBICE) has not only proven benefits as an effective treatment of myocardial infarction but also provides potential avenues for treatment of other diseases such as cancer, PVD and infectious diseases. In a rodent model of myocardial infarction, MagBICE redirects circulating stem cells (either exogenous or endogenous) and promotes myocardial recovery. The combination of magnetic nanoparticles with two types of antibodies adds the potential for imaging and for even higher concentration of the therapeutic cells in the targeted tissue by applying a magnetic field.
Declaration of interest
This work was supported by funding from American Heart Association (grant #12BGIA12040477), NC State University Chancellor’s Faculty Excellence Program, and National Natural Science Foundation of China (#H020381370216). J Tang is supported by China Scholarship Council. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Notes
Bibliography
- Thakur A, Lum LG. Cancer therapy with bispecific antibodies: Clinical experience. Curr Opin Mol Ther 2010;12:340-9
- Lum LG, Davol PA, Lee RJ. The new face of bispecific antibodies: targeting cancer and much more. Exp Hematol 2006;34:1-6
- Cheng K, Shen D, Hensley MT, et al. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat Commun 2014; published online 10 September 2014; 10.1038/ncomms5880
- Choi BD, Cai M, Bigner DD, et al. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin Biol Ther 2011;11:843-53
- De Gast GC, Van Houten AA, Haagen IA, et al. Clinical experience with CD3 x CD19 bispecific antibodies in patients with B cell malignancies. J Hematother 1995;4:433-7
- Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res 2009;69:4941-4
- Davol PA, Smith JA, Kouttab N, et al. Anti-CD3 x anti-HER2 bispecific antibody effectively redirects armed T cells to inhibit tumor development and growth in hormone-refractory prostate cancer-bearing severe combined immunodeficient beige mice. Clin Prostate Cancer 2004;3:112-21
- Reusch U, Sundaram M, Davol PA, et al. Anti-CD3 x anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res 2006;12:183-90
- Lee RJ, Fang Q, Davol PA, et al. Antibody targeting of stem cells to infarcted myocardium. Stem Cells 2007;25:712-17
- Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004;364:14-18
- Nagamine H, Watanabe G, Shiobara S, et al. Intramyocardial CD34+ cell transplantation combined with off-pump coronary artery bypass grafting. Heart Surg Forum 2004;7:285-7
- Zhao TC, Tseng A, Yano N, et al. Targeting human CD34+ hematopoietic stem cells with anti-CD45 × anti-myosin light-chain bispecific antibody preserves cardiac function in myocardial infarction. J Appl Physiol (1985). 2008;104:1793-800
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005;26:3995-4021
- Cheng K, Li TS, Malliaras K, et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res 2010;106:1570-81
- Cheng K, Malliaras K, Li TS, et al. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplant 2012;21:1121-35
