Abstract
Making a diagnosis of pulmonary vasculitis is challenging. The most common cause of pulmonary vasculitis is small vessel anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Pulmonary involvement in other forms of vasculitis such as large vessel vasculitis is rare. Since correct and timely diagnosis is pivotal to start (immunosuppressive) therapy to avoid vasculitic damage, a complete patient history should be obtained and a physical examination performed. Initial laboratory evaluation should include inflammation markers, renal and liver function tests, and the determination of ANCA. New developments in ANCA testing result in tests with excellent predictive value for the diagnosis of AAV-related pulmonary vasculitis. Consequently, ANCA should be tested with these tests of the so-called second (capture ELISA) or third (anchor ELISA) generation. In patients who are ANCA negative, a simple algorithm is presented based on laboratory evaluation of autoantibodies and 18F-FDG-PET-CT scanning. Such an algorithm may be useful for accelerating the diagnostic process needed to make a diagnosis of pulmonary vasculitis, or alternatively, to quickly exclude such a diagnosis.
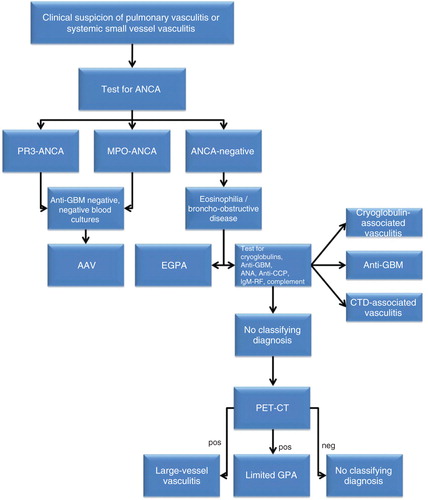
The clinical approach to a patient with possibly pulmonary vasculitis depends on a smart clinician who is astute enough to consider the diagnosis and who is capable to identify the clinical, radiological, laboratory, and pathological abnormalities. In the November 2012 issue of Expert Opinion on Medical Diagnostics, Casian and Jayne review their extensive experience in order to provide readers of the Journal to obtain the knowledge to act when pulmonary vasculitis is clinically suspected Citation[1]. Although diagnosis, generally, requires integration of clinical, laboratory, imaging (CT scan, MRI), lung functional, bronchoscopic and histological findings Citation[1], a simple algorithm () with a central role for laboratory evaluations is in my opinion very helpful in guiding the diagnostic process. This algorithm is the main focus of this Editorial.
Figure 1. Algorithm for the diagnostic process when evaluating a patient clinically suspected for pulmonary vasculitis. Firstly, the patient is evaluated for the presence of anti-neutrophil cytoplasmic antibodies (ANCA). If ANCA directed to proteinase 3 (PR3-ANCA) or ANCA directed to myeloperoxidase (MPO-ANCA) are present, a test for the presence of anti-glomerular basement membrane (GBM) antibodies should be performed to exclude anti-GBM disease. In addition, blood cultures should be obtained to exclude endocarditis. If both are negative, a diagnosis of ANCA-associated vasculitis (AAV) is likely. If ANCA are not found, lung function tests should be performed to evaluate the presence of bronchusobstructive lung disease/asthma. If present in combination with eosinophilia a diagnosis of eosinophilic granulomatosis with polyangiitis (EGPA) is likely. If asthma and eosinophilia are absent, tests for cryoglobulins, anti-GBM antibodies, anti-nuclear antibodies (ANA), anti-cyclic citrullinated peptide (anti-CCP), rheumatoid factor (IgM-RF), and complement should be performed. If after these tests are performed no classifying diagnosis is made, 18F-FDG-PET CT scan can be the next step. The 18F-FDG-PET CT scan may reveal uptake in large vessels suggesting large vessel vasculitis. In addition, uptake in the upper/lower respiratory tract is suggestive of localized granulomatosis with polyangiitis (GPA) when other disorders (such as tuberculosis and lymphoma; see text) can be excluded.
Pulmonary vasculitis mainly occurs in patients with small vessel vasculitides (SVV). Patients with SVV often present with non-specific complaints such as malaise, fever, weight loss, fatigue, anorexia, and arthralgias. In addition, since the vasculitic process can affect blood vessels in any location dysfunction in virtually any organ system can be present. Pulmonary manifestations such as an antibiotic resistant pneumonia, a new-onset asthma, or alveolar lung hemorrhage should alert the physician to the possibility of SVV Citation[1]. Otherwise, pulmonary artery stenoses and/or aneurysms should alert the physician to the possibility of large vessel vasculitis.
Firstly, the clinician should perform laboratory testing including ESR, C-reactive protein, hemoglobulin, leukocyte and eosinophil count, serum creatinin level, transaminases, and urine investigations for the presence of erythrocyturia and proteinuria Citation[2]. In addition, patients should be tested for anti-neutrophil cytoplasmic autoantibodies (ANCA) ().
