Abstract
Cancer is the leading cause of morbidity and mortality in developed countries and the second major cause of death in developing countries. Laminins are crucial proteins in the basal lamina (one of the layers of the basement membrane), and these form a protein network that influences both normal and transformed cell differentiation, migration and adhesion, as well as phenotype and survival. The basement membranes act as a mechanical barrier to tumor growth, but these molecules, including laminins, are also important autocrine factors produced by cancers to promote tumorigenesis. Several studies in cancers have shown the importance of LAMC2, a laminin component. The elevated expression of LAMC2 on cancer cells appears to drive tumorigenesis through its interactions with several cell-surface receptors including α6β4 and α3β1 integrins and EGFRs. The accumulating evidence indicates that LAMC2-mediated signaling network plays an important role in the progression, migration and invasion of multiple types of cancer, suggesting that it might be a potential therapeutic anticancer target for inhibiting tumorigenesis. Furthermore, elevated serum levels of LAMC2 in cancer patients might be an attractive serum-based diagnostic biomarker.
1. Introduction
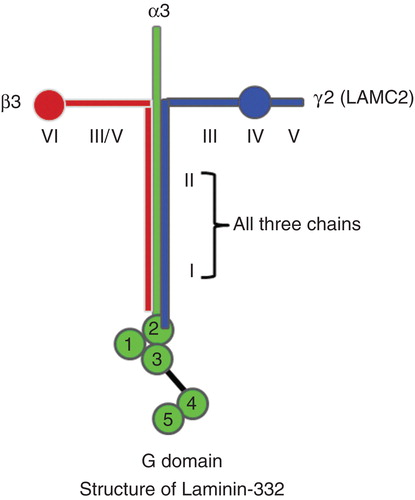
Extracellular matrix proteins, including collagens, laminins, fibronectins and proteoglycans not only create tissue architecture but also regulate complex cellular functions by binding to specific cell-surface receptors. The basement membranes are thin sheets of specialized extracellular matrix proteins supporting epithelial cell layers Citation[1]. Laminin-332 (previously known as laminin-5) is an essential adhesive component of epithelial basement membranes and regulates cell adhesion, differentiation, migration and the invasion of epithelial cells in normal tissues Citation[1]. Laminin-332 consists of three non-identical chains of laminin, α3, β3 and γ2, resulting in a heterotrimeric glycoprotein (). In humans, the γ2 chain is also known as LAMC2. Laminin-332 typically forms a cross-shaped structure when viewed by rotary shadowing electron microscopy Citation[1]. The long arm of the cross consists of domain I and II and has a role in the intermolecular assembly of laminin trimers. A globular structure, known as the G domain, is located at the base of the long arm and contains five EGF repeats ().
Figure 1. Schematic illustrations of Laminin-332 structure: Laminin-332 forms a cruciform shaped structure consisting of three chains (α3, β3 and γ2, also known as LAMC2). Domains I and II in each of the three chains help in forming a trimeric unit. Domain III of laminin γ2 interacts with EGFR. G domain contains five repeating segments with EGF-like sequences at the base of the long arm of the cruciform structure. The first three repeats of EGF-like sequences (G1, G2 and G3 domains) have binding sites for cell-surface integrin receptors. Last two repeats contain heparin binding activity and can interact with extracellular heparin sulfate proteoglycans, such as α-dystroglycan.
The crucial role of γ2 chain of laminin-332 (LAMC2) is reflected by a germ-line inherited mutation of γ2 chain in a disease called Herlitz junctional epidermolysis bullosa (H-JEB), characterized by fragile skin, resulting in widespread blisters and erosions and neonatal death. A homozygous mutation in LAMC2 leads to a premature stop codon (CGA to TGA) in exon 3, resulting in loss of LAMC2 expression in H-JEB disease () Citation[2]. LAMC2-/- mice displayed blistering phenotype with impaired anchorage and detachment of the epidermis on days 1 – 2 of birth similar to human H-JEB. LAMC2-/- mice also have less organized tracheal hemidesmosomes, suggesting its role in normal lung development.
