Abstract
Endometriosis (EMS) is a chronic, estrogen-dependent inflammatory disease characterized by growth of endometrial tissue outside the uterine cavity. Symptoms in EMS patients include severe pelvic pain, dysmenorrhea, dyspareunia and infertility. To date, medical therapies are mostly based on hormonal suppressive drugs that induce a hypoestrogenic state. Although being effective regarding the reduction of endometriotic tissue masses and pelvic pain, this treatment is accompanied by severe side effects. Since EMS is associated with chronic inflammation, novel therapeutic strategies also focus on immune modulating drugs. However, little is known about how and to what extent immune cell subsets contribute to the network of locally produced cytokines, chemokines and other mitogenic factors that modulate the growth of ectopic endometrial implants and the inflammation associated with them. Mast cells (MCs) are known to be key players of the immune system, especially during allergic reactions. However, in recent years MCs have been identified to exhibit a far broader range of functions and to be involved in host defense and wound healing responses. Here, recent reports that imply an involvement of MCs in EMS has been reviewed, while the value of novel mouse models for clarifying their contribution to the pathology of this condition has been discussed.
Keywords::
1. Endometriosis: an estrogen-dependent, chronic inflammatory disease
Endometriosis (EMS) was first described in medical literature more than 300 years ago and has since been recognized as a chronic, painful disease in women. It is characterized by the presence and growth of endometrial tissues in ectopic sites. EMS is the most common cause of pelvic pain, dysmenorrhea, dyspareunia and subfertility in women of reproductive age. Early diagnosis is critical for management but is still difficult due to the lack of suitable non-invasive tools. In fact, the only reliable technique is surgical identification by laparoscopy.
Although EMS is one of the most investigated disorders in gynecology, its causes remain ill-characterized. Among the numerous theories that attempt to explain the mechanisms involved in the development of EMS, Sampson's theory of retrograde menstruation is best supported by evidence Citation[1]. However, retrograde menstruation occurs in almost all women, but only a small percentage actually develops EMS. As a consequence, other factors must be involved allowing retrogradely displaced endometrial tissue to implant and develop into endometriotic lesions. The current consensus is that EMS is associated with a local pelvic inflammatory process with altered functions of immune-related cells in the peritoneal environment.
Besides its inflammatory component, EMS is evidently an estrogen-dependent disease. Therefore, the medical treatment has focused on the use of hormonal suppression including gonadotropin-releasing hormone (GnRH) analogs, continuous oral contraceptives, gestrinone, progestins or danazol leading to a hypoestrogenic state. Although the pharmacological induction of a hypoestrogenic state reduces EMS-associated pain temporarily, the disease generally relapses after the termination of hormonal treatment. As a consequence of the systemically low levels of estrogen (e.g., after treatment with GnRH analogs), patients experience a variety of side effects including osteoporosis, headache, nausea and weight gain and menopausal-type symptoms. Finally, none of these therapies are of any benefit in resolving the infertility associated with EMS.
These aspects raise the need to develop therapies for EMS with a better risk:benefit ratio.
Most recent developments of better EMS therapies are targeting immune cells and their mediators. For example, it has been claimed that, in affected women, refluxed endometrial cells are able to escape T-cell-driven immune surveillance Citation[2] and that T-cell activating drugs should ameliorate the disease. However, in two small randomized clinical trials, the immune-stimulatory cytokines IL-2 and IFN-α had no effect on lesion size or pain Citation[3,4]. These results indicate that these two cytokines cannot repair defects in immune surveillance or that impaired T-cell function is not critical to EMS pathogenesis.
Most recently, the contribution of immune cells to the chronic inflammation associated with the ectopic lesions in EMS has evolved as a promising novel therapeutic target. Many previous reports have demonstrated elevated levels of various pro-inflammatory cytokines (e.g., IL-4, IL-6, IL-8, IL-12, TNF, MCP-1 and others Citation[5]) in the peritoneal fluid and serum of EMS patients, and EMS lesions are characteristically infiltrated by large numbers of different activated leukocytes. Although the contribution of specific immune cell subsets and their mediators to the onset and the course of the inflammatory process in endometrial lesions is still poorly understood, some evidence exists that mast cells (MCs) are crucially involved.
2. Inflammation and EMS: orchestrated by mast cells?
Traditionally, MCs have been most extensively studied for their role as early effector cells of allergic disease but their additional roles such killing of pathogens, degrading of toxic endogenous peptides, regulating the number, viability, distribution, phenotype and ‘non-immune’ functions of structural cells, such as fibroblasts and vascular endothelial cells become more and more evident Citation[6].
