Abstract
Oral colon-targeted drug delivery systems have gained enormous attention among researchers in the last two decades. The significance of this site-specific drug delivery system can be measured by its usefulness for delivering a variety of therapeutic agents, both for the treatment of local diseases or for systemic therapies. With the arrival of newer innovations, a large number of breakthrough technologies have emerged for targeting a drug molecule to the colon. Researchers have attempted various approaches in the development of these formulation technologies, such as pH-dependent, time-dependent and microflora-activated systems. Recently, a number of approaches have been proposed that utilize a novel concept of di-dependent drug delivery systems, that is, the systems in which the drug release is controlled by two factors: pH and time, and pH and microflora of the colon. This Editorial article is not intended to offer a comprehensive review on drug delivery, but shall familiarize the readers with the formulation technologies that have been developed for attaining colon-specific drug delivery.
1. Introduction
Colonic delivery refers to the targeted delivery of drugs into the lower part of the gastrointestinal tract (GIT), that is, the large intestine. Colonic drug delivery can be achieved by either oral or rectal administration. The rectal routes (suppositories and enemas) are not always effective because of high variability in the distribution of the drugs administered by this route. On the other hand, the major drawbacks of administering the drugs by oral route are absorption and degradation of drug in the upper part of the GIT. Hence, in the design of per-oral controlled release drug products, colon-targeted drug delivery systems (CoDDS) have gained a tremendous interest for delivering a variety of drugs Citation[1].
CoDDS are mainly used for localized treatment of certain diseased conditions such as inflammatory bowel disease (IBD), colon cancer, irritable bowel syndrome (IBS) and so on. Recently, much attention has been focused on the colon as a potential site for the absorption of proteins, peptides (such as insulin, calcitonin and vasopressin) and vaccines (since it is rich in lymphoid tissues). Oral pulsatile/delayed systems intended for chronotherapy (of diseases such as arthritis, asthma, cardiac arrhythmias, hypertension or inflammation), when provided with an external enteric film, can offer interesting possibilities for colonic drug delivery. Since onset of these diseases is at night time or early in the morning, it is desirable to have a delayed release delivery system that can provide nocturnal release of a drug, which in turn provides a considerable relief to the patient while resting Citation[1,2].
Over the last few years, various approaches have been used for oral delivery of drugs to the colon, which include a pH-dependent, time-dependent, pressure-controlled system and systems that use bacteria that colonize in the colon or produce enzymes to modulate the drug release Citation[1]. It has been reported that the system coated with pH-dependent polymers lacks site specificity for drug release in the colon and may either lead to a premature release of drug in the small intestine or no drug release in the colon Citation[2-4]. For time-dependent systems, the location of initial drug release predominantly depends on the transit times of the GIT. Although the transit time of small intestine is relatively constant (3 – 4 h), a large variation in the gastric emptying time may lead to either a premature release of drug in small intestine or a delayed release far down in the colon Citation[5-8]. A more precise and accurate strategy for targeting drugs to the colon uses the ecosystem of the specific microflora present in the large intestine, that is , microbially triggered CoDDS. Natural polysaccharides are the most promising and commonly explored carriers for CoDDS, which are specifically hydrolyzed by the colonic microflora. Unfortunately, as most of the natural polysaccharides are highly hydrophilic, there is difficulty in controlling release of drug from these materials Citation[9,10]. Hence in order to overcome the drawback of each individual approach, recently, newer approaches have been proposed that utilizes a novel concept of di-dependent drug delivery systems, that is, the systems in which the drug release is controlled by two factors viz., pH and time, and pH and microflora of the colon. This Editorial article is not intended to offer a comprehensive review on drug delivery, but to highlight the breakthrough technologies that emerged as a milestone in the field of CoDDS. Additionally, an attempt has been made to give a brief about how colon can be exploited for delivering drugs for local and systemic applications.
2. Colon as a potential site for local and systemic delivery of drugs
CoDDS has major advantages in the direct treatment of the local diseases as well as for systemic therapy. In order to achieve a successful colonic delivery, the drug molecule must reach intact to the desired site by overcoming all the barriers of the upper GIT.