Table 1. Summary of LAMC2 lesions in human diseases and malignancies.
2. Role of LAMC2 in cancers
LAMC2 is located on chromosome 1q25-q31. Array-based comparative genome hybridization revealed amplification of LAMC2 in nasopharyngeal carcinoma (NPC) patient samples (33%) as well as cell lines and xenografts () Citation[3]. The gene is also amplified in hepatocellular carcinoma () Citation[4] and squamous lung cancer () Citation[5]. Immunohistochemical analysis revealed that LAMC2 protein is highly expressed in carcinomas of the pancreas, stomach, tongue, bladder, colorectal, lung, squamous cell carcinoma of vulva, cervix and esophagus (squamous) as well as melanoma and anaplastic thyroid carcinoma (ATC) () Citation[6-13]. The elevated levels of LAMC2 are often correlated with poor overall survival, early recurrence and metastasis in human cancers Citation[12]. The cytoplasmic accumulation of the LAMC2 was typically observed in invading or budding tumor cells, which has been considered a specific marker for invasive tumors. LAMC2 is robustly secreted by high expressing cancers as either a monomer or a heterodimer with β3, resulting in its high serum levels in pancreatic cancer patients Citation[14]. LAMC2 serum levels can be elevated in pancreatic cancer patients who have low serum levels of CA19-9 (a commonly used serum diagnostic marker of pancreatic cancer), suggesting that serum measurement of both LAMC2 and CA19-9 serum levels may provide a more sensitive detection of these cancers Citation[14]. The cytoplasmic expression of LAMC2 strongly correlated with postoperative hepatic metastasis and poorer overall survival of the pancreatic ductal adenocarcinoma Citation[6].
Both TGF-β1 and HGF upregulate the cellular expression of LAMC2. In intestinal epithelial cells, TGF-β1 and HGF synergistically stimulate LAMC2 levels via enhanced activation of AP-1 DNA binding sites of the promoter region of LAMC2 Citation[15]. This synergistic activation of the LAMC2 gene results in overproduction of LAMC2 relative to other laminin constituent chains. The enhanced expression of LAMC2 in gastric cancers appears to be induced by Wnt5a Citation[16]. The Wnt5a ligand binds to Fz2 receptor activating β-catenin to induce transcript of JunD, which can bind with JNK at the AP-1 binding site of the LAMC2 promoter gene enhancing gene expression Citation[16]. Furthermore, transcription factor 4 (TCF-4) and β-catenin can also: i) activate the promoter of LAMC2 through TCF binding sites; and ii) stimulate HGF and JunD to enhance LAMC2 transcription in gastric epithelial cells. The expression of endogenous LAMC2 correlated with the amount of nuclear β-catenin in these cells, suggesting that LAMC2 may be the target gene of β-catenin in colorectal carcinomas. Furthermore, thyroid cancer 1 is an upstream regulator of the Wnt/β-catenin pathways and upregulates the expression β-catenin target genes including LAMC2 Citation[17]. Pim1 (a serine/threonine kinase) and Myc may also co-operate with LAMC2 to enhance the tumorigenicity of prostate cancer cells Citation[18]. Pim1 upregulation of c-Myc increased the expression of LAMC2. Selective inhibition of c-Myc using a short hairpin RNA (shRNA) significantly inhibited the expression of LAMC2, suggesting that LAMC2 is a c-Myc target gene. The data suggest that inhibitors of c-Myc could have a role in treating the tumors expressing high levels of both c-Myc and LAMC2 Citation[18]. Periodically, several studies noted decreased expression of LAMC2 in lung, prostrate, breast and basal cell skin carcinoma because of aberrant methylation of the promoter region of the gene () Citation[19,20]. The functional significance of this observation requires further study.
Laminin-332 is known to stimulate cell migration. This might, in part, be mediated by domain III of LAMC2; this region of the protein is composed of EGF-like repeats allowing it to interact with EGFR. This binding stimulates downstream signaling resulting in increased MMP-2 gene expression, which can enhance cell migration in breast carcinoma Citation[21]. Additionally, investigations of LAMC2 in extra-hepatic cholangiocarcinoma showed that the inhibition of LAMC2 significantly inhibited their ability to invade and migrate Citation[22].