More recently, MCs have also been reported to be involved in complex biological functions and pathologies (e.g., wound healing Citation[7] or autoimmune diseases such as rheumatoid arthritis and multiple sclerosis) Citation[8] and peripheral tolerance Citation[9].
MCs exhibit several exclusive characteristics that discriminate them from other leukocytes. First of all, their maturation and differentiation occurs locally, after migration of their precursors to the vascularized tissues in which they will finally reside. Here, they can exert their effector functions through the direct or indirect actions of a wide variety of preformed or newly synthesized and selectively released mediators including histamine, proteases (e.g., tryptase, chymase), leukotrienes, prostaglandins as well as numerous cytokines (e.g., TNF, IL-1, -3, -4, -5, -6, -8, -9, -13), neurotransmitters and growth factors.
This unique mediator profile enables MCs to initiate an inflammatory cascade leading to the observed symptoms of EMS, for example, by modulating the recruitment, survival, development, phenotype or function of other immune cells described to be involved in EMS pathology, including monocytes/macrophages, granulocytes, dendritic cells (DCs), T and B cells Citation[5,10-14].
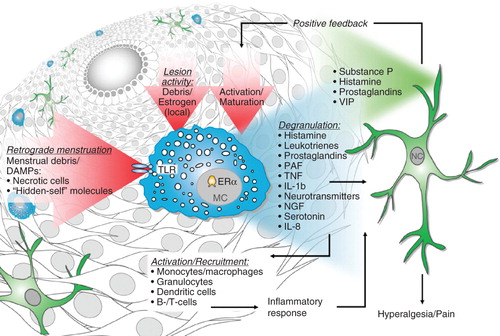
Increasing evidence supports an involvement of MCs also in the inflammatory process of EMS. High numbers of degranulated MCs have been found in endometriotic lesions Citation[15]. Of note, this was not the case at unaffected sites of the peritoneum or eutopic endometrial tissue from EMS patients or healthy controls. Additionally, it has been shown that stem cell factor (SCF), the major growth differentiation and chemoattractant factor for MCs, is found in higher concentrations in the peritoneal fluid of EMS patients, especially in the early stages of the condition Citation[16].
The production of IgE in allergic diseases typically requires the release of Th2 cytokines (e.g., IL-4, IL-5 and IL-13) by T-helper cells. Indeed, in previous reports, the peritoneal fluid and sera of patients with EMS showed enhanced expression of IL-4 Citation[17]. However, whether or not the activation of MCs in EMS lesions is IgE-dependent is yet unclear. The activation of MCs during the early onset of EMS and the tissue damage/remodeling associated with EMS lesions may also occur by multiple other mechanisms. For example, MCs express Toll-like receptors (e.g., TLR2 and TLR4 Citation[18]), that is, pattern recognition receptors (PRRs) that sense damage-associated molecular patterns (DAMPs). DAMPs and so-called ‘hidden-self’ molecules are constituents of menstrual debris Citation[19], which become displaced by retrograde menstruation and may cause MC activation and subsequent ‘sterile’ inflammatory responses through TLR activation Citation[20].
One important feature of EMS is the estrogen-dependent progression of the disease. In EMS patients, the aberrantly high expression of aromatase in ectopic lesions contributes to the increased local concentration of estrogen Citation[21]. Recently, MCs have been reported to express estrogen receptors, and it is generally accepted that estrogen treatment stimulates MCs to release mediators Citation[22]. Importantly, this effect does not require IgE cross-linking and was measurable at physiological estrogen (10-11 to 10-10 M) concentrations. Thus, high levels of locally produced endogenous estrogens may induce and/or facilitate MC activation and MC-driven inflammation by binding to MC estrogen α-receptors. Interestingly, estrogens have attracted significant interest as potential modulators of immune responses that contribute to chronic inflammatory conditions including autoimmune and hypersensitivity diseases, that is, conditions which are widely held to be, at least in part, MC-mediated.