2.1 Local delivery of drugs
CoDDS for local treatment is usually undertaken for various diseased conditions such as IBD, IBS, colon cancer and so on.
IBD is a localized inflammation of the small and large intestines. It mainly comprises two types of specific conditions, that is, ulcerative colitis and Crohn's disease. Use of site-specific drug delivery to the colon can reduce the total amount of drug administered, thereby reducing side effects. Drug therapies for these disorders include aminosalicylates, corticosteroids, immunosuppressive agents, nicotine, cationized antioxidant enzymes and genetically engineered bacteria to produce cytokines Citation[1].
In addition to IBD, IBS is another common chronic gastrointestinal disorder in which the interaction of psychological, luminal and enteric factors results in disorders of the gut functions. The anticholinergics (e.g., dicyclomine, propantheline), anxiolytics, tricyclic antidepressants (e.g., desipramine and trimipramine), 5-hydroxytryptamine antagonists, calcium channel blockers (nifedipine, nicardipine) and calcium antagonist (pinaverium bromide) have been used for IBS treatment Citation[1].
The treatment of colonic cancer using intravenous administration produces severe systemic side effects due to their cytotoxic effect on normal cells. Researchers have reported the use of various lectins and neoglycoconjugates for targeting drugs to cancer cells. They have shown improved specific targeting to the colon on oral administration, with avoidance of the degradation associated with the hepatic first-pass metabolism Citation[11,12]. Recently, ligand-mediated targeted delivery is being explored for improving the selective toxicity of anticancer therapeutics. The delivery systems used for targeting anticancer drugs to tumor cells or tissues can be improved by a combination of binding drugs with molecules that bind to antigens or receptors that are specific on the target cells as compared with normal cells or tissues Citation[13].
Laxatives such as sennosides and related compounds, when targeted to the colon, show improved efficacy because they act specifically in the large intestine. The prodrug laxative sodium sulisatin, an anionic drug, is poorly absorbed and arrives intact to the colon on oral administration. It is then hydrolyzed in the colon by the bacterial arylsulfate sulfohydrolase to a diphenolic derivative, which is an active laxative Citation[14]. Bisacodyl is also cleaved by the esterases in the small intestine to its active metabolite. This causes stimulation of water and secretion of electrolytes and causes a laxative effect Citation[15].
2.2 Systemic delivery of protein/peptide and non-peptide drugs
The colon is also a potential site for delivering therapeutic proteins and peptides. The advantages of colonic delivery of peptide and protein drugs include low metabolic activity, longer residence time, responsiveness to absorption enhancers, good targeting opportunities due to the presence of colonic bacterial enzymes, improved absorption for ionizable/ionized drugs due to transmucosal and membrane potential differences, and scope for solvent drag due to bulk water absorption in this region. If the problems of limited bioavailability and successful targeting to the colon are resolved, the colon could be a potential site for proteins and peptides. Protein and peptide drug candidates exploited for CoDDS are insulin, calcitonin, met-enkephalins, vasopressin, interferons, glucagon and so on Citation[1].
CoDDS are also advantageous to deliver non-peptide drugs when a delay in the absorption is required. They are also useful for the treatment of diseases that have peak symptoms in the early morning and exhibit circadian rhythms, such as arthritis, asthma, cardiac arrhythmias, hypertension or inflammation. These drugs include diclofenac sodium, ibuprofen, pseudoephedrine and others. The colon has also been proven to be a potential site for the absorption of various beta-blockers such as alprenolol hydrochloride, atenolol, bunolol hydrochloride, penbutolol sulfate, pronethalol hydrochloride, metoprolol, oxprenolol, bevantolol, bufuralol, propanolol hydrochloride and timolol maleate, as reported by Vila et al. Citation[16]. These researchers have reported the good penetrability of the drugs through the colonic membrane. Another series of references cited by Godbillon Citation[17] and his research team has demonstrated the colonic absorption of metoprolol. Theophylline, acetaminophen and phenylpropanolamine hydrochloride have also been delivered to the colon with high relative bioavailability of these drugs, which suggests that the colonic absorbability of these drugs is quite good Citation[18].
