
Adults and children are unevenly affected by sudden death. Children under 5 are the main targets of sudden death [Citation1,Citation2]. An infectious cause is detected on average in 50% (15–86% depending on the study) of sudden death in children and infection concerns mostly the respiratory system [Citation3–Citation5]. A viral etiology of respiratory infection is then identified in 20–48% of cases during the postmortem investigations [Citation1,Citation2]. Viral respiratory infections are therefore one of the main etiologies of sudden deaths of children ahead of heart attacks and far ahead of the central nervous system involvement. The viruses then involved are identical to those responsible for high or low respiratory tract infections in the general population. The influenza virus A and B (FluA or FluB), respiratory syncytial virus (RSV), parainfluenza virus, metapneumovirus, adenovirus (ADV), and rhino-enterovirus (EV) are thus classically found () [Citation1,Citation2,Citation6–Citation9]. The epidemiology of sudden deaths associated with respiratory virus responds to obvious seasonal changes and coincides with epidemic winter peaks of these viruses, with the notable exception of the EV group mainly circulating during summer and autumn. Some of these viruses have a specific clinical presentation; EV often associate heart and lung damage; ADV are also responsible for gastroenteritis; finally, the pandemic FluA is responsible for more deaths than seasonal virus due to increased virulence of the pathogen, its circulation within an immunologically naïve population leading to more readily systemic spread through the body [Citation10]. Other viruses involved in common childhood diseases such as herpes simplex, Varicella Zoster virus (VZV), Epstein–Barr virus, cytomegalovirus, human herpes virus type 6, and Parvovirus B19 have also been detected in lungs of deceased infants, but their involvement in the death remained sometimes uncertain [Citation11]. However, the epidemiology is probably still largely incomplete as it totally depends on the methodology used during the postmortem investigations. It is likely also biased since respiratory virus testing is more commonly performed in children than in adults leading to an underestimation in this second group of patients. Furthermore, the use of viral culture, a method known to be less sensitive than PCR and not isolating many pathogenic viruses, probably contributed to distort the epidemiology of sudden respiratory viral deaths. Its profile is thus gradually refined as we move from accumulated case reports using various diagnostic methodologies to standardized cohort studies where broad spectrum molecular biology tools are now conventionally used. Nevertheless, the epidemiological data are still missing for many respiratory viruses.
Table 1. Epidemiology of respiratory viruses involved in sudden death.
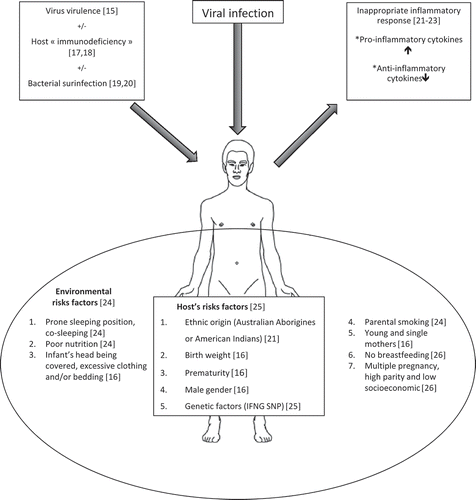
Sudden death caused by respiratory viruses is due to septic shock, obstruction of bronchioles, and impaired breathing responsible for apnea and severe, potentially life-threatening hypoxemia [Citation7,Citation16]. It occurs in the context of an acute infection or an exacerbation of a chronic respiratory disease such as asthma or chronic obstructive pulmonary disease. The pathophysiology of sudden death is double (). It combines an infectious part with an inflammatory part [Citation17]. The infectious side itself is multiple. First, it is related to the own virus virulence. The most pathogenic and epidemic viruses like RSV or influenza viruses are also those commonly identified in sudden death [Citation18]. Pandemic influenza viruses are responsible for a larger number of deaths than the seasonal influenza virus [Citation10]. Second, infectious part is also based on host immunodeficiency that mainly affects children under 2 years at a period of their development where they lose the protection provided by maternal antibodies while still having an immature immune system. Third, pre-existing viral infections have a facilitating role on bacterial infections. Viral infections cause an increase in the number of bacteria receptors promoting adherence and colonization of the respiratory tract by a higher number and greater variety of bacterial species [Citation19]. Moreover, lethal doses of bacterial endotoxins and exotoxins in animal model are reduced during mild or asymptomatic viral infection [Citation20,Citation21]. Inappropriate inflammatory response to viral infection remains, however, the main element likely to trigger sudden death [Citation22]. Well described in children, this immunopathological component, known as cytokine storm hypothesis, is a combination of inadequate secretion in response to viral infection of pro-inflammatory cytokines (IFNγ, IL-1, IL-6, and TNFα) with a deficit in anti-inflammatory