Abstract
Objective: Since the introduction of the routine childhood immunization, a change in epidemiology of pneumococcal disease has been seen in both children and adults. This study aimed to quantify the public health and budget impact of pneumococcal vaccination of the elderly and those in at risk groups in the UK. Methods: The model was adapted from a previous population-based Markov model. At-risk adults and the elderly were assumed to receive PPV23 or PCV13 vaccination or no vaccination. Results: Over the study period (2012–2016), PPV23 vaccination led to a reduction in the number of invasive pneumococcal disease cases in most scenarios. The net budget impact ranged between £15 and £39 million (vs no vaccination) or between −£116 and −£93 million (vs PCV13). Conclusion: PPV23 vaccination program remains the optimal strategy from public health and budgetary perspectives despite epidemiological changes. PCV13 is likely to impose a significant budget with limited health benefits.
Background
Streptococcus pneumoniae
Pneumococcal disease is caused by infection with Streptococcus pneumoniae, a Gram-positive coccus Citation[1]. Invasive forms of the disease, such as bacteremic pneumonia, meningitis and bacteremia, can lead to death or disabling sequelae Citation[2–4].
In 2002, the WHO estimated that 1.6 million patients die annually due to all cause pneumococcal disease, most of them being infants or elderly adults Citation[5]. In England and Wales, an average of 8835 cases per year of invasive pneumococcal disease (IPD) were reported, and the incidence was estimated at 16.1 per 100,000 person-years between 2000 and 2006 Citation[6]. In 2008, up to 12,750 deaths were attributable to all-cause pneumococcal disease in those aged 65 years or older in the UK. The figure is comparable to that of colon cancer Citation[7].
Infants, the elderly and individuals with risk factors are more prone to contracting pneumococcal diseases. Risk factors include chronic pulmonary, cardiac, renal and hepatic diseases and immunosuppression Citation[2–5,8].
Vaccination against pneumococcal diseases
To date, vaccination is considered to be the main approach used in limiting the burden of pneumococcal diseases. Other methods used include improved nutrition, better housing and reduced indoor air pollution, factors which can be difficult to address Citation[4,9]. Two types of vaccines are currently available in the market, pneumococcal polysaccharide vaccines (PPV) and pneumococcal conjugate vaccines (PCV). In Europe, recommendations for vaccination with PPV were issued as early as 1980 (in Denmark), targeting at-risk adults and/or the elderly Citation[10].
In the UK, the only available PPV is a 23-valent vaccine (PPV23) (Pneumovax® II, Sanofi Pasteur MSD), indicated in at-risk individuals aged 2 or older Citation[11]. The Department of Health (DH) has recommended PPV23 in at-risk populations since 1992, and the recommendation was extended to those aged 65 years and older in 2003 Citation[12]. The 23 serotypes covered more than 95% of IPD serotypes observed in England and Wales between 2000 and 2006 Citation[6]. Its efficacy and effectiveness have been confirmed in several clinical studies of immunocompetent or immunosuppressed individuals Citation[5,13–19]. Due to its broad serotype coverage, PPV23 was found to be cost–effective in developed countries when compared to no vaccination Citation[20–28], even in the context of the changing epidemiology of IPD Citation[29].
The other type of vaccine, PCV, was introduced in Europe in 2001 (a 7-valent vaccine [PCV7]; Prevenar®, Pfizer) Citation[30]. A 13-valent vaccine (PCV13) (Prevenar 13®, Pfizer) was authorized for use in those aged from 6 weeks to 5 years Citation[31] in 2009. In many European countries, childhood vaccination with PCV vaccines has been recommended, being publicly funded in some Citation[10].
In the UK, PCV7 vaccination has been included in the routine childhood immunization program since 2006, being replaced by PCV13 in 2010 Citation[12]. Nevertheless, evidence is only available in terms of immunogenicity of PCV13 in children and adults and of PCV10 in adults Citation[31–33]. In England and Wales, since the introduction of the PCV vaccines, the incidence of IPD associated with serotypes covered by PCV7 dropped from 54.2 (2000–2006) to 23.6 (2008–2010) per 100,000 patient-years in those aged younger than 2 years Citation[6]. Between 2008 and 2010, serotypes covered by PCV7 only accounted for 8.0% of IPD-causing serotypes in those aged less than 5 years Citation[6].
Since 2011, the authorization of PCV13 has been extended to the prevention of IPD in all age groups, based on evidence on immunogenicity rather than the number of disease cases avoided Citation[31].
The impact of vaccinating children
The impact of routine childhood immunization with PCV7 has been seen in adults as well as children Citation[6,34–36].
