Abstract
Cardiovascular disease (CVD) is the leading cause of mortality globally. Effective CVD preventive medications are available including statin, blood pressure-lowering and antiplatelet medications; however most people do not take these drugs long term. Fixed-dose combination pills (“polypills”) have been shown, in several clinical trials, to improve adherence to these recommended medications, with corresponding improvements in risk factors such as blood pressure and LDL-cholesterol. In patients not taking all modalities of recommended CVD preventive therapies, polypill-based strategies could importantly contribute to global CVD control strategies. The largest benefits are seen in those who are under-treated at baseline, rather than those who are already taking the individual components separately: simplified step-up is more important than pill count reduction.
Despite the potential benefits for patients and payers, only a few polypills are available due to market failure in the funding of research and development for affordable non-communicable disease medicines. Regulatory paradigms have focused on substitution indications among patients already taking component medications; however, this is the population that is likely to receive the least benefit from a polypill-based strategy. Greater health impact is likely if focus is given to patients who have indications for all polypill components, but currently do not receive the benefits of recommended medicines long term.
1. Background
Large-scale randomized trial evidence has shown that statins,[Citation1] blood-pressure-lowering [Citation2] and antiplatelet [Citation3] drugs reduce cardiovascular events when used in those with established cardiovascular disease (secondary prevention) as well as in a wide range of other patient groups (primary prevention). However, despite usually being started on these medications after an acute event, most people in high-income countries do not take all recommended medications long term.[Citation4] In low-income countries, more than 90% of patients at risk do not receive recommended medications long term.[Citation4] Fixed-dose combination pills (or “polypills”) have been discussed for over a decade as a means of addressing the size of this evidence–practice gap in secondary prevention [Citation5] and also potentially playing a transformational role in primary prevention.[Citation6] In this article, we summarize the evidence that has accrued in the first wave of clinical trials, and speculate as to the reasons why progress has been so slow with this technology, despite the potential scale of its health benefits.
2. Short-term trials assessing effects on risk factors and tolerability
Several short-term (up to 12 weeks) trials comparing polypills to placebo or no treatment have assessed the impact of polypills on risk factors such as blood pressure and cholesterol (). Overall, these trials demonstrated short-term risk-factor reductions consistent with the expected size of effect based on components contained in the polypill. Side effects were also similar to those seen in trials of individual components.
Table 1. Trials assessing the impact of polypills on risk factor levels.
3. Long-term trials assessing events in low- to moderate-risk primary prevention
One of the key areas in which research is required is the use of polypills in primary prevention among patients who are at low to moderate cardiovascular disease (CVD) risk. There are currently no long-term safety or clinical benefit data to support the use of polypills in this setting. Proponents have suggested varying inclusion-targeting strategies such as age alone (e.g., give to everyone >55 years of age),[Citation6] age plus one or other risk factor (e.g., >50 years plus diabetes or raised blood pressure) [Citation7] or simply age plus absolute calculated risk (as in the TIPS-3 trial currently underway). Three large-scale placebo-controlled or minimal-care comparison trials are underway, with results expected over the next few years.
Heart Outcomes Prevention Evaluation-3 – HOPE-3 – (2 × 2 factorial design of 10 mg rosuvastatin, 16/12.5 mg candesartan/hydrocholorothiazide, both or placebo) is due to report in 2016.
Prevention of Cardiovascular Disease in Middle-aged and Elderly Iranians Using a Single PolyPill – PolyIran – [polypill containing aspirin 81 mg, enalapril 5 mg (or valsartan 40 mg), atorvastatin 20 mg and hydrochlorothiazide 12.5 mg vs usual care]
The International Polycap Study 3 – TIPS-3 – (2 × 2 × 2 factorial of polypill containing thiazide 25 mg, atenolol 100 mg, ramipril 10 mg, simvastatin 40 mg, Vitamin D, aspirin and placebo)
4. Long-term trials of polypills in secondary prevention or high-risk primary prevention
The least controversial use of polypills is in patients who have established indications for statins, blood-pressure-lowering and antiplatelet drugs, that is, those with established disease or similarly high calculated CVD risk. Nonetheless, important research questions have to be addressed – principally, whether any disadvantages in terms of reduced flexibility of dosing and components were outweighed by any improvements in uptake and adherence. Four such trials [Citation12–Citation15] have been completed with two larger trials (HOPE-4 and Poly-Iran), with cardiovascular event outcomes ongoing. All four completed trials demonstrated improved adherence to recommended therapy with the use of a polypill. () The UMPIRE trial [Citation13] also demonstrated a significant improvement in systolic blood pressure and LDL-cholesterol as a result of the improved adherenc,e with the other trials under-powered to demonstrate a similar difference.
Figure 1. Adherence to combination therapy at end of study. Adherence defined as taking antiplatelet, statin and ≥2 BP-lowering drugs at least 4 days of the last 7 at end of study in UMPIRE, Kanyini-GAP and IMPACT. Adherence in the FOCUS trial was defined as pill count between 80 and 110% at end of study plus a score of 20/20 on the Morisky–Green questionnaire.