cytokines such as IL-10 [Citation23]. It causes microscopic inflammatory changes of the respiratory tract, frequently observed in sudden deaths, consisting in peribronchial inflammatory infiltrates, increase in IgM-producing cells in trachea, and mast cell degranulation [Citation24,Citation25]. The RSV, in particular, is known to cause a major and inappropriate inflammatory response, which explains its frequent detection in sudden death of the child [Citation26]. Genetic factors are also involved since a recent study showed a single nucleotide polymorphism interferon gamma (IFNG) T + 874A genotype associated with higher IFNγ response that is overrepresented in sudden infant death syndrome infants [Citation27]. It is, of course, mandatory to mention the key role of environmental factors such as exposure to cigarette smoke, poor nutrition, or sleeping in the prone position [Citation28]. All these risk factors will distinguish a mild, self-limiting infection in an individual from a fatal infection in another. Finally, it is important to mention that the respiratory viruses can cause sudden death by extrapulmonary involvement. Influenza viruses are well known to be responsible for cardiac or neurological manifestations that can be fatal [Citation29].
Figure 1. Pathophysiology of sudden death involving respiratory viruses combines infectious and inflammatory parts. The severity of the viral infection is facilitated by the virulence of the virus itself but also by the host immunodeficiency. This, in turn, facilitates super-infections by bacteria producing endo- and exotoxins. The immunopathological part consists of inappropriate pro-inflammatory cytokines secretion conjugated to a deficit in anti-inflammatory cytokines. It causes the appearance of inflammatory lesions commonly seen in the respiratory tract of deceased individuals. These mechanisms in addition to genetic and environmental factors may lead to the sudden death.
Given the frequency of viral causes of sudden death, the completion of a postmortem viral diagnosis is absolutely necessary before concluding, particularly in children, with an unexplained death [Citation30]. However, the quality of virological investigations currently suffers from the lack of decision-tree conducting postmortem examination [Citation31]. These investigations require standardized multidisciplinary approach involving a macroscopic examination performed at autopsy, histological microscopic examination and virological testing [Citation13,Citation32]. They must be preceded by questioning of the family to track down a recent mild respiratory infection that occurred in approximately 40–80% of cases of sudden death [Citation28]. Exploration conditions must then take into account a number of factors likely to influence the results of virological examination [Citation32]. First, the time between death and sampling must be as short as possible; a significant delay may lead to an alteration of viral nucleic acids. Then the choice of the sample to be analyzed is fundamental. Moore and Jones have shown the superiority of postmortem respiratory swabbing to the analysis of lung tissue, without any significant difference between swabs performed at the upper and lower respiratory tract level [Citation15]. Moreover, histological as well as virological investigations performed in lung tissue may be sometimes complicated by the focal character of the infection that can lead to false negative results, if the sampling is done outside of inflammatory lesions. This feature then requires the analysis of multiple samples of tissue, frozen but not paraffin-embedded since this process can cause significant protein–nucleic acid crosslinks as well as fragmentation of nucleic acids reducing the quantity and size of nucleic acids, in particular viral RNA, suitable for amplification [Citation13]. These drawbacks do not exist with the respiratory tract swab only dedicated to virological diagnosis and thereby delivered to the laboratory in a transport medium maintaining viral structures. Finally, the choice of the technique used for the virological diagnosis is crucial. Molecular tools are now regarded as the reference method for postmortem virological investigations. Their performances far exceed those of viral culture, serology, or viral antigen testing by immunohistochemistry [Citation6]. They offer the possibility of very sensitive broad-spectrum viral research from a single sample that has revolutionized the virological diagnosis of respiratory infections, in general, and sudden death, in particular [Citation33]. The contribution of high-throughput sequencing tools, for the identification of new pathogens or known pathogens unintended in this context, is a promising development that should provide, in future years, a comprehensive viral epidemiology of sudden deaths caused by respiratory viruses.
