Abstract
Metabolic reprogramming is one of the hallmarks of cancer. Altered metabolism in cancer cells is exemplified by enhanced glucose utilization, a biochemical signature that is clinically exploited for cancer diagnosis using positron-emission tomography and computed tomography imaging. Accordingly, disrupting the glucose metabolism of cancer cells has been contemplated as a potential therapeutic strategy against cancer. Experimental evidences indicate that targeting glucose metabolism by inhibition of glycolysis or oxidative phosphorylation promotes anticancer effects. Yet, successful clinical translation of antimetabolites or energy blockers to treat cancer remains a challenge, primarily due to lack of efficacy and/or systemic toxicity. Recently, using nanotechnology, Marrache and Dhar have documented the feasibility of delivering a glycolytic inhibitor through triphenylphosphonium (TPP), a mitotropic agent that selectively targets mitochondria based on membrane potential. Furthermore, by utilizing gold nanoparticles the investigators also demonstrated the potential for simultaneous induction of photothermal therapy, thus facilitating an additional line of attack on cancer cells. The report establishes that specific inhibition of tumor glycolysis is achievable through TPP-dependent selective targeting of cancer cells. This nanotechnological approach involving TPP-guided selective delivery of an antiglycolytic agent complemented with photothermal therapy provides a new window of opportunity for effective and specific targeting of tumor glycolysis.
1. Introduction
In cancer, uncontrolled proliferation and survival under unfavorable microenvironments necessitate an uninterrupted supply of cellular energy (e.g., ATP). In order to meet this metabolic requirement, cancer cells increase the rate of glucose uptake and its utilization. This metabolic phenotype is commonly witnessed in vast majority of cancers. Hence, this functional characteristic of cancer is currently exploited in the clinical diagnosis of cancer through positron-emission tomography and computed tomography (PET/CT) imaging Citation[1]. The propensity of cancer cells to accelerate glucose metabolism for uninterrupted growth also suggests that glucose metabolism could potentially be used as a therapeutic target. Consequently, several research laboratories around the world focused on the development of energy blockers that can disrupt glucose metabolism. It is worth recalling that major steps of glucose catabolism are divided into two main phases: i) glycolysis (the process of conversion of glucose into pyruvate) and ii) oxidative phosphorylation. Under normoxic or aerobic conditions, pyruvate is metabolized via oxidative phosphorylation in the mitochondria. However, under hypoxic conditions or mitochondrial dysfunction, pyruvate is converted into lactate (a process known as anaerobic glycolysis), and the lactate thus produced is exported to the extracellular milieu. Interestingly, cancer cells oxidize glucose into pyruvate followed by lactate production even in the presence of sufficient molecular oxygen (a process known as ‘aerobic glycolysis’). Although ‘aerobic glycolytic phenotype’ of cancer cells has long been known Citation[2], only in light of recent research its intricacies and relevance to cancer progression have been increasingly evident Citation[3]. Thus, focus on tumor metabolism, particularly tumor glycolysis, has gained further attention for potential therapeutic intervention.
2. Targeting glycolysis in cancer
Several elegant reviews have highlighted the biochemical and physiological advantages of aerobic glycolysis in cancer cells Citation[4]. It has been well established that aerobic glycolysis in conjunction with pentose phosphate pathway (PPP) provide multiple benefits to cancer cells, which include macromolecular biosynthesis, metabolic maintenance and resistance to therapy. Moreover, glycolytic enzymes have also been known to be multifunctional due to their participation in several non-glycolytic processes Citation[5]. Thus, targeting tumor glycolysis is likely to impact cancer cells beyond glycolysis.
Preclinical studies have identified several antiglycolytic agents that target various enzymes of glycolysis. Although many candidate drugs have been developed and evaluated in preclinical tumor models, majority of them were unsuccessful in clinical translation. Lack of efficacy (e.g., chemoresistance) and/or systemic toxicity are the major impediments that prevent successful clinical translation of any potential anticancer agent. Despite detailed validation of molecular specificity and mechanism of action in animal models, the selective targeting of tumor remains a major challenge. Conceivably, selective targeting and specific inhibition of cancer cells could improve the efficacy while reducing, if not eliminating, unwanted toxicities.