1. Anti-neutrophil cytoplasmic autoantibodies
SVV can be classified as ANCA-associated vasculitis (AAV) or non-ANCA associated SVV. Whereas pulmonary involvement in non-ANCA associated vasculitis is rare (less than 5% of patients), most attention should go to making a diagnosis of AAV. ANCA in AAV recognize two different antigens, proteinase 3 (PR3) or myeloperoxidase (MPO) Citation[3].The AAV syndromes comprise granulomatosis with polyangiitis (GPA, Wegener’s), eosinophilic granulomatosis with polyangiitis (EGPA; Churg-Strauss syndrome), and microscopic polyangiitis (MPA). For classification of patients, however, we have proposed that ANCA serotype, that is, either MPO-ANCA or PR3-ANCA, is more important than classifying patients according to their clinical subtype, that is, GPA, EGPA, or MPA Citation[3,4]. This proposal is supported by genetic findings, clinical manifestations, and response to therapy which are all more related to ANCA serotype than to clinical subtype Citation[3-5].
So, the next step for the patient clinically suspected of pulmonary vasculitis is testing the serum for the presence of MPO-ANCA and PR3-ANCA. Since this testing is crucial for the further diagnostic and therapeutic process, the quality of ANCA testing should be optimal. ANCA are traditionally detected by an indirect immunofluorescence (IIF) technique. In patients with AAV, either a cytoplasmic (C-ANCA; due to PR3-ANCA) or a perinuclear (P-ANCA; due to MPO-ANCA) staining pattern is observed. According to the international consensus on ANCA testing, ANCA should be tested both by an IIF test and subsequently by antigen-specific tests Citation[6]. During the last decade, the quality of these antigen-specific tests improved substantially. Antigen-specific tests of the first generation (so-called “direct” enzyme-linked immunosorbent assays = ELISAs) can now be replaced by superior assays of the second (i.e., capture ELISA) Citation[3,7] or third (i.e., anchor ELISA) Citation[3,8] generation. Based on the results obtained with these newer ANCA test systems, we proposed that these ANCA tests of the second or third generation replace the need to perform IIF assays for ANCA detection Citation[3].
In patients with MPA, generalized GPA or limited (“early generalized”) GPA, virtually all patients have either PR3 or MPO-ANCA Citation[3,9]. In patients with loco-regional GPA, however, ANCA is clearly not detected in all patients Citation[3,10]. In addition, in EGPA most patients are ANCA negative whereas in about 40% of the patients MPO-ANCA can be detected Citation[3,11]. Although MPO-ANCA and PR3-ANCA when tested with these new ANCA assays are highly specific for either GPA, EGPA, and/or MPA, it must be stressed that these ANCA serotypes are also found in patients with anti-GBM disease or endocarditis with or without a complicating vasculitis Citation[3]. Also, MPO-ANCA and/or PR3-ANCA can be found during cocaine use and/or use of certain drugs such as propylthiouracil Citation[3].
If a patient is both clinically suspected of pulmonary vasculitis and ANCA positive, a histological confirmation is frequently sought but not invariably needed to make the diagnosis of AAV. Preferred biopsy spots are affected organs such as skin, nose, and/or kidney. An open lung biopsy is only needed in extremely rare occasions Citation[12].
2. What diagnostic tests should be performed in patients clinically suspected of pulmonary vasculitis that are ANCA negative?
If a patient clinically suspected of pulmonary vasculitis does not test positive for ANCA, he/she may suffer from EGPA, cryoglobulin-associated vasculitis, anti-glomerular basement membrane (GBM) disease, vasculitis secondary to connective tissue diseases, localized GPA, or (occasionally) one of the other forms of vasculitis such as large vessel vasculitis, Henoch Schonlein purpura, Behcet disease, or a secondary form of vasculitis () Citation[1,12].
These patients should undergo lung function tests to evaluate the presence of bronchusobstructive lung disease/asthma. If present in combination with eosinophilia, a diagnosis of EGPA is likely and special attention should be paid to the peripheral nervous system (is polyneuropathy or mononeuritis multiplex present?), to the skin (are purpura or Churg-Strauss nodules present?), and to cardiac involvement Citation[2,11].
If eosinophilia and asthma are absent, the following laboratory evaluations should be performed: cryoglobulins, complement levels, rheumatoid factor IgM (IgM-Rf), anti-cyclic citrullinated peptide (anti-CCP) antibodies, anti nuclear antibodies, and anti-GBM antibodies. Special attention should be paid to the detection of cryoglobulins. Since testing of these antibodies is very sensitive to cooling, we perform this test with preheated tubes. Furthermore, the tubes are kept at 37°C during transport to the laboratory to prevent cryoprecipitation prior to arriving in the laboratory Citation[13]. When these measures are not taken, the presence of cryoglobulins can be missed but suspected by the presence of low levels of complement in combination with high levels of IgM-Rf in the absence of anti-CCP antibodies.