LAMC2 and EGFR appear to be co-activated in a variety of cancers. The binding of EGF to EGFR enhances the expression of the LAMC2 in a several human cancer cell lines including oral (squamous) carcinoma, breast and ATC cell lines Citation[13,21]. Another study also identified hyperactivation of the EGFR/MAPK (extracellular signal-regulated kinase [ERK])-pathway as the driver of LAMC2 overexpression by neoplastic cells and correlated this stimulation with increased phosphorylation (activation) of the translation factors S6 and eIF4B Citation[23]. Recently, we showed that LAMC2 co-localized and interacted with EGFR causing stimulation of the EGFR pathway including ERK1/2 and Akt in ATC. shRNA-mediated stable knockdown of LAMC2 expression in these cells decreased their cell growth in liquid culture, soft agar and in human xenograft murine model Citation[13]. In addition, inhibition of LAMC2 showed reduced cell migration, invasion, wound healing and cell cycle arrest (G1/S phases of cell cycle) of ATC cells Citation[13]. Interestingly, LAMC2 knockdown significantly suppressed the EGF-induced invasion of epidermoid carcinoma cells (A431 cells) Citation[24]. Moreover, cetuximab (EGFR blocking antibody) or EGFR small interfering RNA additively enhanced the antiproliferative effect of LAMC2 knockdown compared to control cells.
MicroRNA (miR) research has recently added a new layer of complexity to LAMC2 regulation. miRs are small, endogenous and evolutionary highly conserved RNA molecules that modulate the expression of their target genes post-transcriptionally by binding to the 3′UTR (untranslated region) of target mRNA blocking translation and often mediating degradation of target RNA. The expression patterns showed significant downregulation of miR-29a/b/c in various cancers including head and neck. Recent studies showed that LAMC2 and α6 integrin (ITGA6) mRNA are targets regulated by miR-29s Citation[25]. Therefore, decreased expression of miR29 in selected cancers may result in high expression of LAMC2, which may enhance cancer progression.
3. Expert opinion for LAMC2 to be a biomarker and therapeutic target of cancer
Currently, available serum markers are insufficient for diagnosis of early or recurrent cancers. As mentioned, the measurements of serum levels of LAMC2 may augment measuring CA19-9 serum levels allowing earlier detection of primary or recurrent pancreatic cancer. This raises the question, can serum measurement of LAMC2 be used as a reliable diagnostic biomarker for the detection of other solid cancers. To determine if this might be the case requires further study on large cohorts of different cancers. Emerging understanding of the role of the LAMC2 in enhancing cancer progression, migration and invasion raise the possibility that inhibiting LAMC2 may be an attractive therapeutic target for anticancer therapy. A neutralizing monoclonal antibody against LAMC2 may be an intriguing therapeutic approach.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
- Marinkovich MP. Laminin 332 in squamous-cell carcinoma. Nat Rev Cancer 2007;7:370-80
- Aberdam D, Galliano MF, Vailly J, et al. Herlitz’s junctional epidermolysis bullosa is linked to mutations in the gene (LAMC2) for the gamma-2 subunit of nicein/kalinin (Laminin−5). Nat Genet 1994;6:299-304
- Hui AB, Lo KW, Teo PM, et al. Genome wide detection of oncogene amplification in nasopharyngeal carcinoma by array based comparative genomic hybridization. Int J Oncol 2002;20:467-73
- Hashimoto K, Mori N, Tamesa T, et al. Analysis of DNA copy number aberrations in hepatitis C virus associated hepatocellular carcinoma by conventional CGH and array CGH. Mod Poathol 2004;17:617-722