The question of the causal link between the detected virus and the sudden death is a recurring problem. The use of molecular techniques of great sensitivity and detecting a wide range of more or less pathogenic viruses can make delicate interpretation of virological data. If this interpretation is relatively easy in case of detection of a virus whose virulence is well recognized and which is only responsible for acute infections such as FluA or RSV, it is much more difficult when it is a virus whose involvement in respiratory infection is discussed, describing latency and potentially passively transported by inflammatory cells such as viruses of the Herpesviridae family. Testing for markers of active viral replication would be necessary but is not routinely performed. Moreover, in both cases (virus of well-known or debatable pathogenicity), a/ pauci-symptomatic infections are always possible. Case-control studies are then needed to prove a significantly higher detection in individuals who died from unexplained sudden death suggesting virus involvement. The controversy is therefore inevitable if the detection rate is equivalent between individuals who suddenly died and healthy individuals or died from other explained cause [Citation34]. However, if the virus is not considered as the main cause of death, it can still be a triggering or aggravating factor according to the context or the genetic background. Overall, the interpretation of virological results must be done in connection with autopsy and histological data. Inflammatory abnormalities only lend weight to the detected virus. The absence of consistent histological abnormalities renders much more hypothetical the role of the virus in the death. Nevertheless, the potential focal nature of the infection and the delayed onset of the inflammatory infiltrate in children do not totally exclude a causal link in the absence of visible inflammatory abnormalities [Citation35]. All these points explain why a large number of viruses detected are not ultimately retained as the probable cause of death [Citation15]. Among the tools that could help interpreting virological data, viral genome quantitation in lung tissue or respiratory swab would enable to distinguish between a low-level detection matching with latent or past infection from a high level of nucleic acids corresponding to active infection likely associated with death. Localization of respiratory virus antigens in the lung tissue would allow both demonstrating an in situ viral replication and improving the understanding of the pathogenesis of sudden death by identifying infected cells [Citation36]. In addition to the virological side, genetic explorations and pro-inflammatory cytokines secretion profile in serum or tissue would explore the host response to infection that would be consistent with the sudden death pathophysiology [Citation37].
Prevention of sudden death due to respiratory viruses is the prevention of respiratory viral infections in general with the objective to limit the transmission of the virus to younger children through air and contact containment. It is also possible to act on the environmental risk factors (sleeping position, the room temperature, smoking, etc.). Vaccination against the viruses responsible for respiratory diseases such as Flu, measles, or VZV is obviously desirable either for the child itself or its relatives. Finally, the treatment of benign viral infections that frequently precede sudden death faces two obstacles, for now insurmountable, (i) the absence of antiviral treatment for respiratory viruses other than Flu and (ii) the high frequency of respiratory infections in the general population compared to the very low number of sudden deaths. This second point forces first to identify genetic risk factors for sudden death and second to develop suitable screening methods.
