Abstract
The HPV types 16/18-AS04-adjuvanted cervical cancer vaccine, Cervarix® (HPV-16/18-vaccine, GlaxoSmithKline, Belgium) was first approved in 2007 and is licensed in 134 countries for the prevention of persistent infection, premalignant cervical lesions and cervical cancer caused by oncogenic HPV. Benefit–risk status requires continual re-evaluation as vaccine uptake increases, as the epidemiology of the disease evolves and as new information becomes available. This paper provides an example of benefit–risk considerations and risk-management planning. Evaluation of the benefit–risk of HPV-16/18-vaccine post-licensure includes studies with a range of designs in many countries and in collaboration with national public agencies and regulatory authorities. The strategy to assess benefit versus risk will continue to evolve and adapt to the changing HPV-16/18-vaccine market.
New vaccines are licensed for use after their rigorous evaluation in clinical trials that characterize the safety profile and efficacy in defined study populations and conditions. The randomized controlled clinical trial design minimizes potential biases and is widely used for hypothesis testing during vaccine development. Because such trials are conducted in near ‘ideal’ circumstances, their results may not ultimately translate into vaccine use under everyday practice. There are important aspects of safety and effectiveness that cannot be measured or detected during pre-licensure clinical trials. These include indirect vaccine effects on unvaccinated populations (herd immunity), effects on disease epidemiology including transmission patterns, strain distribution and replacement, rare adverse events and medium- to long-term vaccine effects that might be unforeseen: together, the effects of vaccination on a population are referred to as the ‘vaccine impact’ Citation[1]. Post-licensure studies conducted under ‘real-world’ conditions are therefore pivotal in monitoring and informing on population-level effects and end points that could not be fully assessed in pre-licensure studies.
Individual vaccines are licensed based on a positive assessment of benefits versus risks. However, many benefits (namely indirect vaccine effects and population impact) and potential risks (rare adverse events) remain uncharacterized at the time of licensure. Determining the benefit–risk ratio of vaccination involves assessing a complex interplay of factors that include the frequency and severity of the disease and the safety and effectiveness of the vaccine Citation[2]. It is important to note that a benefit–risk ratio may differ according to the population for whom the vaccine is intended, and may change over time as the disease epidemiology is influenced by vaccination. Regulatory agencies have introduced increasingly formalized approaches to perform adequate continuous monitoring and benefit–risk evaluation during the post-authorization period. Regulatory agencies require manufacturers to conduct post-authorization safety studies (PASS) Citation[3], and legislation for the conduct of post-authorization efficacy studies (extended to effectiveness and impact studies) is being considered Citation[4]. The main aims of post-licensure pharmacovigilance activities include further characterization of the vaccine safety profile, with specific investigation of potential and new risks, ensuring that safety hazards are minimized and the benefits of immunization are maximized by appropriate actions. The outcomes of these activities are managed by risk mitigation or minimization strategies.
Challenges unique to the benefit–risk evaluation of HPV vaccines
HPV vaccines are the first vaccines licensed for which the primary indication is the prevention of cervical cancer. As such, HPV vaccines pose unique challenges for the design and conduct of post-marketing studies Citation[5,6]. One major challenge is that the ultimate clinical end point of interest (prevention of cancer) is rare and occurs in mid-to-late life, whereas the target population for vaccination is primarily adolescent girls. Furthermore, cervical cancer as a clinical study end point is not ethically acceptable for clinical trials because effective screening and treatment are available. Pre-licensure clinical studies therefore assessed surrogate end points of efficacy (HPV pre-cancerous lesions and persistent HPV infection).
Randomized controlled clinical trials conducted to date evaluated the efficacy of HPV in vaccinated girls and women, but assessment of the overall benefits in the entire population, including unvaccinated women and also men, could not be assessed. These effects may include herd protection (vaccine impact) but also potential changes in HPV-16 and HPV-18 prevalence in unvaccinated women and in oncogenic HPV types other than those contained in the vaccine, and potential effects on non-cervical infection end points such as oral and anal HPV infections. Post-marketing studies may also show unforeseen benefits. For example, an ecologic study in England suggested that an observed 13.3% decrease in genital warts among 16- to 19-year olds was likely due, at least partially, to HPV-16/18-vaccination Citation[7].
Monitoring of HPV-associated cancers many years after vaccination also poses particular logistic challenges in terms of tracking vaccination history (vaccine registries are frequently limited to recording of pediatric immunization), ensuring accurate and comparable baseline data and accounting for potential confounding factors such as availability of new vaccines, changes in risk factors such as sexual activity and the evolution of HPV screening practices and treatment protocols that might all influence the primary outcome. These issues are magnified in low- and medium-income countries where there are generally fewer resources and less-established health infrastructure, but where the cervical cancer burden is highest.