- Ma J, Gao M, Lu Y, et al. Gain of 1q25-32, 12q23-24.3, and 17q12-22 facilitates tumorigenesis and progression of human squamous cell lung cancer. J Pathol 2006;210:205-13
- Takahashi S, Hasebe T, Oda T, et al. Cytoplasmic expression of laminin gamma2 chain correlates with postoperative hepatic metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma. Cancer 2002;94:1894-901
- Koshikawa N, Moriyama K, Takamura H, et al. Overexpression of laminin gamma 2 chain monomer in invading gastric carcinoma cells. Cancer Res 1999;59:5596-601
- Ono Y, Nakanishi Y, Ino Y, et al. Clinicopathologic significance of laminin-5 c2 chain expression in squamous cell carcinoma of the tongue: immunohistochemical analysis of 67 lesions. Cancer 1999;85:2315-21
- Smith SC, Nicholson B, Nitz M, et al. Profiling bladder cancer organ site-specific metastasis identifies LAMC2 as a novel biomarker of hematogenous dissemination. Am J Pathol 2009;174:371-9
- Hlubek F, Jung A, Kotzor N, et al. Expression of the invasion factor laminin gamma 2 in colorectal carcinomas is regulated by b-catenin. Cancer Res 2001;61:8089-93
- Skyldberg B, Salo S, Eriksson E, et al. Laminin-5 as a marker of invasiveness in cervical lesions. J Natl Cancer Inst 1999;91:1882-7
- Yamamoto H, Itoh F, Iku S, et al. Expression of the gamma 2 chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human oesophageal squamous cell carcinoma. Clin Cancer Res 2001;7:896-900
- Garg M, Kanojia D, Okamoto R, et al. Laminin 5 gamma 2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration, and invasion by modulating signaling of EGFR. J Clin Endocrinol Metab 2014;99(1):E62-72
- Kosanam H, Prassas I, Chrystoja CC, et al. Laminin, gamma 2 (LAMC2): a promising new putative pancreatic cancer biomarker identified by proteomic analysis of pancreatic adenomacarcinoma tissues. Mol Cell Proteomics 2013;12:2820-32
- Olsen J, Lefebvre O, Fritsch C, et al. Involvement of activator protein 1 complexes in the epithelium-specific activation of the laminin gamma 2 chain gene promoter by hepatocyte growth factor (scatter factor). Biochem J 2000;347:407-17
- Yamamoto H, Kitadai Y, Yamamoto H, et al. Laminin gamma 2 mediates Wnt5a-induced invasion of gastric cancer cells. Gastroenterology 2009;137:242-52
- Jung Y, Bang S, Choi K, et al. TC1 (C8orf4) enhances the Wnt/beta-catenin pathway by relieving antagonistic activity of Chibby. Cancer Res 2006;66:723-8
- Kim J, Roh M, Abdulkadir SA. Pim1 promotes human prostate cancer cell tumorigenicity and c-MYC transcriptional activity. BMC Cancer 2010;10:248
- Sathyanarayana UG, Padar A, Huang CX, et al. Aberrant promoter methylation and silencing of laminin-59-encoding genes in breast carcinoma. Clin Cancer Res 2003;9:6389-94
- Hao J, Yang Y, McDaniel KM, et al. Differential expression of laminin 5 (alpha 3 beta 3 gamma 2) by human malignant and normal prostrate. Am J Pathol 1996;149:1341-9
- Schenk S, Hintermann E, Bilban M, et al. Binding to EGF receptor of a laminin-5 – like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol 2003;161:197-209
- Liu W, Tian F, Jiang P, et al. Aberrant expression of LAMC2 correlates with poor prognosis and promote invasion in extra-hepatic Cholangiocarcinoma. J Surg Res 2014;186:150-6
- Degen M, Natarajan E, Barron P, et al. MAPK/ERK-dependent translation factor hyperactivation and dysregulated laminin gamma2 expression in oral dysplasia and squamous cell carcinoma. Am J Pathol 2012;180:2462-78
- Hamasaki H, Koga K, Aoki M, et al. Expression of laminin 5-gamma2 chain in cutaneous squamous cell carcinoma and its role in tumor invasion. Br J Cancer 2011;105:824-32
- Kinoshita T, Nohata N, Hanazawa T, et al. Tumor-suppressive microRNA-29s inhibits cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma. Br J Cancer 2013;109:2636-45