In conclusion, respiratory viral infections are a common cause of a fortunately rare disease, the sudden death. The systematic implementation of a postmortem respiratory viral diagnosis combined with autopsy and histological examinations thus seem evident and should be mandatory in order to improve the elucidation rate of unexplained sudden death in child as in adult. Better understandings of viral epidemiology but also of the pathophysiological mechanisms through genetic and immunological explorations aim to better understand the risk factors and thus better prevent sudden death caused by respiratory viruses.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- An SF, Gould S, Keeling JW, et al. Role of respiratory viral infection in SIDS: detection of viral nucleic acid by in situ hybridization. J Pathol. 1993;171(4):271–278.
- Bajanowski T, Rolf B, Jorch G, et al. Detection of RNA viruses in sudden infant death (SID). Int J Legal Med. 2003;117(4):237–240.
- Eisenstein EM, Haklai Z, Schwartz S, et al. Investigation of unexplained infant deaths in Jerusalem, Israel 1996–2003. Arch Dis Child. 2007;92(8):697–699.
- Sadler DW, Nakano R. The value of a thorough protocol in the investigation of sudden infant deaths. J Clin Pathol. 1998;51(9):689–694.
- Vennemann MM, Bajanowski T, Butterfass-Bahloul T, et al. Do risk factors differ between explained sudden unexpected death in infancy and sudden infant death syndrome? Arch Dis Child. 2007;92(2):133–136.
- Burger MC, Dempers JJ, de Beer C. Profiling the approach to the investigation of viral infections in cases of sudden unexpected death in infancy in the Western Cape Province, South Africa. Forensic Sci Int. 2014;239:27–30.
- Lucas JR, Haas EA, Masoumi H, et al. Sudden death in a toddler with laryngotracheitis caused by human parainfluenza virus-1. Pediatr Dev Pathol. 2009;12(2):165–168.
- Siani V, Netter JC, Gorguet B. [Detection of respiratory syncytial virus using immunohistochemistry. Report of 53 cases of sudden infant death]. Ann Pathol. 1999;19(2):99–102.
- Weber MA, Hartley JC, Ashworth MT, et al. Virological investigations in sudden unexpected deaths in infancy (SUDI). Forensic Sci Med Pathol. 2010;6(4):261–267.
- Nakajima N, Sato Y, Katano H, et al. Histopathological and immunohistochemical findings of 20 autopsy cases with 2009 H1N1 virus infection. Mod Pathol. 2012;25(1):1–13.
- Álvarez-Lafuente R, Aguilera B, Suárez-Mier MP, et al. Detection of human herpesvirus-6, Epstein-Barr virus and cytomegalovirus in formalin-fixed tissues from sudden infant death: a study with quantitative real-time PCR. Forensic Sci Int. 2008;178(2–3):106–111.
- Cecchi R, Cucurachi N, Mencarelli R, et al. CMV-DNA detection in parenchymatous organs in cases of sudden infant death syndrome. Int J Legal Med. 1995;107(6):291–295.
- Desmons A, Terrade C, Boulagnon C, et al. Post-mortem diagnosis, of cytomegalovirus and varicella zoster virus co-infection by combined histology and tissue molecular biology, in a sudden unexplained infant death. J Clin Virol. 2013;58(2):486–489.
- Dettmeyer R, Sperhake JP, Müller J, et al. Cytomegalovirus-induced pneumonia and myocarditis in three cases of suspected sudden infant death syndrome (SIDS): diagnosis by immunohistochemical techniques and molecular pathologic methods. Forensic Sci Int. 2008;174(2–3):229–233.
- Moore C, Jones R. The use of coroner’s autopsy reports to validate the use of targeted swabbing rather than tissue collection for rapid confirmation of virological causes of sudden death in the community. J Clin Virol. 2015;63:59–62.
- Samuels M. Viruses and sudden infant death. Paediatr Respir Rev. 2003;4(3):178–183.
- Blackwell CC Moscovis SM, Gordon AE., Cytokine responses and sudden infant death syndrome: genetic, developmental, and environmental risk factors. J Leukoc Biol. 2005;78(6):1242–1254.