3. Cutting-edge technologies developed for CoDDS
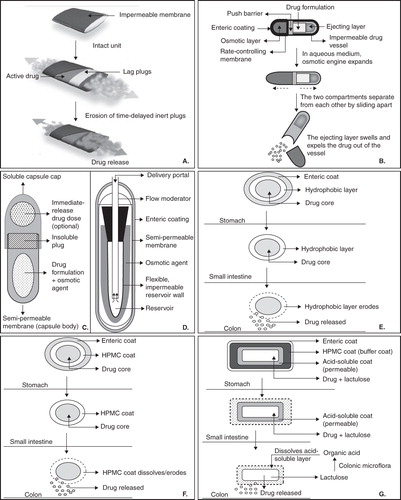
With the advancements in the ongoing research in CoDDS, large numbers of formulation technologies have been reported. These technologies may be broadly classified based on the formulation approaches that have been exploited for the development of CoDDS, viz, pH-dependent, time-dependent, microflora-activated, pH- and time-dependent, and pH- and microflora-activated systems. Among them, pH-dependent systems are most widely used as far as commercial availability of CoDDS is concerned Citation[2]. Furthermore, in early 2007, the first and only FDA-approved once daily oral formulation of mesalamine, MMX (Multi-Matrix System™, Shire Pharmaceuticals Inc., Pennsylvania, USA) was developed for the induction of remission of mild to moderate ulcerative colitis. In Europe, MMX mesalazine (Mezavant XL in the UK; Mezavant elsewhere in the EU) has been approved for the induction and maintenance of clinical and endoscopic remission in patients with active, mild to moderate ulcerative colitis. In order to understand the execution of some of the time-dependent systems, the readers are requested to have thorough knowledge regarding the maneuver of osmotic drug delivery systems. The breakthrough technologies that have been developed for CoDDS are summarized in (a detailed schematic diagram of some of the technologies and their functioning is shown in and ).
Table 1. Breakthrough technologies developed for achieving colon-targeted drug delivery systems.
Figure 1. Schematic diagram of (A) pulsatile hydrogel capsule, (B) Pulsincap, (C) erodible plug time-delayed capsule, (D) hydrophilic sandwich capsule, (E) OROS-CT, (F) time controlled explosion system and (G) Eudracol.
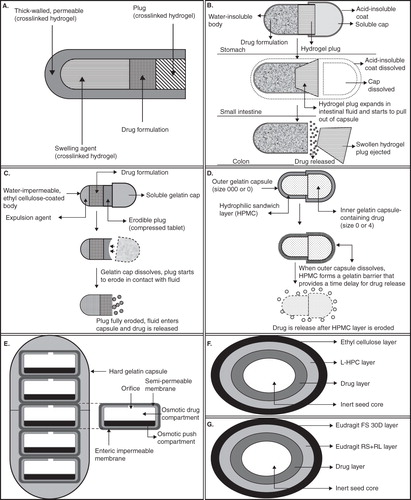
Figure 2. Schematic diagram of (A) Egalet, (B) Chronset™, (C) PORT System, (D) Osmet™, (E) Time Clock system, (F) Chronotopic system (G) CODES™.