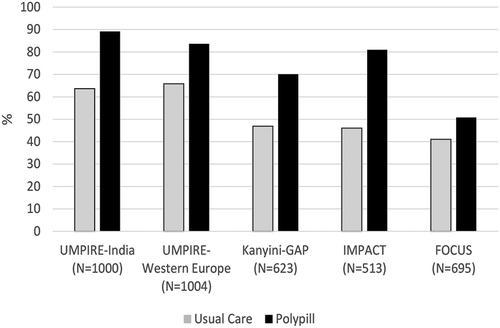
The Fixed Dose Combination Drug for Secondary Cardiovascular Prevention (FOCUS) trial [Citation12] was conducted in Spain, Argentina, Brazil and Paraguay and randomized 695 patients to either a polypill (containing aspirin 100 mg; simvastatin 40 mg; and ramipril 2.5, 5 or 10 mg) or individual therapies. The primary endpoint of adherence showed an absolute improvement of 10% (50.8 vs 41%, p = 0.019) in those taking the polypill. No difference was seen in mean SBP (129.6 vs 128.6 mmHg) or LDL-cholesterol (90 vs 92 mg/dl). In the FOCUS trial, participants in the control arm were provided the same component drugs and doses as contained in the polypill – therefore, this is a more direct comparison of a fixed-dose combination versus separate pills, rather than a polypill strategy.
Three of the completed trials [Citation13–Citation15] were part of the Single Pill to Avert Cardiovascular Events collaboration (www.spacecollaboration.org). SPACE collaboration trials compared polypill-based care with usual care and made no attempt to control patient management in the usual care arm; therefore, it was a comparison of a treatment strategy versus routine clinical practice (within the constraints of a clinical trial). To be eligible, participants had to be indicated for all the polypill components, but may not necessarily have been currently taking them. The rationale for this, and one of the motivating factors behind polypill development, is that large numbers of patients do not actually take recommended medications long term, despite indications. Relative risk for adherence to combination therapy at end of study in those who were already taking all component medications at baseline ranged from 1.04 (95% CI: 1.01 – 1.08) in the UMPIRE trial [Citation13] to 1.08 (95% CI: 0.97 – 1.21) in Kanyini-GAP.[Citation14] However, the relative risks for adherence in those who were under-treated at baseline were 3.35 (95% CI: 2.74 – 4.09) for UMPIRE, 3.7 (95% CI: 2.48 – 5.53) for Kanyini-GAP and 5.09 (95% CI: 3.40 – 7.63) for IMPACT.[Citation15] Therefore, the primary value of the polypill seems to lie in improving uptake of all recommended medications among those who are undertreated – that is, as a tool to commence or re-commence all recommended medicines. The safety and efficacy of the approach of not requiring prior stabilization has, therefore, now been confirmed in these trials.
It is worth noting, furthermore, that patient adherence in the usual care arm in the SPACE trials was better than normally seen in the general population. For example, in high-income countries, approximately 40% of patients with prior coronary heart disease or stroke do not receive antiplatelet, statin and BP-lowering drugs long term [Citation4], whereas, in these trials, ~15% of patients were not receiving all three treatment modalities. Moreover, in at least one of the studies, two-thirds of statin prescriptions in the usual care arm were for atorvastatin or rosuvastatin [Citation14], which is considerably higher than the average. Furthermore, in contrast to general population observations, treatment rates initially rose slightly in the usual care group and remained higher than baseline throughout the trials. As patients most in need of strategies to improve treatment uptake and adherence are under-represented in these clinical trials, the overall results from the trials will underestimate the population-level impact of the introduction of such treatments.
5. Conclusion
The use of polypills has the capacity to significantly improve use of effective CVD medications and, thereby, reduce the global burden of cardiovascular events and has been proven to be superior to use of individual component medications. However, lack of clearly defined regulatory paths and market failure in funding affordable medicines has hampered efforts to make polypills widely available for use in clinical practice. Once available on the market, effort will need to be invested into implementation strategies to change current treatment paradigms to incorporate use of polypills in routine clinical practice. The next decade should see a turnaround in the fortunes of the polypill, as a pragmatic solution to a major global health issue.