HPV-16/18-AS04-adjuvanted vaccine
The HPV-16/18-AS04-adjuvanted cervical cancer vaccine, Cervarix® (HPV-16/18-vaccine, GlaxoSmithKline, Belgium) contains HPV-16 and HPV-18 virus-like particles formulated with the AS04 adjuvant. AS04 contains aluminum salt and monophosphoryl lipid A, a purified, detoxified derivative of the lipopolysaccharide molecule from the bacterial wall of Salmonella minnesota. HPV-16/18-vaccine demonstrated very high (more than 90%) efficacy in preventing vaccine-type and non-vaccine-type persistent HPV infection and cervical intraepithelial neoplasia (CIN) grade 2 and above in young women Citation[8–11]. HPV-16/18-vaccine as a three-dose course was first approved in 2007 and is licensed in 134 countries for use in girls and women from 9 years of age, for the prevention of persistent infection, premalignant genital (cervical, vulvar and vaginal) lesions and cervical cancer causally related to certain oncogenic HPV types. Extensive experience on the use of HPV-16/18-vaccine in routine national immunization programs has been acquired in several countries, for example, the UK, The Netherlands, regions of Spain and Italy, Finland, Japan, Malaysia, Mexico and South Africa.
The extensive clinical trial program including all data that have been reviewed since its first market introduction and post-marketing safety surveillance conducted to date show that the previously established favorable benefit–risk profile for HPV-16/18-vaccine remains unchanged Citation[10,12–15]. Post-marketing safety experiences with HPV-16/18-vaccine are in line with independent safety assessments made by key regulatory bodies and public health networks, in the UK, The Netherlands and Italy Citation[16–19].
GlaxoSmithKline’s strategy to further characterize the benefit–risk profile of the HPV-16/18-vaccine in the post-licensure setting encompasses studies conducted in a range of countries with a variety of designs and in collaboration with national public agencies and regulatory authorities. Strategies employed include long-term follow-up of cohorts vaccinated in clinical trials, pooling of clinical trial data, signal detection evaluation from spontaneous adverse events reports received by GlaxoSmithKline, post-marketing surveillance of safety and clinical outcomes and review of large health databases for evaluation of specific, rare events that are difficult to capture . Here, we review major post-marketing initiatives developed to support the safety and effectiveness of the product and to inform about population impacts of vaccination with HPV-16/18-vaccine. Key features of these activities are provided in .
Figure 1. Determinants of the HPV-16/18-vaccine benefit–risk with highlights of investigative strategies.
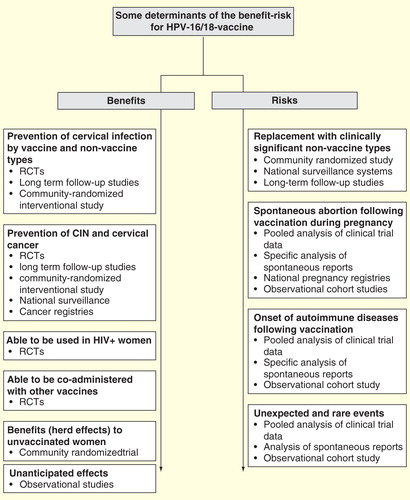
Table 1. Summary of post-licensure studies to evaluate the benefit–risk of HPV-16/18-vaccination.
Table 2. Summary of post-licensure national surveillance activities to evaluate the benefit–risk of HPV-16/18-vaccination.
Use of HPV-16/18-vaccine in special populations & settings
Post-licensure clinical trials allow vaccine evaluation in populations with specific needs who might benefit from vaccination, but who were not studied in pre-licensure studies.
HIV+ women
Women who are positive for HIV are at higher risk of HPV infection and CIN Citation[20,21]. Two randomized controlled studies have evaluated HPV-16/18-vaccine in HIV+ women. The first study evaluated HPV-16/18-vaccine safety and immunogenicity and the effect of vaccination on CD4+ T-cell count and HIV viral load in asymptomatic HIV+ women aged 15–25 years in South Africa Citation[22]. All HPV-16/18-vaccinated women were seropositive for HPV-16/18 antibodies after two doses, and remained antibody-positive at month 12 Citation[22]. Twelve months after vaccination, CD4+ T-cell counts and viral load were unchanged compared to baseline. The safety profile of HPV-16/18-vaccine in HIV+ women was generally consistent with that of healthy women. This study is the first to evaluate HPV-16/18-vaccine in HIV+ women and provides evidence that HPV-16/18-vaccine in this population is immunogenic, with an acceptable safety profile similar to that observed in HIV– individuals, and without apparent effects on HIV disease.