The use of nanoparticles for targeted oral drug delivery to the inflamed gut tissue in IBD appears to be a promising approach. Such a strategy of local drug delivery would be a distinct improvement compared with the existing colon delivery devices for this disease. For colonic pathologies, it was shown that nanoparticles tend to accumulate at the site of inflammation in IBD. This is because in the case of colitis, a strong cellular immune response occurs in the inflamed regions due to increased presence of neutrophils, natural killer cells, macrophages and so on. It has been reported that microspheres and nanoparticles could be efficiently taken up by these macrophages. This results in the accumulation of the particulate carrier system resulting in prolonged residence time in the desired area. Lamprecht et al. proved an increased nanoparticle deposition in the inflamed tissue of the colon compared with the healthy control Citation[19,20]. Recently, researchers at the Georgia Institute of Technology and Emory University have designed and fabricated nanoparticles, which could be ingested orally, encapsulate anti-inflammatory drugs and dissolve only in the presence of inflamed tissue. The nanoparticles are composed of thioketals, organic molecules containing sulfur, which have the property that they remain stable in the presence of acids and bases, such as that occur in different regions of the digestive tract. But they break down in the presence of reactive oxygen molecules, which are secreted by macrophages and other white cells that cause inflammation. Thioketal nanoparticles containing anti-inflammatory drugs could be consumed orally and would remain intact as they pass through the digestive tract but would release the drugs as they encounter inflamed tissue. Such a drug delivery system would be ideal for treating Crohn's disease or ulcerative colitis Citation[21].
4. Expert opinion
CoDDS have gained remarkable importance for the treatment of local disease as well as for systemic therapies. For the development of a successful CoDDS, the triggering mechanism in the delivery system must respond only to the physiological conditions particular to the colon. Due to the lack of continuity in physiological parameters along the GIT, few mechanisms can be incorporated into a delivery system to affect colon-specific drug release. Of the three principal approaches used for the development of colon-targeted technologies, microflora- or enzyme-activated systems appear more promising since the abrupt increase in the bacterial population and associated enzyme activity in the colon represent a non-continuous event independent of GI transit time. In this regard, formulations that employ di-dependent system based on the combination of dual approaches also represent a significant technological advancement. Taking into consideration the above-mentioned merits of the microflora- or enzyme-activated systems, among the two di-dependent approaches proposed, the combination of pH and microflora appears to be the most promising. The proposed advantages delivered by the technologies discussed in this article include commonly used pharmaceutical excipients and feasible processing, site specificity of drug release and versatile drug release kinetics.
In the past 20 years, the pharmaceutical literatures have been flooded with numerous experimental techniques for targeting the large bowel via the oral route. Unfortunately, very few of them succeeded in reaching the doors of clinical phase. The main reasons for the failure to move from promise to reality are i) the lack of appealing medical opportunities (excluding IBD, which employs old technologies, old drugs and large doses for topical colonic treatment) to justify complicated developments; ii) nonrealistic assumptions regarding the therapeutic advantage of targeting the colon with molecules that probably would have worked after systemic administration (in other words: the lack of accompanying in-depth pharmacological research) and iii) the ongoing difficulty to launch oral protein products (biologics), which impacts on the development of colonic platforms for this complicated group of molecules Citation[22].
The future direction in achieving effective colonic delivery for the treatment of diseases associated with the colon may involve the use of various receptors present on the surfaces of diseased cells and drugs may be taken up via receptor-mediated endocytosis after reaching the colon. The success for such approaches will require the drug carrier to be stable in the lumen while capable of releasing the active agent in close proximity to, or within, the target cells. Thus, it is about time to explore realistic, efficient and safe modes of targeted drug delivery to colon. The search for colon-specific drug carriers has just begun.
Declaration of interest
The author states no conflict of interest and has received no payment in preparation of this manuscript.