As seen in the USA, Germany and Spain, as well as in the UK, the incidence of IPD associated with PCV7 serotypes has decreased in adults as a result of a herd protection effect. However, such reduction was partially offset by an increase in the incidence of IPD associated with serotypes not covered by the conjugate vaccine Citation[6,34–36]. In England and Wales, a study conducted by the Health Protection Agency (HPA) (now Public Health England) showed that the incidence of IPD associated with serotypes not covered by PCV7 in those aged 65 years and above increased significantly after the inclusion of the conjugate vaccine in the routine childhood immunization program Citation[6]. An emerging trend, suggesting a similar effect, has also been observed in the regions that have switched the immunization from PCV7 to PCV13 Citation[37].
Based on such observations, the recent switch from PCV7 to PCV13 may further affect the epidemiology of pneumococcal diseases. The benefits of vaccinating adults with PPV23 or PCV13 on the burden of pneumococcal diseases need to be re-evaluated.
Objective
This study aimed to address three questions. First, what is the net public health and budget impact of vaccinating the elderly and at-risk adults in the UK with PPV23 when compared to no vaccination or PCV13? Second, in the context of changing epidemiology of IPD and tight budgetary constraint, is the role of PCV13 relevant in providing further control of pneumococcal diseases? Third, is it important to consider the changing epidemiology of IPD in the analysis?
Methods
Model structure
A model used in a previous analysis was adapted to the UK settings Citation[38]. The population-based, multi-cohort Markov model tracked UK adults who were eligible for vaccination (regardless of receiving vaccination or not).
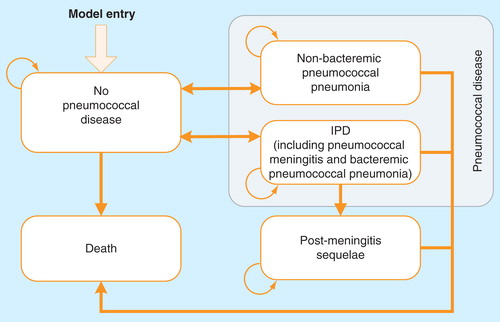
Each year, incident cohorts of individuals who became eligible for vaccination in that calendar year, regardless of receiving the vaccination or not, were tracked until they reached the age of 100 years or die, at which point they left the model. The numbers of disease events and costs were recorded by calendar year and by single year of age ( for model schematics).
As shown in , for vaccinated and unvaccinated cohorts, individuals with no pneumococcal disease can develop IPD or non-bacteremic pneumococcal pneumonia (NBPP). Following an episode of IPD (including pneumonia and meningitis), patients who have developed meningitis face a risk of permanent post-meningitis sequelae (PMS). After recovery from IPD or NBPP, individuals may contract the disease a second time. It was assumed that patients with PMS would not develop another episode of IPD or NBPP due to its rarity Citation[6]. IPD and NBPP were associated with excess mortality, whereas the mortality rate of the general population was applied to other health states.
The model had a cycle length of 1 year, and results were reported for a period of 5 years, that is, between 2012 and 2016. However, the model was initiated in 2005, allowing the analysis to capture those who were vaccinated before 2012. Other assumptions were discussed in a previous paper on the cost–effectiveness assessment of PPV23 in the UK setting Citation[39].
Target population & assessed strategies
The analysis focused on: at-risk adults (immunocompetent or immunosuppressed) and the elderly aged 65 years and older. According to the recommendation on PPV vaccination issued by DH, at-risk adults were defined as those with asplenia or splenic dysfunction, chronic pulmonary, cardiac, renal or hepatic disease, immunosuppression (such as HIV infection, undergoing chemotherapy or systemic steroid use), with cochlear implants or with cerebrospinal fluid leaks Citation[12].
The analysis compared vaccinating with PPV23 alone to two strategies, no vaccination or vaccination with PCV13 alone. For PCV13, it was assumed that it would be used in the same target population as PPV23, so that they were assessed on a comparable basis.
Revaccination was only considered for PPV23 at 5 years with no revaccination for PCV13, following DH recommendations Citation[12]. The proportion of the vaccinated receiving revaccination was estimated from a study conducted by the Royal College of General Practitioners (RCGP) [Sanofi Pasteur msd, Data On File]. It is estimated that 5.0% of the vaccinated were revaccinated and 68.0% received the revaccination between 5 and 7 years (i.e., appropriately revaccinated), between 2010 and 2012. In the model, 3.4% was therefore assumed as the rate of revaccination 5 years after the primary vaccination.
Invasive pneumococcal diseases
The model assessed different vaccination strategies in an environment where the epidemiology of IPD is changing due to serotype replacement stemming from the routine childhood immunization program.