3. Triphenylphosphonium
Although phosphonium salts (e.g., triphenylphosphonium [TPP]) were primarily used in chemistry (for labeling, synthesis, etc.), the earliest indication of their anticancer potential was documented in the late 1970s Citation[6]. Soon several reports supported the anticancer activity of phosphonium salts Citation[7,8]. Mechanistically, the cancer-targeting principle of TPP has been known to rely on membrane potential of cells. Since cancer cells have been known to exhibit distinctive membrane potential, more so on the mitochondria, the lipophilic nature and cationic property of TPP enable it to selectively or preferentially target cancer cells Citation[9]. Besides its therapeutic potential, TPP has also been investigated for its possible role in cancer detection using tracers/imaging technology Citation[10]. Taken together, TPP demonstrates marked potential for its use in cancer therapy and/or detection.
The cancer-targeting ability of TPP has been substantiated in multiple cancer cell lines in vitro and in vivo Citation[11]. Biochemically, TPP-mediated anticancer effects involve its localization to mitochondria followed by impairment of mitochondrial electron transport resulting in the disruption of oxidative phosphorylation Citation[12]. Furthermore, it has been shown that the efficacy of TPP cations to affect mitochondrial respiration increased proportionately with the length of the alkyl side chains. Thus, the hydrophobicity of TPP moiety impacts the effective targeting of mitochondria Citation[12]. Intriguingly, it is the chemical capacity of TPP to conjugate with other anticancer chemotherapeutic agents that expanded its therapeutic potential and relevance in cancer treatment. Experimental evidences demonstrate that TPP-conjugated chemotherapeutics could achieve concurrent inhibition of mitochondrial function as well as the molecular target of interest Citation[13]. For example, conjugation of the antineoplastic alkylating agent, chlorambucil with TPP resulted in the generation of Mito-Chlor. Remarkably, Mito-Chlor showed selective accumulation in the mitochondria of cancer cells due to the intrinsic difference in membrane potential between cancer and normal cells. While the TPP facilitated selective delivery of Mito-Chlor to cancer mitochondria, the chlorambucil alkylated mitochondrial DNA thus promoting anticancer effects. Similar, TPP-guided delivery approach has been validated for other anticancer agents such as doxorubicin Citation[14] and paclitaxel Citation[15]. Although potential challenges to cellular uptake of TPP by resistance mechanisms (e.g., P-glycoprotein and breast cancer resistance protein) have been indicated Citation[16], compelling data demonstrate that TPP-conjugated chemotherapeutic could overcome resistance and target cancer cells effectively Citation[17,18]. Thus, the cancer specificity and anticancer potential of TPP revitalizes the current enthusiasm to target cancer metabolism.
4. Expert opinion
The seminal discovery made by the German scientist Otto Warburg disclosed for the first time that cancer cells produce lactate even in the presence of oxygen. This process of production of lactate known as fermentation was referred as ‘aerobic fermentation’, also commonly known as ‘aerobic glycolysis’. However, the notion that carcinogenesis, that is, the origin of cancer is due to a dysfunctional or mutated mitochondria remains challenged. In the words of Douglas Wallace Citation[19], eminent scientist of mitochondrial biology, “Contrary to conventional wisdom, functional mitochondria are essential for the cancer cell. Although mutations in mitochondrial genes are common in cancer cells, they do not inactivate mitochondrial energy metabolism but rather alter the mitochondrial bioenergetics and biosynthetic state”. In a detailed review, Zhivotovsky and collaborators have opined that “the Warburg effect is more closely related to alterations in signaling pathways that govern glucose uptake and utilization than to mitochondrial defects per se” Citation[20]. Irrespective of whether mitochondrial respiration contributes or governs aerobic glycolysis, the role of mitochondria in cancer growth and survival is indispensable. Almost four decades ago, in one of the earliest reviews Pedersen has deciphered the cancer-associated significance of mitochondria Citation[21]. In fact, disruption of glycolysis in cancer cells by lactate dehydrogenase inhibition has been shown to promote a metabolic switch leading to mitochondria-dependent oxidative phosphorylation Citation[22]. In other words, glycolytic cancer cells are capable of switching to mitochondrial oxidation if possible and necessary. Thus, in principle, a complete abrogation of (glucose-dependent) energy metabolism to affect cancer cells must involve concurrent inhibition of glycolysis and mitochondrial respiration. The electrical potential of plasma membrane of cancer cells has been known to be distinctive compared with normal cells Citation[23,24]. Next, the cellular delivery of TPP relies on the plasma membrane potential, and its mitochondrial accumulation depends upon mitochondrial membrane potential. As evident from the literature, it is possible to image or identify cancers using TPP-guided tracers Citation[25]. Thus, using TPP as a delivery system for selective targeting of tumor cells (based on differential membrane potential) is feasible and perhaps safe for normal, healthy cells.