The second study will assess HPV-16/18-vaccine safety and immunogenicity in HIV+ women 15–25 years of age in India, Thailand, Brazil and Estonia, compared with HPV-6/11/16/18-vaccine Citation[23]. A comparative study design will identify if HIV+ women might benefit from a more immunogenic vaccine because vaccine immunogenicity is typically lower in immunocompromised than immunocompetent individuals Citation[24]. Immune responses in healthy individuals have been demonstrated to be higher following HPV-16/18-vaccine than HPV-6/11/16/18-vaccine Citation[25].
HIV+ women and a group of HIV– women subjects are randomized to receive HPV-16/18-vaccine or HPV-6/11/16/18-vaccine, allowing evaluation of HIV+ status on immunogenicity and safety of both vaccines in HIV+ women. The study aims to demonstrate non-inferiority of the immune response of HPV-16/18-vaccine versus HPV-6/11/16/18-vaccine in HIV+ women, and superiority of the immune response following HPV-16/18-vaccine versus HPV-6/11/16/18-vaccine. Results are anticipated in 2016.
Co-administration
Vaccines that can be co-administered at the same visit reduce the number of office visits required to complete the vaccination course, and improve coverage and compliance Citation[26]. Since licensure, four clinical trials have evaluated immunogenicity and safety of HPV-16/18-vaccine when co-administered with other vaccines likely to be used in adolescents and adults Citation[26–29]. Study results indicate that HPV-16/18-vaccine can be co-administered safely with meningococcal serogroups A, C, Y and W-135 polysaccharide diphtheria toxoid conjugate vaccine (Menactra™, Sanofi-Pasteur), combined diphtheria-tetanus-acellular pertussis vaccine (with or without inactivated poliomyelitis) (Boostrix™, Boostrix™ Polio, GlaxoSmithKline Biologicals, Belgium), hepatitis B vaccine (HBV, Engerix™B, GlaxoSmithKline Biologicals, Belgium) or combined hepatitis A and HBV vaccine (Twinrix™, GlaxoSmithKline Biologicals, Belgium) Citation[26–29].
HPV type replacement
It is not definitively established whether clinically relevant non-vaccine HPV types will emerge following the widespread use of HPV vaccines. Follow-up 4 years after HPV-16/18 vaccination showed no evidence of type replacement after vaccination in the short term Citation[30]. A large, community-randomized controlled trial being conducted in Finland will evaluate type replacement Citation[31,32]. The three study arms (A, B, C) are each composed of 11 communities in which: 90% of males and females are vaccinated with HPV-16/18-vaccine and 10% are vaccinated with HBV vaccine (A); 90% of females are vaccinated with HPV-16/18-vaccine and 10% are vaccinated with HBV vaccine while all males are vaccinated with HBV vaccine (B); and males and females are vaccinated with HBV vaccine (C). Effectiveness evaluation will start when subjects reach 18.5 years of age. Communities were selected to ensure minimal risk of mixing among young people from the other communities. Approximately 32,000 adolescents have been enrolled and vaccinated (20,500 females and 11,600 males), of which around 60% of females and 20% of males received HPV-16/18-vaccine.
This large, community-randomized controlled study design will evaluate the overall impact of HPV-16/18-vaccination on HPV-16/18 prevalence in a population setting, as well as the risk of type replacement. Recruitment for the immunization phase was completed in 2010 with final results expected by 2015/2016.
Refinements to vaccination schedules
Booster doses
Based on current data and mathematical modeling projections of antibody persistence Citation[33], booster vaccination with HPV-16/18-vaccine is not recommended. One clinical study has investigated the administration of a booster dose of HPV-16/18-vaccine Citation[34]. The availability of studies on antibody persistence and efficacy many years after vaccination will contribute to understanding kinetics of immunity, exposure and protection over the long-term.
Two-dose schedule
Initially licensed for use in a three-dose schedule, recent clinical trials indicate that a two-dose schedule of HPV-16/18-vaccine in 9- to 14-year-old girls achieves similar immunogenicity and antibody persistence as three doses in 15- to 25-year-old women, the population in which efficacy was demonstrated Citation[35]. A two-dose schedule provides significant benefits by facilitating greater vaccine acceptance and higher uptake. The HPV-16/18-vaccine given as a two-dose schedule in 9- to 14-year-old adolescents has been approved by the EMA Citation[35] and other countries. Licensure for this indication required new appraisal and adaption of the benefit–risk strategy.