The aforementioned HPA study provided the incidence of IPD associated with serotypes covered or not covered by PCV7 and the incidence of IPD by serotype for the periods 2000–2006 and 2008–2010 Citation[6]. Additional data were obtained from the HPA website for the distribution between other groups of serotypes (e.g., included in PCV13 not PCV7) Citation[40]. Numbers were estimated from the digitalized (GetData Graph Digitizer version 2.24, S. Federov, Moscow, Russia) figures, where results were only reported graphically, not numerically, and could not be obtained from the authors and health authorities.
It was assumed that the serotype replacement effect associated with PCV7 had been fully captured by the HPA study Citation[6], and the incidence associated with these seven serotypes remained stable during the study period. As PCV13 replaced PCV7 in 2010, a reduction was expected in the incidence of IPD associated with the additional six serotypes included in PCV13. Based on the effect of routine vaccination with PCV7, an increase in the incidence of IPD associated with serotypes not covered by PCV13 was assumed. Using the same approach as in previous studies [Sanofi Pasteur msd, Data On File] Citation[38], the model assumed that the proportional change in the incidence of IPD was a function of the cumulative vaccination rate in children. The function was selected as a cumulative gamma distribution due to its goodness of fit. As per the conclusion of the HPA study, the change was only applied to those aged 65 years and older. The change was applied for a period of 4 years since no data were available beyond this period of time. Constant incidence was assumed afterward. It should be noted that although PPV23 has been used in the past, no back calculation was carried out for the historical absolute incidence of IPD, therefore assuming no vaccination in the historical data. The approach was considered as a limitation of the analysis. However, the approach used was conservative since the total number of IPD cases had a downward bias, and hence the effectiveness of the vaccination had a downward bias.
The incidence of IPD in at-risk adults was based on English and Welsh data published by the HPA Citation[41], adjusted to account for the excess risk in at-risk adults . The source also provided the proportions of patients by risk group and the case–fatality rate of IPD. The proportion of IPD patients suffering from meningitis was obtained from another HPA study Citation[42]. The proportion of meningitis patients developing PMS was based on a meta-analysis on the outcomes of pneumococcal meningitis survivors Citation[43].
Figure 3. Epidemiological change in incidence of invasive pneumococcal disease induced by routine childhood immunization.
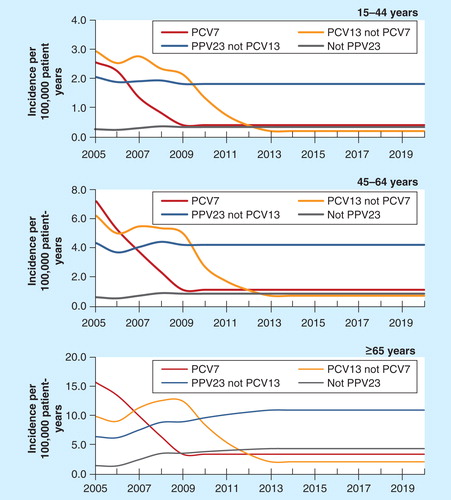
Table 1. Clinical and demographical parameters.
Natural course of other pneumococcal diseases
The overall incidence of NBPP was estimated as the incidence of hospitalized non-invasive pneumococcal pneumonia observed in Greater Nottingham Citation[44] divided by the hospitalization rate of patients with pneumonia Citation[45]. The case fatality rate for hospitalized pneumonia was obtained from the same observational study Citation[44] and 0% was assumed for non-hospitalized cases Citation[45]. No serotype replacement was considered for NBPP.
Demography
Population size and life tables by age over time were obtained from the Office for National Statistics Citation[46,47]. The distribution by risk group was based on English and Welsh data from the aforementioned HPA study Citation[41].
Observational data from DH for the period before 2011 were used for vaccination coverage rates of PPV23 Citation[48]. The rates of 2011 were applied from 2012, and the same vaccination coverage rates were assumed for PCV13. reports the proportion of target population vaccinated every year.
Table 2. Vaccine coverage rate.
Vaccine effectiveness
In terms of the effectiveness of the two vaccines, immunological properties of PPV23 had been compared to the conjugate vaccines in order to estimate their likely clinical efficacy. Nevertheless, past studies have failed to demonstrate the superiority of PCV13 in adults Citation[49–51]. In order to address the uncertainty around the comparative effectiveness, four sets of scenarios were considered . In scenario A, the effectiveness of PPV23 against IPD in the normal risk and the at-risk immunocompetent was based on a Cochrane review of 10 clinical trials Citation[14]. For the at-risk immunosuppressed, this was based on a clinical trial of HIV-infected patients Citation[15]. The effectiveness against NBPP in the normal risk and the immunocompetent was based on a Spanish cohort study in the elderly Citation[52], whilst no protection was assumed for the immunosuppressed. For PCV13, due to a lack of effectiveness data, the same effectiveness was assumed as PPV23 (against vaccinated serotypes). In scenarios B, C and D, alternative values were tested Citation[14,15,18,52], favoring PCV13. In addition, no epidemiological change was assumed following the switch from PCV7 to PCV13 in routine childhood immunization in scenarios B and D.