Bibliography
- Patel M, Shah T, Amin A. Therapeutic opportunities in colon specific drug delivery systems. Crit Rev Ther Drug Carrier Syst 2007;24:147-202
- Patel MM, Amin AA. Design and optimization of colon targeted system of theophylline for chronotherapy of nocturnal asthma. J Pharm Sci 2011;100:1760-72
- Ashford M, Fell JT, Attwood D, An in vivo investigation into the suitability of pH dependent polymers for colonic targeting. Int J Pharm 1993;95:193-9
- McConnell EL, Short MD, Basit AW. An in vivo comparison of intestinal pH and bacteria as physiological trigger mechanisms for colonic targeting in man. J Control Release 2008;130:154-60
- Matsuda KI, Takaya T, Shimoji F, Effect of food intake on the deliver of fluorescein as a model drug in colon delivery capsule after oral to beagle dogs. J Drug Target 1996;4:59-67
- Patel MM, Shah TJ, Amin AF, Design, development and optimization of a novel time and pH-dependent colon targeted drug delivery system. Pharm Dev Technol 2009;14:62-9
- Patel MM, Patel SL, Bhadani MN, A synchronous colon specific drug delivery system for orally administered mesalamine. Acta Pharm Sci 2009;51:251-60
- Patel MM, Amin AA. Formulation and development of release modulated colon targeted system of meloxicam for potential application in the prophylaxis of colorectal cancer. Drug Deliv 2011;18:281-93
- Ji C, Xu H, Wu W. In vitro evaluation and pharmacokinetics in dogs of guar gum and Eudragit® FS30D-coated colon-targeted pellets of indomethacin. J Drug Target 2007;15:123-31
- Patel MM, Amin AF. Process, optimization and characterization of mebeverine hydrochloride loaded guar gum microspheres for irritable bowel syndrome. Carbohydr Polym 2011; published online 7 May 2011; doi:10.1016/j.carbpol.2011.04.068.
- Moreto M, Gonalons E, Mylonakis N, 3,3-Bis-(4-hydroxyphenyl)-7-methyl-2-indolinone (BHMI), the active metabolite of the laxative sulisatin. Arzneimittel Forsch Drug Res 1979;29:1561-4
- Csaky TZ. Pharmacology of intestinal permeation II. Springer-Verlag; Heidelberg: 1984
- Wroblewski S, Rihova B, Rossmann P, The influence of a colonic microbiota on HPMA copolymer lectin conjugates binding in rodent intestine. J Drug Target 2001;9:85-94
- Galanina O, Hallouin F, Goupille C, Detection of a potential receptor for the H-blood-group antigen on rat colon carcinoma cells and normal tissues. Int J Cancer 1998;76:136-40
- Theresa MA. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2002;2:750-63
- Vila JI, Calpena AC, Obach R, Gastric intestinal and colonic absorption of a series of beta-blockers in the rat. Int J Clin Pharmacol Ther Toxicol 1992;30:280-6
- Godbillon J, Evard D, Vidon N, Investigation of drug absorption from the gastrointestinal tract of man. III. Metoprolol in the colon. Br J Clin Pharmacol 1985;19:113S-8S
- Ishibashi T, Ikegami K, Kubo H, Evaluation of colonic absorbability of drugs in dogs using a novel colon targeted delivery capsules. J Control Release 1999;59:361-76
- Lamprecht A, Scaffer U, Lehr CM. Size dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res 2001;18:788-93
- Lamprecht A, Ubrich N, Yamamoto H, Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J Pharmacol Exp Ther 2001;299:775-81
- Wilson DS, Dalmasso G, Wang L, Orally delivered thioketal nanoparticles loaded with TNF-alpha–siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater 2010;9:923-8
- Rubinstein A. Colonic drug delivery. Drug Discov Today Technol 2005;2:33-7
- Rashid A. Dispensing device. EP0384642; 1990
- Krogel I, Bodmeier R. Pulsatile drug release from an insoluble capsule body controlled by an insoluble plug. Pharm Res 1998;15:474-81
- Stevens HNE, Ross AC, Johnson JR. The hydrophilic sandwich (HS) capsule: a convenient time-delayed oral probe device. J Pharm Pharmcol 2000;52:S41
- Ueda S, Hata T, Asakura S, Development of a novel drug delivery system. Time-controlled Explosion System (TES). I. Concept and design. J Drug Target 1994;2:35-44
- Pozzi F, Furlani P, Gazzaniga A, The Time Clock system: a new oral dosage form for fast and complete release of drug after a predetermined lag time. J Control Release 1994;31:99-108
- Gazzaniga A, Sangalli ME, Giordano F. Oral Chronotopic drug delivery systems: achievement of time and/or site specificity. Eur J Pharm Biopharm 1994;40:246-50