Long-term vaccine efficacy
Statistical modeling based on conservative mathematical assumptions predicts that mean anti-HPV-16 and anti-HPV-18 antibody titers will persist substantially above natural infection levels for at least 20 years Citation[33,36]. Follow-up of cohorts vaccinated in clinical trials provides the earliest information on long-term HPV-16/18-vaccine immunogenicity and efficacy.
15 -year assessment of efficacy in Finland
Long-term assessment of effectiveness underway in Finland will evaluate cumulative incidence of carcinoma in situ, CIN3 and invasive cervical cancer for 15 years after vaccination. The study cohorts include women vaccinated with HPV-16/18-vaccine or control vaccine in a previous clinical trial, and a matched unvaccinated cohort. The cumulative incidence of CIN3+ and invasive cervical cancer will be retrieved from the National Registry. The study design, method and objectives have been published Citation[37–39]. Final results are anticipated in 2020.
Extended follow-up of a clinical trial population
Sustained efficacy was demonstrated 6.4 years after the commencement of a Phase IIb efficacy study Citation[40] against incident HPV infection, 6- and 12-month persistent infections, cytological abnormalities and HPV-16/18 histopathological lesions Citation[41,42]. Substantial efficacy against incident infections associated with other oncogenic HPV types (HPV-31 and HPV-45) and against cytological abnormalities associated with all oncogenic types, and CIN lesions regardless of HPV type, was also shown. An extension conducted in Brazilian centers showed sustained immune responses to HPV-16 and HPV-18 (100% seropositivity) and 95.6% efficacy (95% CI: 61.4–100%) against incident HPV-16 or HPV-18 infection in HPV-16/18-vaccinees followed for 9.4 years Citation[43].
Vaccine effectiveness & population impact activities
Several large, well-designed studies evaluating HPV-16/18-vaccine effectiveness/impact have been independently set up by public health authorities in countries in which HPV vaccination is well established in national immunization programs. GSK Vaccines has supported these programs by transferring some methods/materials (e.g., virus-like particles required for serosurveillance activities) in England and The Netherlands.
UK national surveillance
England
In September 2008, routine vaccination with HPV-16/18-vaccine was introduced in UK girls aged 12–13 years, including catch-up for girls aged up to 17 years of age. To evaluate program impact, Public Health England (PHE, formerly Health Protection Agency) instituted a two-phase national surveillance program. Phase I (2009–2014) focused on surveillance of infection with vaccine and non-vaccine HPV types in young women. The second phase will begin in 2015 when the first vaccinated cohorts will enter routine cervical screening at age 25. As well as continuing infection surveillance in young women, Phase II will include HPV-related cervical disease outcomes, assessing type replacement and cross-protection via monitoring of the prevalence of vaccine and non-vaccine types in immunized and unimmunized women with normal and abnormal cervical cytology/histology. The potential for a large sample size and the use of existing systems should enable monitoring of non-vaccine types to detect the net effects of HPV type replacement and cross-protection.
Data from PHE from the first 4 years after commencement of the immunization program suggest that the prevalence of HPV-16/18 in residual vulva-vaginal swabs from women undergoing Chlamydia screening was reduced in young women, corresponding to age-specific immunization estimates Citation[44]. A study of diagnoses from genitourinary medical clinics in England suggested that an apparent decrease in genital warts was likely due, at least in part, to HPV-16/18-vaccination Citation[7].
Scotland
The first results from the longitudinal national surveillance program conducted by National Services Scotland assessed HPV prevalence among women vaccinated in the UK catch-up program (45–89% coverage) attending for their first routine cervical smear at age 20 Citation[45]. Vaccination with three HPV-16/18-vaccine doses was associated with a more than 50% reduction in HPV-16 and HPV-18 prevalence Citation[45]. Significant reductions in non-vaccine types (31, 33 and 45), and a statistically significant reduction in CIN3 diagnoses associated with vaccination were observed Citation[46].
Longitudinal cohort study in the Netherlands
HPV-16/18-vaccination of 12-year-old girls with catch-up of 13- to 16-year-old girls was introduced into the Netherlands’ National Immunization Program in 2009. Coverage of approximately 60% has been achieved Citation[47], and a two-dose schedule was introduced in 2014. In 2009, the National Institute for Public Health and the Environment established a longitudinal cohort study to evaluate the effect of vaccination on HPV-16/18 persistent infection and epigenetic alterations suggestive of high-grade CIN lesions in girls born in 1993. A total of 11,300 girls aged 14–16 years were enrolled in 2009 and 2010. HPV testing and administration of a behavioral questionnaire was performed yearly, initially for 5 years.