When these laboratory evaluations are not helpful for making a diagnosis, the patient clinically suspected of pulmonary vasculitis may still suffer from localized GPA, large vessel vasculitides, and/or other forms of vasculitis. In these cases, a 18F-FDG-PET-CT scan can be the next step (). The 18F-FDG-PET-CT scanning has been used successfully for making a diagnosis of large vessel vasculitis Citation[1]. In addition, we found more recently that this technique is also useful for diagnosing active SSV and a negative 18F-FDG-PET-CT scan virtually excludes active pulmonary vasculitis Citation[14]. Besides, the extent of (sometimes unsuspected) Citation[15] organ involvement can be ascertained using this technique. A positive 18F-FDG-PET-CT scan is, however, not a specific finding and other diagnoses such as malignancy and tuberculosis still should be excluded. Therefore, in a patient clinically suspected of localized pulmonary GPA histological confirmation of vasculitis is mandatory.
3. Conclusion
Making a diagnosis of pulmonary vasculitis is challenging. Since correct and timely diagnosis is pivotal to start (immunosuppressive) therapy to avoid vasculitic damage, a complete patient history should be obtained and a physical examination performed. Initial laboratory evaluation should include inflammation markers, renal and liver function tests and the determination of ANCA. New developments in ANCA testing result in tests with excellent predictive value for the diagnosis of AAV-related pulmonary vasculitis. Consequently, ANCA should be tested with these tests of the so-called second or third generation. In patients who are ANCA negative, a simple algorithm is presented based on laboratory evaluation of autoantibodies and 18F-FDG-PET-CT scanning. Such an algorithm may be useful for accelerating the diagnostic process needed to make a diagnosis of pulmonary vasculitis, or alternatively, to quickly exclude such a diagnosis.
Declaration of interest
The author states no conflict of interest and have received no payment in preparation of this manuscript.
Acknowledgment
The author would like to thank B Wilde, MD, Department of Immunology, Maastricht University, Maastricht, the Netherlands for help with making the figure.
Bibliography
- Casian A, Jayne D. Current modalities in the diagnosis of pulmonary vasculitis. Expert Opin Med Diagn 2012;6(6):499-516
- Tervaert JW, Kallenberg CG. Neurologic manifestations of systemic vasculitides. Rheum Dis Clin North Am 1993;19:913-40
- Cohen Tervaert JW, Damoiseaux J. Antineutrophil cytoplasmic autoantibodies: how are they detected and what is their use for diagnosis, classification and follow-up? Clin Rec Allerg Immunol 2012; Epub ahead of print
- Franssen CF, Stegeman CA, Kallenberg CG, Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int 2000;57:2195-296
- Lyons PA, Rayner TF, Trivedi S, Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 2012;367:214-31
- Savige J, Gillis D, Benson E, International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA). Am J Clin Pathol 1999;111:507-13
- Csernok E, Holle J, Hellmich B, Evaluation of capture ELISA for detection of antineutrophil cytoplasmic antibodies directed against proteinase 3 in Wegener's granulomatosis: first results from a multicentre study. Rheumatology (Oxford) 2004;43:174-80
- Damoiseaux J, Dahnrich C, Rosemann A, A novel enzyme-linked immunosorbent assay using a mixture of human native and recombinant proteinase-3 significantly improves the diagnostic potential for antineutrophil cytoplasmic antibodies directed against proteinase 3. Ann Rheum Dis 2009;68:228-33
- Tervaert JWC, Goldschmeding R, Hene RJ, Kallenberg CGM. Neutrophil cytoplasmic autoantibodies and Wegener's granulomatosis. Lancet 1989;333:270
- Holle JU, Gross WL, Holl-Ulrich K, Prospective long-term follow-up of patients with localised Wegener's granulomatosis: does it occur as persistent disease stage? Ann Rheum Dis 2010;69:1934-9
- Dennert RM, van Paassen P, Schalla S, Cardiac involvement in Churg-Strauss syndrome. Arhritis Rheum 2010;62:627-34
- Cohen Tervaert JW, van der Werf TS, Stegeman CA, Pulmonary manifestations of systemic vasculitides. In: Isenberg DA, Spiro SG, editors. Autoimmune aspects of lung disease. Birkhauser Verlag; Basel Switzerland: 1998. p. 53-85
- Cohen Tervaert JW, van Paassen P, Damoiseaux J. Type II cryoglobulinemia is not associated with Hepatitis C infection: the Dutch experience. Ann NY Acad Sci 2007;1107:251-8
- Voo S, Kemna M, van Paassen P, Clinical value of 18F-fluorodeoxyglucose PET-CT in patients with small- and medium-size vessel vasculitis, such as Wegener s granulomatosis. J Nucl Med 2012;53(Suppl 1):353
- Van Durme CM, Kisters JM, van Paassen P, Multiple endocrine abnormalities. Lancet 2011;378(9790):540