Table 3. Scenario analysis.
Vaccine effectiveness was assumed to wane over time, with no protection remaining 8 years after the initial vaccination Citation[53,54].
Table 4. Waning function.
Costs
Costs were estimated from the perspective of UK NHS and Personal Social Services Citation[55]. All costs were estimated in 2011 values in Pounds Sterling.
The unit price of the vaccine was retrieved from the British National Formulary 63 (as of March 2012) Citation[56]: £8.32 for a vial of PPV23 (Pneumovax II, Sanofi Pasteur MSD) and £49.10 for a vial of PCV13 (Prevenar 13, Pfizer). Neither of the vaccines is centrally procured, and therefore these prices are assumed to be a reasonably accurate reflection of true costs. Administration cost was assumed to be 10 min’ time of a band 7 advanced nurse, as seen in a HPA study on seasonal influenza vaccination program Citation[57]. It is estimated that 54.5% of pneumococcal vaccination will be co-administered with influenza vaccination based on the RCGP data [Sanofi Pasteur msd, Data On File], therefore not incurring additional administration cost. The average administration cost was estimated at £6.92 per vaccine. Costs of and assumptions on managing pneumococcal diseases and PMS were retrieved from previous cost–effectiveness studies Citation[58], the NHS Reference Costs Citation[59] and the Unit Costs of Health and Social Care report published by the Personal Social Services Research Unit Citation[60]. Costs were inflated to 2011 where necessary Citation[60]. Estimates used in scenario A are reported in .
Table 5. Costs.
Public health & budget impact
The study endpoints included the number of IPD and NBPP cases over time and the cumulative number of PMS cases over time in the general population, as well as the total costs and costs by category over time. The budget impact was defined as the difference in total costs between two assessed strategies. Compared to cost–effectiveness analyses, public health and budget impact analyses look at the effect of a healthcare intervention and affordability at the population level, rather than its value for money.
Results
Overview
The model tracked the UK population eligible for pneumococcal vaccination over time. 78.0% of eligible patients were aged 65 years or older while the rest were at-risk adults. Between 2012 and 2016, a total of 2.67 million people would receive the initial vaccination, 72.2% of whom being aged 65 years or older.
Public health impact
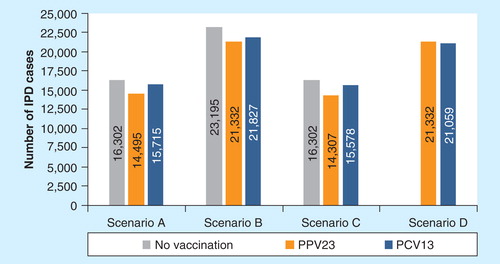
Compared to no vaccination, vaccinating the target population with PPV23 was associated with a decrease in the total number of IPD cases, of between 1807 (−11.1%) in scenario A to 1995 (−12.2%) in scenario C, as reported in . The strategy was also associated with a reduction in the number of NBPP cases in scenarios in which PPV23 was assumed efficacious, ranging between 14,975 (−6.4%) in scenario A and 24,591 (−10.5%) in scenario C. Due to the reduction in the incidence of IPD, the number of PMS cases also decreased with PPV23 (see Online Supplementary Appendix [supplementary material can be found online at www.informahealthcare.com/suppl/10.1586/14737167.2014.953932]).
In scenarios A, B and C, using PCV13 instead of PPV23 was associated with more IPD cases as PPV23 covers more IPD-causing serotypes. The difference ranged from 495 cases (+2.3%) in scenario B to 1271 cases (+8.9%) in scenario C. In scenario D, which assumed high effectiveness for PCV13 and no change in the epidemiology of IPD (i.e., more IPD cases of serotypes covered by PCV13), PCV13 moderately decreased the total number of IPD cases by 273 (−1.3%) compared with PPV23. No evident difference in the number of NBPP cases over the study period was predicted between the two vaccines, except in scenario D, in which no protection was assumed for PPV23 but high effectiveness was assumed for PCV13. The change in the number of PMS cases followed that of IPD.
Budget impact analysis
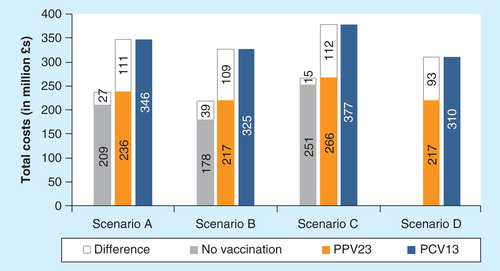
Between 2012 and 2016, vaccinating UK at-risk adults with PPV23 is predicted to cost the national healthcare system between £15 million (+5.9%) in scenario C and £39 million (+21.7%) in scenario B, when compared to no vaccination. The cost of administration accounted for 45.4% of the total cost of vaccination (i.e. vaccine and its administration). As a result of preventing pneumococcal diseases, the cost of vaccination was offset by 8.1% (scenario B) to 64.6% (scenario C) (see Online Supplementary Appendix).