- Hardelid P, Pebody R, Andrews N. Mortality caused by influenza and respiratory syncytial virus by age group in England and Wales 1999–2010. Influenza Other Respir Viruses. 2013;7(1):35–45.
- Raza MW, Guhathakurta B, Ghosh AN, et al. Infection with respiratory syncytial virus enhances expression of native receptors for non-pilate Neisseria meningitidis on HEp-2 cells. FEMS Immunol Med Microbiol. 1999;23(2):115–124.
- Lundemose JB, Lepore DA, Horne RS, et al. Cytokine release from human peripheral blood leucocytes incubated with endotoxin with and without prior infection with influenza virus: relevance to the sudden infant death syndrome. Int J Exp Pathol. 1993;74(3):291–297.
- Sarawar SR, Blackman MA, Doherty PC Superantigen shock in mice with an inapparent viral infection. J Infect Dis. 1994;170(5):1189–1194
- Blackwell C, Moscovis S, Hall S, et al. Exploring the risk factors for sudden infant deaths and their role in inflammatory responses to infection. Front Immunol. 2015;6:44.
- Schultz C, Temming P, Bucsky P, et al. Immature anti-inflammatory response in neonates. Clin Exp Immunol. 2004;135(1):130–136.
- Holgate ST, Walters C, Walls AF, et al. The anaphylaxis hypothesis of sudden infant death syndrome (SIDS): mast cell degranulation in cot death revealed by elevated concentrations of tryptase in serum. Clin Exp Allergy. 1994;24(12):1115–1122.
- Howat WJ, Moore IE, Judd M, et al. Pulmonary immunopathology of sudden infant death syndrome. Lancet. 1994;343(8910):1390–1392.
- Openshaw PJ. Antiviral immune responses and lung inflammation after respiratory syncytial virus infection. Proc Am Thorac Soc. 2005;2(2):121–125.
- Moscovis SM, Gordon AE, Al Madani OM, et al. Virus infections and sudden death in infancy: the role of interferon-γ. Front Immunol. 2015;6:107.
- Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results of the national institute of child health and human development SIDS cooperative epidemiological study. Ann N Y Acad Sci. 1988;533:13–30.
- Boulagnon C, Leveque N, Renois F, et al. Influenza A/H1N1 (2009) infection as a cause of unexpected out-of-hospital death in the young. J Forensic Sci. 2012;57(6):1650–1655.
- Rambaud C, Guibert M, Briand E, et al. Microbiology in sudden infant death syndrome (SIDS) and other childhood deaths. FEMS Immunol Med Microbiol. 1999;25(1–2):59–66.
- Brooks EG, Gill JR. Testing for infectious diseases in sudden unexpected infant death: a survey of medical examiner and coroner offices in the United States; National Association of Medical Examiners NAME Ad Hoc Committee for bioterrorism and infectious disease. J Pediatr. 2015;167(1):178–182.
- la Grange H, Verster J, Dempers JJ, et al. Review of immunological and virological aspects as contributory factors in sudden unexpected death in infancy (SUDI). Forensic Sci Int. 2014;245C:12–16.
- Renois F, Talmud D, Huguenin A, et al. Rapid detection of respiratory tract viral infections and coinfections in patients with influenza-like illnesses by use of reverse transcription-PCR DNA microarray systems. J Clin Microbiol. 2010;48(11):3836–3842.
- Williams AL, Uren EC, Bretherton L. Respiratory viruses and sudden infant death. Br Med J (Clin Res Ed). 1984;288(6429):1491–1493.
- Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–683.
- Lazara M, Perelygina L, Martines R, et al. Immunolocalization and distribution of rubella antigen in fatal congenital rubella syndrome. EBioMedicine. 2015;3:86—92. DOI:10.1016/j.ebiom.2015.11.050.
- Weber MA, Sebire NJ. Molecular diagnostic techniques in the post-mortem investigation of sudden unexpected infant deaths: current and future applications. Open Pathol J. 2010;4:110–119.