3. Mast cells in EMS: a painful liaison?
EMS is the most common cause of chronic pelvic pain in women. The correlation between pain intensity and the anatomy and biochemistry of the ectopic implants is still poorly understood. However, parallels have been found between pain severity and both the depth of infiltration into the peritoneum or pelvic organs. Anaf et al. found increased numbers of activated MCs near endometriotic lesions, often close to nerve fibers Citation[23]. Importantly, the pain intensity in EMS patients with increased lesional MC numbers was higher as compared with patients with normal lesional MC numbers. MCs are likely to sensitize primary nociceptive neurons by the release of nerve growth factor (NGF) and pro-inflammatory cytokines. Lesional NGF can, in turn, attract MCs and trigger their degranulation Citation[24].
With regard to endometriotic pain, the pro-inflammatory cytokine IL-1 has some important properties. IL-1, which can be released by MCs on TLR triggering induces the synthesis of prostaglandins, important pain mediators and stimulates fibroblasts activation and proliferation, which contribute to fibrosis and adhesion formation by fibrinogen and subsequent collagen depositions Citation[18,25]. Adhesions are frequently found in the area of endometriotic lesions Citation[26,27]. Depending on the severity of adhesion formation, chronic pain arises from organ immobilizations and dysfunctions Citation[28].
MCs can release preformed and de novo synthesized TNF, a pain-mediating pro-inflammatory cytokine which can activate nociceptive primary afferent nerve fibers by eliciting a dose-dependent rapid-onset increase in c-fiber discharge independent of peripheral receptor involvement Citation[29]. Moreover, MCs can secrete the pro-inflammatory chemokine IL-8, which is also known to induce prostaglandin-independent hyperalgesia in vivo Citation[30].
The stimulation of sensory nerve fibers by inflammatory substances can lead to the release of neurotransmitters such as substance P (SP), endothelin, histamine, glutamate, prostaglandins and vasointestinal peptide (VIP). SP is known to provide a positive feedback on MCs by causing degranulation and the release of pro-inflammatory mediators that, in turn, can attract other inflammatory cells Citation[31-33].
Thus, MCs may contribute directly to the development of pain in EMS by releasing mediators that can sensitize/activate sensory nerve fibers, or indirectly by recruitment of other pro-inflammatory leukocytes ().
4. Expert opinion
MCs are known potent mediators of inflammatory immune responses, yet their precise role in inflammation and pain induction in EMS has not been fully elucidated. However, their role in several other pathologies associated with chronic pelvic pain (e.g., irritable bowel syndrome, interstitial cystitis, chronic pelvic pain syndrome and chronic prostatitis) Citation[34,35], especially their role as pain mediators, has been analyzed in more detail. For example, in a murine model of interstitial cystitis it has been shown that the disease-associated pain is MC-dependent Citation[36]. This study took advantage of the so-called MC ‘knock-in’ mouse model. Here, C57BL/6-KitW-sh/W-sh mice, which are genetically MC-deficient due to a mutation in the Kit gene, were used. To exclude the differences between Kit-mutant mice and wild-type mice due to MC-independent abnormalities in these animals, the C57BL/6-KitW-sh/W-sh mice were selectively ‘repaired’ by the adoptive transfer of in vitro-derived MCs Citation[37]. Using this mouse model could clarify the contribution of MCs to the establishment of EMS-associated inflammation and pain.
Furthermore, the therapeutic potential of targeting MCs in murine EMS can be analyzed in a novel transgenic mouse expressing Cre recombinase under the control of the MC protease (Mcpt) 5 promoter Citation[38]. Crossing of these mice with the recently generated iDTR mice Citation[39] generates a mouse in which MCs exclusively express a high-affinity diphtheria toxin (DT) receptor. The subsequent application of DT then leads to the selective depletion of these cells. This conditional MC-ablated mouse allows the analysis of the role of MCs in therapeutic disease models without affecting other cell subsets.
Guilty or not – many new tools are available that will help to better characterize the role of MCs in murine EMS models and potentially open new therapeutic avenues for the treatment of EMS.
Declaration of interest
All authors except M Maurer are employees of Bayer Pharma AG. The work has been supported by Bayer Pharma AG.