Safety surveillance of potential risks
Two adverse events of special interest are being evaluated for HPV-16/18-vaccine: the first relates to use of novel Adjuvant Systems, exemplified by Adjuvant System 04 (AS04) contained in HPV-16/18-vaccine, which enhances the innate immune response and guides the resulting adaptive immune responses Citation[48,49]. The immunostimulatory effects of AS04 are localized and transient Citation[50]. However, there are theoretical concerns of the occurrence of potential vaccine-induced immune-mediated diseases (pIMDs) in susceptible individuals after vaccination with an adjuvant system-containing product Citation[51]. Thus, post-marketing activities with the specific aim of evaluating new onset/exacerbation of pIMDs after HPV-16/18-vaccination have been initiated.
HPV-16/18-vaccine is indicated for young women at an age when sexual activity is beginning or has begun. Inadvertent administration in early pregnancy is possible, and pregnancy outcomes in the event of HPV-16/18-vaccine exposure are of special interest.
New onset & exacerbation of potential immune-mediated disease
Data from analyses of large safety databases of subjects who received AS04-adjuvanted vaccines including HPV-16/18-vaccine Citation[52] signal detection analysis of available post-marketing data Citation[14], and specific pooled analyses of clinical trial data including long-term follow-up of safety in individual subjects Citation[13,15] have not identified any excess risk of pIMD onset after HPV-16/18 vaccination. A large observational study was initiated using the Clinical Practice Research Datalink General Practitioner OnLine Database (CPRD GOLD) in the UK to assess risk of new onset of autoimmune disease following administration of HPV-16/18-vaccine Citation[53]. Four cohorts of 65,000 subjects each were defined: an exposed female cohort who received at least one dose of HPV-16/18-vaccine according to local practice (recommended age in the UK is 12–13 years), an unexposed concurrent male cohort, an historical male cohort and an historical female cohort. The male cohorts are internal controls for changes in reporting new onset of autoimmune diseases over time. The primary end point is incidence of neuro-inflammatory/ophthalmic autoimmune diseases and other pre-specified autoimmune diseases within 12 months of vaccination. Results are anticipated in 2015.
Pregnancy safety surveillance
Based on a pre-licensure clinical trial in which a non-statistically significant numerical imbalance suggested higher incidence of spontaneous abortion (SA) in a specific risk window (i.e., date of onset of the last menstrual period [LMP] between 30 days before until 45 days [–30 to +45] after HPV-16/18 vaccination) Citation[54]. In a pooled analysis of 42 clinical trials in which more than 31,000 girls and women received HPV-16/18-vaccine, the rate of SA was 15.3% in HPV-vaccinated women and 11.1% in controls (risk ratio: 1.37; 95% CI: 0.94–2.01) Citation[15]. The rate in those exposed within 60 days before pregnancy onset was 15.1% in HPV-vaccinated women versus 9.5% in controls (risk ratio: 1.60; 95% CI: 0.99–2.61).
An observational cohort study was conducted using data from the CPRD GOLD database to assess the risk of SA in women aged 15–25 years in whom first day of LMP occurred between an identified potential risk period of –30 to +45 days or +90 days (secondary analysis) after any HPV-16/18-vaccine dose (Citation[55], manuscript in preparation). Two definitions of SA enabled assessment of risk relevant for the UK (SA defined as fetal loss between weeks 1 and 23 of gestation) and the USA (1–19 weeks of gestation). Other pregnancy outcomes were also assessed. An unexposed cohort comprised HPV-16/18-vaccinated women in whom date of LMP onset was between 120 days and 18 months after the third HPV-16/18-vaccine dose.
Pregnancy Registries have been established in the UK in collaboration with PHE, and in the USA (managed by GlaxoSmithKline). The registries record spontaneously reported cases of HPV-16/18-vaccine administered in pregnancy. Each registry aims to record information and further analyze information on women vaccinated with HPV-16/18-vaccine during pregnancy. Global data from routine passive surveillance and from the pregnancy registries have shown no adverse pregnancy outcomes beyond expected background rates Citation[14] (PHE personal communication, manuscript in preparation).
Expert commentary
After licensure, vaccines are usually administered to very large numbers of people, including populations with features or risk factors different to the populations studied in clinical trials, and under conditions of routine use and in schedules that are less controlled than in a clinical trial environment. Studies targeting safety and effectiveness/impact end points provide important information about the vaccine impact in the general population and enable continual evaluation of the benefit–risk ratio. The package to evaluate the benefit–risk of HPV-16/18-vaccine includes all available clinical trials including follow-up phases, as well as a post-marketing strategy to more broadly evaluate effectiveness measures and specific safety events of interest.