6. Expert opinion
Despite recommendations for research and development of polypills almost 15 years ago from two of the most influential bodies in global health (WHO and The Wellcome Trust), progress has been glacial. Only one polypill, created by a public–private partnership with Ferrer and the National Center for Cardiovascular Research in Spain, has reached the market in more than one country, and this is only in the last few years. Market failure for developing high-volume, low-to-moderate margin novel medicines is one key reason: Big Pharma has the budget, but not the incentive; generics Pharma have the incentive, but lack budget and requisite expertise (clinical trials and marketing of novel concepts) and government research agencies lack the remit and budget (usually focusing on discovery science, and not devoting the $10–20 million needed to take a new polypill to regulatory approval). However, an equally significant hurdle has been the lack of a clearly defined path to regulatory approval, and the mismatch between regulatory and clinical approaches to use of fixed-dose combinations. Regulatory agencies have been primarily focused on “straight substitution” indications, that is, polypills can be used once patients are stabilized on the individual component medications. However, the trials have shown that this patient group has the least to gain from treatment – those with the most to gain are, almost by definition, those least likely to be stabilized on exact drugs and doses with separate medications. Thus, an indication for “step-up” therapy or even initial therapy for patients who have established indications for all component medications would be more appropriate. This situation is further complicated by the fact that approved indications for many cardiovascular medications are now decades out of date – for example, none of the beta-blocker labels have been updated to include an indication for use after myocardial infarction, despite universal guideline recommendations. Thus, while all guidelines recommend concomitant use of aspirin, statin, beta-blocker and angiotensin-converting enzyme inhibitor after myocardial infarction, there is no overlap of the approved indications for these four medicines. Finally, current regulatory guidelines have no capacity to incorporate improved adherence into their metrics. Factors affecting adherence in the real world – such as cost, complexity and patient preference – are not considered, but, in some ways, this is the same as deciding not to factor in the chance of drug interactions or the impact of low renal function – it would be simpler and better if those factors do not exist, but they always do. With no clear regulatory pathway to follow or a pathway to a limited market, companies will not invest in the development of such medications. Additional flow on effects also exist, whereby limited marketing indications mean that reimbursers, such as government funding agencies or health insurers, will only offer reimbursement for polypills for straight substitution, again overlooking the population where polypills are likely to be most effective. On a positive note however, the recent European approvals for Ferrer and the US FDA advisory committee meeting on polypills suggest the pathway will become clearer.
It must also be acknowledged that the availability of polypills in the market is just the “race to the start line” – implementation and appropriate scale-up of polypills into routine clinical practice is the ultimate goal. In particular, use of polypills in low- and middle-income countries faces additional implementation issues including health system availability as well as cost of medications.
Declaration of Interest
The authors have received grants from several research charities and national funding agencies for research on cardiovascular fixed-dose combination medications, and from Dr Reddys Ltd. for coordination of the SPACE program. The George Institute for Global Health obtained an exclusive global license in December 2012 for the fixed-dose combinations used in the SPACE trials following a decision by Dr Reddy’s Ltd. not to proceed with taking the products to market because of uncertainty in regulatory requirements. A Rodgers is supported by an NHMRC Principle Research Fellowship. R Webster is supported by a Heart Foundation of Australia post-doctoral fellowship. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article apart from those disclosed.
Bibliography
- Papers of special note have been highlighted as
- either of interest (•) or of considerable interest
- (••) to readers.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665.
- Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860.
- Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378(9798):1231–1243.
- World Health Organization. Secondary prevention of non-communicable disease in low and middle income countries through community-based and health service interventions. Geneva: World Health Organization; 2002 August 1–3.
- Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ. 2003;326(7404):1419.
•Seminal paper on polypills.
- Indian Polycap Study, Yusuf S, Pais P, et al. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. [see comment]. Lancet. 2009;373(9672):1341–1351.
••First reported randomized trial of effect of a polypill on short-term risk factors.
- Malekzadeh F, Marshall T, Pourshams A, et al. A pilot double-blind randomised placebo-controlled trial of the effects of fixed-dose combination therapy (‘polypill’) on cardiovascular risk factors. Int J Clin Pract. 2010;64(9):1220–1227.
- Pill Collaborative Group, Rodgers A, Patel A, et al. An international randomised placebo-controlled trial of a four-component combination pill (“polypill”) in people with raised cardiovascular risk. Plos One. 2011;6(5):e19857.
•Short-term randomized, placebo controlled trial of the effect of a polypill on short-term risk factors.
- Wald DS, Morris JK, Wald NJ. Randomized polypill crossover trial in people aged 50 and over. PLoS One. 2012;7(7):e41297.
•Short-term randomized, placebo-controlled trial of the effect of a polypill on short-term risk factors.
- Yusuf S, Pais P, Sigamani A, et al. Comparison of risk factor reduction and tolerability of a full-dose polypill (with potassium) versus low-dose polypill (polycap) in individuals at high risk of cardiovascular diseases: the Second Indian Polycap Study (TIPS-2) investigators. Circ Cardiovasc Quality Outcomes. 2012;5(4):463–471.
- Castellano JM, Sanz G, Penalvo JL, et al. A polypill strategy to improve adherence: results from FOCUS (Fixed-dose Combination Drug for Secondary Cardiovascular Prevention) Project. J Am Coll Cardiol. 2014;64(20):2071–2082.
•Randomized trial of a polypill vs. individual components.
- Thom S, Poulter N, Field J, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310(9):918–929.
••Randomized trial of a polpyill-based strategy vs. usual care.
- Patel A, Cass A, Peiris D, et al. A pragmatic randomized trial of a polypill-based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. Eur J Prev Cardiol. 2015;22(7):920–930.
••Randomized trial of a polpyill-based strategy vs. usual care.
- Selak V, Elley CR, Bullen C, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ. 2014;348:g3318.