Notes
Bibliography
- Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927;3:93-110; 43
- Christodoulakos G, Augoulea A, Lambrinoudaki I, Pathogenesis of endometriosis: the role of defective 'immunosurveillance'. Eur J Contracept Reprod Health Care 2007;12:194-202
- Acien P, Quereda F, Campos A, Use of intraperitoneal interferon alpha-2b therapy after conservative surgery for endometriosis and postoperative medical treatment with depot gonadotropin-releasing hormone analog: a randomized clinical trial. Fertil Steril 2002;78:705-11
- Acien P, Quereda FJ, Gomez-Torres MJ, GnRH analogues, transvaginal ultrasound-guided drainage and intracystic injection of recombinant interleukin-2 in the treatment of endometriosis. Gynecol Obstet Invest 2003;55:96-104
- Herington JL, Bruner-Tran KL, Lucas JA, Immune interactions in endometriosis. Expert Rev Clin Immunol 2011;7:611-26
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 2010;10:440-52
- Weller K, Foitzik K, Paus R, Mast cells are required for normal healing of skin wounds in mice. FASEB J 2006;20:2366-8
- Gregory GD, Brown MA. Mast cells in allergy and autoimmunity: implications for adaptive immunity. Methods Mol Biol 2006;315:35-50
- Lu LF, Lind EF, Gondek DC, Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 2006;442:997-1002
- Maurer M, Metz M. The status quo and quo vadis of mast cells. Exp Dermatol 2005;14:923-9
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol 2008;8:478-86
- Dudeck A, Suender CA, Kostka SL, Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur J Immunol 2011;41:1883-93
- Osuga Y, Koga K, Hirota Y, Lymphocytes in endometriosis. Am J Reprod Immunol 2011;65:1-10
- Saito A, Osuga Y, Yoshino O, TGF-beta1 induces proteinase-activated receptor 2 (PAR2) expression in endometriotic stromal cells and stimulates PAR2 activation-induced secretion of IL-6. Hum Reprod 2011;26:1892-8
- Sugamata M, Ihara T, Uchiide I. Increase of activated mast cells in human endometriosis. Am J Reprod Immunol 2005;53:120-5
- Osuga Y, Koga K, Tsutsumi O, Stem cell factor (SCF) concentrations in peritoneal fluid of women with or without endometriosis. Am J Reprod Immunol 2000;44:231-5
- Hsu CC, Yang BC, Wu MH, Enhanced interleukin-4 expression in patients with endometriosis. Fertil Steril 1997;67:1059-64
- Supajatura V, Ushio H, Nakao A, Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest 2002;109:1351-9
- Flowers CE Jr, Wilborn WH. New observations on the physiology of menstruation. Obstet Gynecol 1978;51:16-24
- Mrabet-Dahbi S, Metz M, Dudeck A, Murine mast cells secrete a unique profile of cytokines and prostaglandins in response to distinct TLR2 ligands. Exp Dermatol 2009;18:437-44
- Bulun SE, Fang Z, Imir G, Aromatase and endometriosis. Semin Reprod Med 2004;22:45-50
- Zaitsu M, Narita S, Lambert KC, Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol 2007;44:1977-85
- Anaf V, Chapron C, El Nakadi I, Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril 2006;86:1336-43
- Anaf V, Simon P, El Nakadi I, Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod 2002;17:1895-900
- Liu W, Ding I, Chen K, Interleukin 1beta (IL1B) signaling is a critical component of radiation-induced skin fibrosis. Radiat Res 2006;165:181-91
- Nezhat F, Nezhat C, Allan CJ, Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med 1992;37:771-6
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update 2005;11:595-606
- Liakakos T, Thomakos N, Fine PM, Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg 2001;18:260-73
- Sorkin LS, Xiao WH, Wagner R, Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 1997;81:255-62
- Cunha FQ, Lorenzetti BB, Poole S, Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol 1991;104:765-7
- Kulka M, Sheen CH, Tancowny BP, Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008;123:398-410
- Tancowny BP, Karpov V, Schleimer RP, Substance P primes lipoteichoic acid- and Pam3CysSerLys4-mediated activation of human mast cells by up-regulating Toll-like receptor 2. Immunology 2010;131:220-30
- Siebenhaar F, Magerl M, Peters EM, Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol 2008;121:955-61
- Mehik A, Leskinen MJ, Hellstrom P. Mechanisms of pain in chronic pelvic pain syndrome: influence of prostatic inflammation. World J Urol 2003;21:90-4
- O'Sullivan M, Clayton N, Breslin NP, Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil 2000;12:449-57
- Rudick CN, Bryce PJ, Guichelaar LA, Mast cell-derived histamine mediates cystitis pain. PLoS One 2008;3:e2096
- Grimbaldeston MA, Chen CC, Piliponsky AM, Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 2005;167:835-48
- Scholten J, Hartmann K, Gerbaulet A, Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res 2008;17:307-15
- Buch T, Heppner FL, Tertilt C, A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2005;2:419-26