The studies listed in this strategy document are diverse in design and are being conducted among diverse groups and in several countries. In epidemiological studies, ways to minimize the effects of bias and confounding have been actively sought and the results are likely to be valid and generally applicable. While many of the studies listed above are ongoing, the available data confirm the benefit of HPV-16/18-vaccination in terms of prevention of HPV persistent infection, premalignant cervical lesions and cervical cancer caused by oncogenic HPV, and suggest additional benefits such as reduced occurrence of genital warts Citation[7]. The safety profile of the vaccine and the balance of its benefits and risks continue to be acceptable following the ongoing systematic review of safety data Citation[14,15].
Five-year view
Benefit–risk status is not static, but requires continual re-evaluation as vaccine uptake increases, as the epidemiology of the disease evolves, and as new information becomes available. Future plans under consideration include impact studies to assess infection end points and cancer rates (using cancer registries). Countries with high vaccination coverage for more than 5 years, such as Panama, or where established laboratory networks exist, such as countries under the umbrella of the Pan-American Health Organization, offer an environment where vaccine impact is likely to be maximal and more easily measured. Means to improve the quality of national databases, such as pregnancy registries, would aid in the collection of vaccine adverse event and impact data for all vaccines. Safety surveillance in terms of autoimmune diseases and adverse pregnancy outcomes will remain under close monitoring. Should a new signal arise, appropriate investigations will define subsequent actions on how to minimize any potential risk associated with vaccine use. Such actions could include changes to the label, communication to healthcare professionals and the public, and means by which to monitor the effectiveness of those measures Citation[14].
The next 5 years will see the availability of results from studies that are currently ongoing, providing important vaccine impact data. Comprehensive investigation of specific safety outcomes and evaluation of vaccine impact at a population level will continue to validate the benefit–risk ratio, providing accurate and relevant information for vaccine providers across a range of clinical settings. The strategy to assess benefit versus risk will evolve and adapt to the changing HPV-16/18-vaccine market and to new information as it becomes available.
Key issues
Rigorous evaluation of HPV-16/18-vaccine continues in the post-licensure setting.
Licensure of HPV-16/18-vaccine was based on a positive benefit–risk established by clinical trial data demonstrating efficacy and a clinically acceptable safety profile. Benefit–risk status requires continual re-evaluation as vaccine uptake increases, as the epidemiology of the disease evolves and as new information becomes available.
GlaxoSmithKline’s strategy to further characterize the benefit–risk profile of the HPV-16/18-vaccine in the post-licensure setting encompasses studies conducted in a range of countries, with a variety of designs, and in collaboration with national public agencies and regulatory authorities.
Recently completed or ongoing clinical trials are evaluating the use of HPV-16/18-vaccine in HIV+ women, co-administration with other vaccines, the use of a two-dose vaccination schedule and long-term immunogenicity and efficacy. Large, well-designed population-based epidemiological studies are examining vaccine effectiveness, herd immune effects and potential HPV type replacement.
Rare adverse events of special interest relating to the theoretical risk of immune-mediated disease and to outcomes if exposure occurs during pregnancy are being specifically evaluated in large studies using existing population-based databases, as well as pregnancy registries.
The extensive clinical trial program including all data that have been reviewed since its first market introduction, and post-marketing safety surveillance conducted to date show that the previously established favorable benefit–risk profile for HPV-16/18-vaccine remains unchanged.
The strategy to assess benefit versus risk will continue to evolve and adapt to the changing HPV-16/18-vaccine market.
Trademark ownership
Cervarix® is a trademark of the GlaxoSmithKline group of companies.
Acknowledgements
The authors wish to thank T Verstraeten who provided guidance and support in the overall functioning of surveillance systems during his term at GlaxoSmithKline Vaccines. From GlaxoSmithKline Vaccines, Wavre, Belgium, the authors thank C Galindo, epidemiologist, J Zima the safety physician responsible for review of all Cervarix® safety data irrespective of source, and who leads the Cervarix Safety Review Team, D Decamps who led the Cervarix clinical development program and D Bi, B Geeraerts and F Thomas-Jooris who checked parts of the manuscript for accuracy. The authors also thank S Hernández-Díaz, Director of the Pharmacoepidemiology Program and Associate Professor of Epidemiology, Harvard School of Public Health, Boston, MA, USA, for expert advice in methodology and analysis of pregnancy exposure data and for analytical observational study on this subject.
Writing support services were provided by J Wolter (independent medical writer, Brisbane, Australia); editing and publication coordinating services were provided by V Delpire and M Payne (Words & Science, Brussels, Belgium).