The use of PCV13 was found to lead to a substantial increase in healthcare budget because of its high price, as reported on . Over the 5-year study period, the switch to PCV13 would increase the total health care budget for pneumococcal diseases by £93 million (+43.0%; scenario D) or £112 million (+42.0%; scenario C). The cost of PCV13 vaccination was only offset by averted treatment cost in scenario D (£14 million; −13.4%), whereas in other cases, vaccination with PCV13 was predicted to increase the costs of managing the disease.
Discussions
Summary of findings
The current analysis examined the budget impact of vaccinating at-risk adults and the elderly with PPV23 compared to no vaccination or vaccination with PCV13 in the UK context. The analysis took into account the evolving epidemiology of IPD induced by the routine childhood immunization program against pneumococcal diseases. Four scenarios tested different assumptions on vaccine effectiveness, costs and presence of epidemiological change of IPD. Despite no concrete evidence being available at the time of this analysis, PCV13 was assumed to have a higher effectiveness than PPV23, except in scenario A.
Between 2012 and 2016, when compared to no vaccination, using PPV23 was predicted to avoid 1807 to 1995 IPD cases with a net budget impact between £15 million and £39 million. Despite a decreasing trend in the incidence of IPD Citation[6], this study demonstrated that PPV23 vaccination still provides protection to at-risk adults and the elderly.
Compared with PPV23, the use of PCV13 was associated with an increase in the number of IPD cases unless favorable effectiveness and no decrease in the incidence of IPD associated with serotypes covered by PCV13 were assumed. Switching from PPV23 to PCV13 would require an additional budget of approximately £100 million even with the most favorable assumptions. Such additional cost almost matches the healthcare budget for skin cancer in England (£119 million; 2010–2011) and corresponds to one-fifth of the total health budget for Camden Primary Care Trust in Greater London (£529.3 million; 2009–2010) Citation[61].
Strengths
The analysis modeled the epidemiological change induced by routine childhood immunization to try to reflect the disease burden in the near future. As a result of herd protection and serotype replacement effects, the annual number of IPD cases associated with serotypes covered by PCV7 decreased from 1635 in the pre-mass vaccination era (2000–2006) to 446 post the introduction of mass vaccination (2008–2010) in the elderly Citation[6]. Although the number of IPD cases associated with the additional six serotypes included in PCV13 increased over this period, the recent switch from PCV7 to PCV13 in children is likely to reverse this trend, leading to a more limited effect of the conjugate vaccine in adults. Epidemiological factors are indeed important to consider in any future analysis to better reflect the context.
This study applied UK data whenever available. The use of national data from HPA ensures quality and reliability of the parameterization of the analysis. The data on co-administration and revaccination were based on a recent study conducted by the RCGP and the estimates reflect the current vaccination practice in England and Wales. This information is very important to gauge the actual cost of administration.
No effectiveness data are currently available for PCV13; hence four scenarios were assumed. Except in one scenario in which effectiveness of PCV13 was comparable to PPV23, all scenarios assumed inferior effectiveness for PPV23.
Limitations
The current study adopted a static approach and did not capture the dynamics of disease transmission, that is, the effect of vaccination on unvaccinated adults. However, at the population level, quantifying such dynamics requires a large number of additional parameters which are not currently available in the literature.
Several scenarios were assumed for the effectiveness of PCV13; nevertheless these estimates were arbitrarily selected. The CAPITA trial compared PCV13 to placebo in adults Citation[62]. However, the preliminary results were only published when the analysis had been completed Citation[63], and these were analogous to the assumptions used in scenario A. The analysis should be updated when the final estimates are fully released and published.
Despite being less severe than IPD, NBPP contributed to a large proportion of the burden of pneumococcal disease due to its commonness. In this study, the incidence was based on that of community-acquired pneumonia of pneumococcal original, which is not equivalent to NBPP. More research is needed to update the study. Further surveillance data on IPD should also be collected to verify the other epidemiological assumptions of this analysis.
Furthermore, the estimation of the absolute incidence of IPD was not based on back calculation, which may bias down the actual burden of the disease. Nevertheless, this approach was conservative in terms of disease avoided and costs saved.