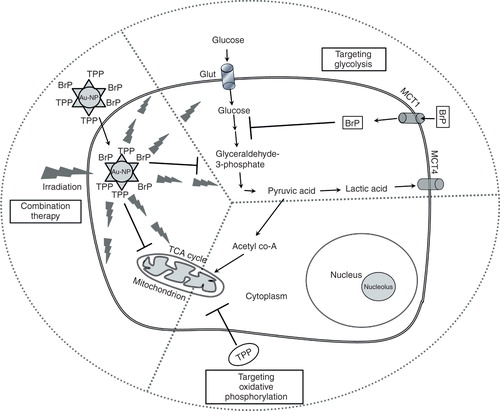
With the advent of nanotechnology, especially chemically compatible microspheres and nanoparticles, the concept of selective targeting of cancer has gained momentum. Micro- and nanocarriers can distribute and deliver potent anticancer, cytotoxic agents with minimal or no systemic toxicity. Recently, using TPP and polyethylene imine hyperbranched polymer, Theodossiou et al. Citation[14]. have demonstrated the feasibility of generation of a mitotropic nanocarrier. In fact, this mitotropic nanocarrier has been verified for the delivery of the chemotherapeutic, doxorubicin. The superior efficacy as well as the specificity of TPP-based drug delivery underscores the therapeutic potential and relevance of such mitotropic nanocarriers. Earlier, Dhar and collaborators have also shown the effectiveness of TPP-guided delivery of the glycolytic inhibitor, dichloroacetate (DCA) Citation[26]. DCA-dependent inhibition of pyruvate dehydrogenase kinase (PDK) enables pyruvate dehydrogenase (PDH) to oxidize pyruvate via mitochondrial respiration. Thus, DCA is a facilitator of mitochondrial oxidation which indirectly suppresses or minimizes glycolysis. Notably, as DCA treatment rewires the metabolic circuitry of cancer cells to oxygen-dependent, mitochondrial oxidation of glucose, such cancer cells are known to be sensitive to hypoxia and demonstrate low tolerance for hypoxic conditions. However, data on DCA’s effect on PPP, another critical metabolic pathway, remain obscure. The antiglycolytic agent, 3-bromopyruvate used by Marrache and Dhar Citation[27] has been known to target glycolytic enzymes (e.g., glyceraldehyde-3-phosphate dehydrogenase) that impact PPP as well. Ideally, inhibition of glycolysis at the initial steps of glucose oxidation is desirable as it can affect both glycolysis as well as PPP. Therefore, combining antiglycolytic agent with mitotropic TPP enables us to achieve specific and effective abrogation of tumor glycolysis.
A potent inhibitor or therapeutic that is capable of dual function (inhibition glycolysis and mitochondrial oxidation) yet specific to cancer cells remains to be known. Nevertheless, the report of Marrache and Dhar Citation[27] demonstrates the feasibility of combining antiglycolytic and antimitochondrial effects via nanotechnology (). The use of gold nanoparticles by the investigators provided additional therapeutic opportunity via photothermal therapy. Thus, the generation of TPP-conjugated gold nanoparticle as a carrier for the delivery of antiglycolytic agent could invigorate current enthusiasm, and underscores the therapeutic potential of targeting tumor metabolism. Further characterization with a focus on dosing regimen, maximum tolerated dose and bioavailability in animal models of varieties of human cancers would throw more light in understanding the translational potential and clinical relevance of this nanotechnology-based inhibition of cancer metabolism.