Financial & competing interests disclosure
GlaxoSmithKline Biologicals SA was the funding source and was involved in all surveillance activities, overall data management (collection, analysis and interpretation); GlaxoSmithKline also funded all costs associated with the development and the publishing of the present manuscript. All authors were employed by the GlaxoSmithKline group of companies at the time of study, and hold/held shares in the company as part of employee remuneration. All authors had full access to the data and the corresponding author was responsible for submission of the publication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- Hanquet G, Valenciano M, Simondon F, Moren A. Vaccine effects and impact of vaccination programmes in post-licensure studies. Vaccine 2013;31:5634-42
- Benefit-Risk balance for marketed drugs: evaluating safety signals. Report of CIOMS Working Group IV, 1998. Available from: www.cioms.ch/publications/g4-benefit-risk.pdf [Last accessed 9 May 2014]
- Guideline on good pharmacovigilance practices (GVP). EMA/838713/2011 European Medicines Agency, 2012. Available from: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129134.pdf [Last accessed 25 January 2014]
- Delegated act on post-authorisation efficacy studies. Article 10B of Regulation (EC) No 726/2004 and Article 22B of Directive 2001/83/EC). European Commission, Health Consumers Directorate-General. 2012. Available from: http://ec.europa.eu/health/files/pharmacovigilance/2012_11_28_pc_paes.pdf [Last accessed 22 October 2013]
- Wong CA, Saraiya M, Hariri S, et al. Approaches to monitoring biological outcomes for HPV vaccination: challenges of early adopter countries. Vaccine 2011;29:878-85
- Chang Y, Brewer NT, Rinas AC, et al. Evaluating the impact of human papillomavirus vaccines. Vaccine 2009;27:4355-62
- Howell-Jones R, Soldan K, Wetten S, et al. Declining genital warts in young women in England associated with HPV 16/18 vaccination: an ecological study. J Infect Dis 2013;208:1397-403
- Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009;374:301-14
- Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012;13:89-99
- Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012;30(Suppl 5):F123-38
- Wheeler CM, Castellsagué X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012;13:100-10
- Macartney KK, Chiu C, Georgousakis M, Brotherton JM. Safety of human papillomavirus vaccines: a review. Drug Saf 2013;36:393-412
- Descamps D, Hardt K, Spiessens B, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin 2009;5:332-40
- Angelo M-G, Zima J, Tavares Da Silva F, et al. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf 2014;23:456-65
- Angelo M-G, David M-P, Zima J, et al. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf 2014;23:466-79
- Gasparini R, Bonanni P, Levi M, et al. Safety and tolerability of bivalent HPV vaccine: an Italian post-licensure study. Hum Vaccin 2011;7(Suppl):136-46
- MHRA Public assessment report Cervarix (HPV vaccine): update on UK safety covering the first two years of the HPV immunisation programme. Medicines and Healthcare products Regulatory Agency (MHRA), October 2010. Available from: www.mhra.gov.uk/PrintPreview/DefaultSplashPP/CON023340?ResultCount=10&DynamicListQuery=&DynamicListSortBy=xCreationDate&DynamicListSortOrder=Desc&DynamicListTitle=&PageNumber=1&Title=Human%20papillomavirus%20(HPV)%20vaccine [Last accessed 7 September 2012]
- Van’t Klooster T, Kemmeren J, Vermeer-de-Bondt P, et al. Adverse events following vaccination against human papillomavirus: Results of the 2010 campaign in the Netherlands. RIVM, 2011. Available from: www.rivm.nl/en/Library/Scientific/Reports/2011/december/Adverse_events_following_vaccination_against_human_papillomavirus_Results_of_the_2010_campaign_in_the_Netherlands?sp=cml2bXE9ZmFsc2U7c2VhcmNoYmFzZT01OTAzMDtyaXZtcT1mYWxzZTs=&pagenr=5904 [Last accessed 7 September 2014]
- The current state of introduction of human papillomavirus vaccination into national immunisation schedules in Europe: first results of the VENICE2 2010 survey. European Centre for Disease Prevention and Control (ECDC) Health Communication Unit. Eurosurveillance, 2010. Available from: www.eurosurveillance.org/viewarticle.aspx?ArticleId=19730 [Last accessed 10 September 2012]
- De Sanjosé S, Palefsky J. Cervical and anal HPV infections in HIV positive women and men. Virus Res 2002;89:201-11
- Adjorlolo-Johnson G, Unger ER, Boni-Ouattara E, et al. Assessing the relationship between HIV infection and cervical cancer in Côte d’Ivoire: a case-control study. BMC Infect Dis 2010;10:242
- Denny L, Hendricks B, Gordon C, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine 2013;31:5745-53