Conclusion
The current study confirms the results from the previous study Citation[38] that the target population for pneumococcal vaccination would continue to benefit from PPV23 despite the epidemiological change. Since PCV13 is used in children, its use in at-risk adults and the elderly is unlikely to bring additional benefit when focusing on the severe forms of pneumococcal disease, that is, IPD. Nevertheless, such results cannot be simply applied to other countries, since the magnitude of the impact of childhood PCV vaccination varied across countries Citation[64]. Adaptation of the model is required to reflect local context of epidemiology.
No head-to-head clinical study has compared PPV23 with PCV13. In the current analysis, efficacy of PCV13 was therefore based on assumptions. In the model scenarios used, excluding scenario A, the conjugate vaccine was assumed to have a higher efficacy than PPV23, despite evidence suggesting this may not be the case Citation[49–51]. It has to be pointed out that uncertainties exist in the underlying assumptions across all scenarios analyzed. Nevertheless, even in the most biased scenario in favor of PCV13 (scenario D), the budget impact of vaccination with PCV13 was associated with a higher total cost. In order to make an informed decision, the healthcare policy-maker will need to further assess the vaccine effectiveness in real life and the epidemiological impact of using either vaccine across different age groups against the prevailing budget constraint. Additionally, the decision process should also be supplemented by the relative cost–effectiveness of the two vaccines, which, although not addressed by the current study, is the topic of an associated piece of analysis Citation[39].
It is important and necessary to incorporate the ever-changing epidemiology of the disease in studies on public health interventions. For diseases like the one discussed in this study, the use of several interventions focusing on different target populations is not uncommon. Although one intervention may be assessed independently in a specific population, other interventions need to be accounted for whenever they have a direct or indirect effect on the assessed population.
The co-administration of influenza and pneumococcal vaccines is recommended in England and Wales by the HPA Citation[65]. Based on data from the RCGP, more than half of the vaccinated received the pneumococcal vaccine at the same visit for the influenza vaccination. Interestingly, when comparing PPV23 to no vaccination in the current analysis, the cost of administration (∼£19 million) ranges from 49 to 128% of the total budget impact in different scenarios. If co-administration was not accounted for, the budget impact would have been approximately 1.5 to more than 2 times higher than the current estimates. Therefore, it is important to appropriately estimate the cost of administration when assessing vaccination strategies so that the actual expenditure can be accurately reflected.
A change in the epidemiology of IPD has been witnessed following the inclusion of pneumococcal conjugate vaccine in the routine childhood immunization program. Nevertheless, PPV23 still affords protection to at-risk adults and the elderly against the disease due to its broad serotype coverage. When compared to PPV23, the results showed that PCV13 would provide limited, if any, public health benefit over PPV23 and require a substantial additional budget to maintain the program.
It is crucial for future studies to consider the drastic change in the epidemiology of IPD. Continuous monitoring of these changes would help to better understand the extent of the impact of the routine childhood immunization program, while studies on the effectiveness of the conjugate vaccine and the epidemiology of NBPP will also refine the model.
Key issues
In the UK, it is estimated that pneumococcal diseases caused up to 12,750 deaths in 2008. Vaccination is considered as the only approach to reduce the disease burden.
The routine childhood immunization program has led to a drastic change in the epidemiology of invasive pneumococcal disease in vaccinated children, as well as in unvaccinated adults.
The current analysis shows that vaccination with PPV23 continues to afford protection to at-risk adults and the elderly from pneumococcal diseases due to its broad serotype coverage. The use of PCV13 would lead to very limited benefits in terms of public health and impact healthcare budget significantly.
It is important to consider the effect of the changing epidemiology of invasive pneumococcal diseases in the analysis. It is also important to appropriately estimate the cost of administration.
Further studies on the dynamics of pneumococcal diseases and comparative effectiveness of the two vaccines would refine the analysis.