Acknowledgements
The author apologizes for not citing several relevant literatures due to space limitations.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
Notes
Bibliography
- Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol 2001;2(3):157-64
- Warburg O, Posener K, Negelein E. Über den stoffwechsel der tumoren. Biochem Z 1924;152:319-44
- Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol Cancer 2013;12:152, 4598-12-152
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27:441-64
- Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 2005;30(3):142-50
- Dubois RJ, Lin CC, Beisler JA. Synthesis and antitumor properties of some isoindolylalkylphosphonium salts. J Med Chem 1978;21(3):303-6
- Rideout DC, Calogeropoulou T, Jaworski JS, et al. Phosphonium salts exhibiting selective anti-carcinoma activity in vitro. Anticancer Drug Des 1989;4(4):265-80
- Manetta A, Gamboa G, Nasseri A, et al. Novel phosphonium salts display in vitro and in vivo cytotoxic activity against human ovarian cancer cell lines. Gynecol Oncol 1996;60(2):203-12
- Modica-Napolitano JS, Aprille JR. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev 2001;49(1-2):63-70
- Li Z, Lopez M, Hardy M, et al. 99m)tc-labeled triphenylphosphonium derivative for the early detection of breast tumors. Cancer Biother Radiopharm 2009;24(5):579-87
- Millard M, Pathania D, Shabaik Y, et al. Preclinical evaluation of novel triphenylphosphonium salts with broad-spectrum activity. PLoS One 2010;5:10
- Trnka J, Elkalaf M, Andel M. Lipophilic triphenylphosphonium cations inhibit mitochondrial electron transport chain and induce mitochondrial proton leak. PLoS One 2015;10(4):e0121837
- Millard M, Gallagher JD, Olenyuk BZ, Neamati N. A selective mitochondrial-targeted chlorambucil with remarkable cytotoxicity in breast and pancreatic cancers. J Med Chem 2013;56(22):9170-9
- Theodossiou TA, Sideratou Z, Katsarou ME, Tsiourvas D. Mitochondrial delivery of doxorubicin by triphenylphosphonium-functionalized hyperbranched nanocarriers results in rapid and severe cytotoxicity. Pharm Res 2013;30(11):2832-42
- Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium-PEG-PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J Control Release 2012;159(3):393-402
- Porteous CM, Menon DK, Aigbirhio FI, et al. P-glycoprotein (Mdr1a/1b) and breast cancer resistance protein (bcrp) decrease the uptake of hydrophobic alkyl triphenylphosphonium cations by the brain. Biochim Biophys Acta 2013;1830(6):3458-65
- Han M, Vakili MR, Soleymani Abyaneh H, et al. Mitochondrial delivery of doxorubicin via triphenylphosphine modification for overcoming drug resistance in MDA-MB-435/DOX cells. Mol Pharm 2014;11(8):2640-9
- Marrache S, Pathak RK, Dhar S. Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc Natl Acad Sci USA 2014;111(29):10444-49
- Wallace DC. Mitochondria and cancer. Nat Rev Cancer 2012;12(10):685-98
- Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: What is so special about them? Trends Cell Biol 2008;18(4):165-73
- Pedersen PL. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res 1978;22:190-274
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006;9(6):425-34
- Marino AA, Morris DM, Schwalke MA, et al. Electrical potential measurements in human breast cancer and benign lesions. Tumour Biol 1994;15(3):147-52
- Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol 2013;4:185
- Wang J, Yang CT, Kim YS, et al. 64Cu-labeled triphenylphosphonium and triphenylarsonium cations as highly tumor-selective imaging agents. J Med Chem 2007;50(21):5057-69
- Pathak RK, Marrache S, Harn DA, Dhar S. Mito-DCA: A mitochondria targeted molecular scaffold for efficacious delivery of metabolic modulator dichloroacetate. ACS Chem Biol 2014;9(5):1178-87
- Marrache S, Dhar S. The energy blocker inside the power house: Mitochondria targeted delivery of 3-bromopyruvate. Chem Sci 2015;6(3):1832-45