- Evaluation of safety and immunogenicity of a human papillomavirus (HPV) vaccine in human immunodeficiency virus (HIV) infected females. Available from: http://clinicaltrials.gov/show/NCT01031069
- General recommendations on immunization–recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60:1-64
- Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009;5:705-19
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012;50:38-46
- Garcia-Sicilia J, Schwarz TF, Carmona A, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine coadministered with combined diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine to girls and young women. J Adolesc Health 2010;46:142-51
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011;30:e225-34
- Schmeink CE, Bekkers RLM, Josefsson A, et al. Co-administration of human papillomavirus-16/18 AS04-adjuvanted vaccine with hepatitis B vaccine: randomized study in healthy girls. Vaccine 2011;29:9276-83
- Palmroth J, Merikukka M, Paavonen J, et al. Occurrence of vaccine and non-vaccine HPV types in adolescent Finnish females four years post vaccination. Int J Cancer 2012;131:2832-8
- Lehtinen M, French K, Dillner J, et al. Sound implementation of human papillomavirus vaccination as a community-randomized trial. Therapy 2008;5:289-94
- Effectiveness, safety and immunogenicity of GSK biologicals’ HPV vaccine GSK580299 (Cervarix TM) administered in healthy adolescents. Available from: http://clinicaltrials.gov/show/NCT00534638
- David M-P, Van Herck K, Hardt K, et al. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol 2009;115:S1-6
- Moscicki A-B, Wheeler CM, Romanowski B, et al. Immune responses elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in previously vaccinated adult women. Vaccine 2012;31:234-41
- GSK Cervarix® two-dose schedule receives European marketing authorisation. GSK Media, 2013. Available from: http://www.gsk.com/media/press-releases/2013/gsk-cervarix–two-dose-schedule-receives-european-marketing-auth.html [Last accessed 8 February 2014]
- Aregay M, Shkedy Z, Molenberghs G, et al. Model-based estimates of long-term persistence of induced HPV antibodies: a flexible subject-specific approach. J Biopharm Stat 2013;23:1228-48
- Lehtinen M, Apter D, Dubin G, et al. Enrolment of 22,000 adolescent women to cancer registry follow-up for long-term human papillomavirus vaccine efficacy: guarding against guessing. Int J STD AIDS 2006;17:517-21
- Lehtinen M, Idänpään-Heikkilä I, Lunnas T, et al. Population-based enrolment of adolescents in a long-term follow-up trial of human papillomavirus vaccine efficacy. Int J STD AIDS 2006;17:237-46
- Lehtinen M, Paavonen J. Effectiveness of preventive human papillomavirus vaccination. Int J STD AIDS 2003;14:787-92
- Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004;364:1757-65
- Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006;367:1247-55
- Romanowski B, de Borba PC, Naud PS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009;374:1975-85
- Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: Final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccines Immunother 2014;10
- Mesher D, Howell-Jones R, Panwar K, et al. Reduction in HPV infection in young women following introduction of HPV immunisation in England. Vaccine 2013;32:26-32
- Kavanagh K, Pollock KGJ, Potts A, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer 2014;110:2804-11
- Pollock K, Potts A, Love J, et al. Early effect of the HPV bivalent vaccine on high-risk HPV prevalence and high-grade cervical abnormalities in Scotland. EUROGIN; Florence, Italy: 2013
- Fewer municipalities with low vaccination coverage. RIVM, 2013. Available from: www.rivm.nl/en/Documents_and_publications/Common_and_Present/Newsmessages/2013/Fewer_municipalities_with_low_vaccination_coverage [Last accessed 8 May 2014]
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 2007;6:723-39
- Garçon N, Segal L, Tavares F, Van Mechelen M. The safety evaluation of adjuvants during vaccine development: the AS04 experience. Vaccine 2011;29:4453-9
- Didierlaurent AM, Morel S, Lockman L, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol Baltim Med 1950;183:6186-97
- Tavares Da Silva F, De Keyser F, Lambert P-H, et al. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine 2013;31:1870-6
- Verstraeten T, Descamps D, David M-P, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008;26:6630-8
- Study assessing risk of autoimmune diseases in females (9–25 Years) exposed to Cervarix® in United Kingdom. Available from: http://clinicaltrials.gov/show/NCT01953822
- Wacholder S, Chen BE, Wilcox A, et al. Risk of miscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ 2010;340:c712
- Post-marketing safety study to assess the risk of spontaneous abortions in women exposed to Cervarix residing in UK. Available from: http://clinicaltrials.gov/show/NCT01905462