Supplementary Material
Download MS Word (497 KB)Financial & competing interests disclosure
This study was conducted by Amaris and funded by Sanofi Pasteur MSD. Y Jiang & A Gauthier are employees of Amaris. A Gauthier, S Keeping & S Carroll are employees of Sanofi Pasteur MSD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Notes
References
- Musher DM. Streptococcus pneumoniae. In: Mandell GL, editor. Principles. and practice of infectious diseases. Churchill Livingstone Elsevier; Philadelphia, PA, USA: 2010. p. 2623-42
- Fedson DS. Pneumococcal vaccination for older adults: the first 20 years. Drugs Aging 1999;15(Suppl 1):21-30
- Lynch JP III, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med 2009;30(2):189-209
- Prato R, Tafuri S, Fortunato F, Martinelli D. Why it is still important that countries know the burden of pneumococcal disease. Hum Vaccin 2010;6(11):918-21
- 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008;83(42):373-84
- Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011;11(10)):760-8
- Pneumococcal Awareness. Fact sheet: don’t underestimate pneumococcal disease (2011). Available from: www.pneumo.co.uk/Portals/0/pdf/Factsheet_Download2.pdf [Last accessed 27 December 2012]
- Cartwright K. Pneumococcal disease in western Europe: burden of disease, antibiotic resistance and management. Eur J Pediatr 2002;161(4):188-95
- Mulholland K. Strategies for the control of pneumococcal diseases. Vaccine 1999;17(Suppl 1):S79-84
- Pebody RG, Leino T, Nohynek H, et al. Pneumococcal vaccination policy in Europe. Euro Surveill 2005;10(9):174-8
- European Medicines Agency. Summary of product characteristics: Pneumovax II (2011). Available from: www.medicines.org.uk/EMC/medicine/1446/SPC [Last accessed 7 December 2012]
- Pneumococcal. In: Salisbury D, editor. Immunisation against infectious disease (the green book). The Stationery Office; London, UK: 2012. p. 295-313
- Spindler C, Hedlund J, Jasir A, et al. Effects of a large-scale introduction of the pneumococcal polysaccharide vaccine among elderly persons in Stockholm, Sweden. Vaccine 2008;26(43):5541-6
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008(1):CD000422
- Rodriguez-Barradas MC, Goulet J, Brown S, et al. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis 2008;46(7):1093-100
- Mooney JD, Weir A, McMenamin J, et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis 2008;8:53
- Kawakami K, Ohkusa Y, Kuroki R, et al. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine 2010;28(43):7063-9
- Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 2010;340:c1004
- Andrews NJ, Waight PA, George RC, et al. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine 2012;30(48):6802-8
- Ament A, Fedson DS, Christie P. Pneumococcal vaccination and pneumonia: even a low level of clinical effectiveness is highly cost-effective. Clin Infect Dis 2001;33(12):2078-9
- Ament A, Baltussen R, Duru G, et al. Cost-effectiveness of pneumococcal vaccination of older people: a study in 5 western European countries. Clin Infect Dis 2000;31(2):444-50
- Sisk JE, Whang W, Butler JC, et al. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann Intern Med 2003;138(12):960-8
- Evers SM, Ament AJ, Colombo GL, et al. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 Western European countries. Eur J Clin Microbiol Infect Dis 2007;26(8):531-40
- Smith KJ, Zimmerman RK, Lin CJ, et al. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine 2008;26(11):1420-31
- Ogilvie I, Khoury AE, Cui Y, et al. Cost-effectiveness of pneumococcal polysaccharide vaccination in adults: a systematic review of conclusions and assumptions. Vaccine 2009;27(36):4891-904
- Smith KJ, Lee BY, Nowalk MP, et al. Cost-effectiveness of dual influenza and pneumococcal vaccination in 50-year-olds. Vaccine 2010;28(48):7620-5
- Fedson DS, Nicolas-Spony L, Klemets P, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines 2011;10(8):1143-67
- Akin L, Kaya M, Altinel S, Durand L. Cost of pneumococcal infections and cost-effectiveness analysis of pneumococcal vaccination at risk adults and elderly in Turkey. Hum Vaccin 2011;7(4):441-50
- Jiang Y, Gauthier A, Annemans L, et al. Cost-effectiveness of vaccinating adults with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in Germany. Expert Rev Pharmacoecon Outcomes Res 2012;12(5):645-60
- European Medicines Agency. Summary of product characteristics: Prevenar (2012). Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000323/WC500041563.pdf [Last accessed 7 December 2012]
- European Medicines Agency. Summary of product characteristics: Prevenar 13 (2013). Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf [Last accessed 7 December 2012]
- Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010;59(RR-11):1-18
- European Medicines Agency. Summary of product characteristics: Synflorix (2012). Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000973/WC500054346.pdf [Last accessed 7 December 2012]
- Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201(1):32-41
- van der Linden M, Imohl M. R2762 Serotype distribution among bacteraemic pneumococcal pneumonia in adults in Germany. Clin Microbiol Infect 2011;17(Suppl 4):S832
- Ardanuy C, Domenech A, Rolo D, et al. Molecular characterization of macrolide- and multidrug-resistant Streptococcus pyogenes isolated from adult patients in Barcelona, Spain (1993-2008). J Antimicrob Chemother 2010;65(4):634-43
- Gladstone RA, Jefferies JM, Faust SN, Clarke SC. Pneumococcal 13-valent conjugate vaccine for the prevention of invasive pneumococcal disease in children and adults. Expert Rev Vaccines 2012;11(8):889-902
- Jiang Y, Gauthier A, Annemans L, et al. A public health and budget impact analysis of vaccinating at-risk adults and the elderly against pneumococcal diseases in Germany. Expert Rev Pharmacoecon Outcomes Res 2012;12(5):631-43
- Jiang Y, Gauthier A, Keeping S, Carroll S. Cost–effectiveness of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res 2014;14(6). In press
- Health Protection Agency. Current epidemiology of invasive pneumococcal disease (IPD) (2012). Available from: www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/EpidemiologicalDataPneumococcal/CurrentEpidemiologyPneumococcal/ [Last accessed 7 December 2012]
- van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect 2012;65(1):17-24
- Trotter CL, Waight P, Andrews NJ, et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: England and Wales, 1996-2006. J Infect 2010;60(3):200-8
- Jit M. The risk of sequelae due to pneumococcal meningitis in high-income countries: a systematic review and meta-analysis. J Infect 2010;61(2):114-24
- Bewick T, Sheppard C, Greenwood S, et al. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax 2012;67(6):540-5
- Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64(Suppl 3):iii1-55
- Office for National Statistics. National population projections, 2010-based projections (2011). Available from: www.ons.gov.uk/ons/rel/npp/national-population-projections/2010-based-projections/index.html [Last accessed 7 December 2012]
- Office for National Statistics. Historic and projected mortality data (1951 to 2060) from the UK life tables, 2010-based (2012). Available from: http://ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-257453 [Last accessed 7 December 2012]
- Begum F, Pebody R. Pneumococcal polysaccharide vaccine (PPV) uptake report (2012). Available from: http://immunisation.dh.gov.uk/ppv-uptake-report-29-feb-2012/ [Last accessed 7 December 2012]
- Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis 2011;52(5):633-40
- Ridda I, Musher DM. Is there a potential role for protein-conjugate pneumococcal vaccine in older adults? Australas Med J 2012;5(4):231-5
- Fedson DS, Guppy MJ. Pneumococcal vaccination of older adults: conjugate or polysaccharide? Hum Vaccin Immunother 2013;9(6):1382-4
- Vila-Corcoles A, Ochoa-Gondar O, Hospital I, et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006;43(7):860-8
- Middleton DB, Lin CJ, Smith KJ, et al. Economic evaluation of standing order programs for pneumococcal vaccination of hospitalized elderly patients. Infect Control Hosp Epidemiol 2008;29(5):385-94
- Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991;325(21):1453-60
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal (2008). Available from: www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf [Last accessed 7 December 2012]
- British National Formulary. British National Formulary 63 (2012). Available from: www.bnf.org [Last accessed 7 December 2012]
- Baguelin M, Jit M, Miller E, Edmunds WJ. Health and economic impact of the seasonal influenza vaccination programme in England. Vaccine 2012;30(23):3459-62
- Trotter CL, Edmunds WJ. Modelling cost effectiveness of meningococcal serogroup C conjugate vaccination campaign in England and Wales. BMJ 2002;324(7341):809
- Department of Health. NHS Reference Cost 2010/11 (2011). Available from: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140 [Last accessed 7 December 2012]
- Curtis L. Unit costs of health and social care 2011. 2011. Available from: www.pssru.ac.uk/archive/pdf/uc/uc2011/uc2011.pdf [Last accessed 7 December 2012]
- Department of Health. 2003-04 to 2010-11 programme budgeting data (2012). Available from: www.dh.gov.uk/health/2012/08/programme-budgeting-data/ [Last accessed 27 December 2012]
- ClinicalTrials.gov. Study evaluating a 13-valent pneumococcal conjugate vaccine (13vPnC) in adults (CAPITA) (2012). Available from: http://clinicaltrials.gov/ct2/show/NCT00744263 [Last accessed 7 December 2012]
- Bonten M, Bolkenbaas M, Huijts S, et al. Community acquired pneumonia immunisation trial in adults (CAPITA). Presented at 9th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD); Hyderabad, India, 9-13 March 2014. Available from: http://isppd.meetingxpert.net/ISPPD_433/poster_90453/program.aspx/anchor90453 [Last accessed 24 March 2014]
- Grabenstein JD, Weber DJ. Pneumococcal serotype diversity among adults in various countries, influenced by pediatric pneumococcal vaccination uptake. Clin Infect Dis 2014;58(6):854-64
- Health Protection Agency. Frequently asked questions about pneumococcal vaccine (2012). Available from: www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/GuidelinesPneumococcal/pneumoFAQs/ [Last accessed 7 December 2012]
- McIntosh ED, Conway P, Willingham J, et al. Pneumococcal pneumonia in the UK–how herd immunity affects the cost-effectiveness of 7-valent pneumococcal conjugate vaccine (PCV). Vaccine 2005;23(14):1739-45
- Mangtani P, Roberts JA, Hall AJ, Cutts FT. An economic analysis of a pneumococcal vaccine programme in people aged over 64 years in a developed country setting. Int J Epidemiol 2005;34(3):565-74