SUMMARY
Increasing evidence suggests that esophagogastric junction (EGJ) distensibility is predictive of long-term clinical success after achalasia treatment. A new commercially available hydraulic dilation balloon is capable of measuring EGJ opening diameters whilst simultaneously dilating the EGJ. Deployed alongside the endoscope under direct visualization, it is used for dilation of the lower esophageal sphincter in patients with achalasia. Impedance measurement electrodes are incorporated in the catheter shaft in the dilation balloon, which allows measuring the diameter of the EGJ and displaying it in real time before, during and after dilation. This obviates the need for fluoroscopy during the dilation procedure. The extent of recoil of the EGJ after dilation potentially provides a measurement that could be incorporated into a clinical rule for predicting therapeutic success after dilation.
Introduction
Idiopathic achalasia is a primary motor disorder of the esophagus, characterized by a failure of the lower esophageal sphincter (LES) to relax when swallowing combined with the absence of peristalsis in the esophageal body [Citation1]. Currently, no curative treatment is available to halt or restore the degeneration of the esophageal myenteric plexus that causes achalasia [Citation2]. Treatment focuses on relieving the functional outflow obstruction by decreasing the LES pressure. Esophageal decompensation reduced esophageal sensitivity and dietary adjustments reduce symptomatology and often delay the identification of patients in need of retreatment [Citation3]. Timely identification of these patients is important to prevent esophageal decompensation. Recent studies suggest that esophagogastric junction (EGJ) distensibility may be useful to assess technical success and to predict clinical success. Recently, a hydraulic dilation catheter has become available that measures EGJ opening while dilating the LES; the esophageal functional lumen-imaging probe (EsoFLIP, Crospon Ltd., Galway, Ireland).
Device characteristics and technique
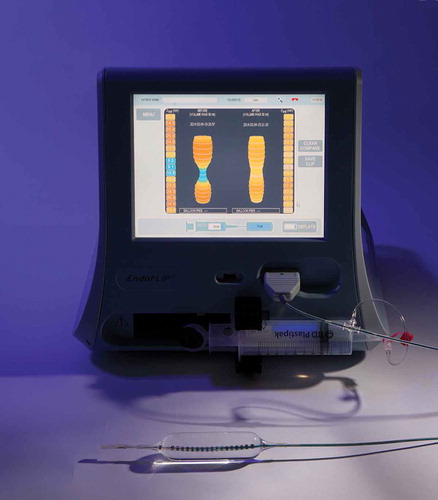
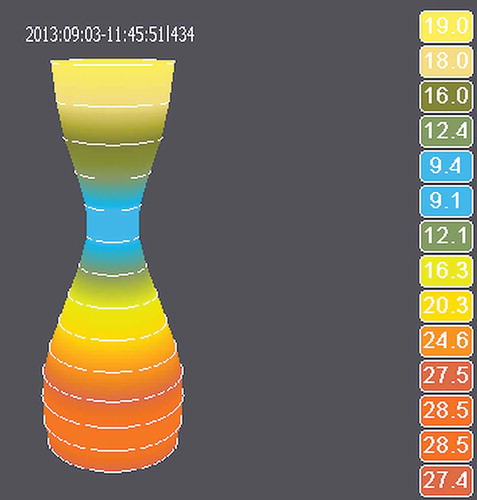
The EsoFLIP is a 2.3-m-long dilation balloon catheter with a 7F shaft (). The currently available 30 mm dilator is designed for dilation of the LES in achalasia patients. The tapered nylon balloon is 8 cm long and can be operated at a pressure of up to 1.5 atm. The catheter is deployed transorally alongside the endoscope and inflated under direct endoscopic visualization. A guidewire with a diameter up to 0.035 in. can be used to direct the catheter beyond the EGJ. A central unit with a display and a motor syringe (Crospon Ltd., Galway, Ireland, ) is connected to the disposable catheter. A conductive saline solution is injected at a controlled rate (up to 60 mL/min) to inflate the balloon [Citation4]. The central unit uses impedance planimetry to perform 14 simultaneous cross-sectional area (CSA) measurements during the dilation procedure, 5 mm apart, over a length of 7 cm [Citation5]. The diameter measurements are displayed on the central unit (). The pressure within the balloon may be measured using an external manometer (Dwyer Instruments Inc., Michigan City, IN), connected to the proximal part of the catheter using a three-way valve. By connecting a manometer, EGJ distensibility can be calculated.
EGJ distensibility
The EGJ distensibility (mm2/mmHg) is a measurement of the relationship between CSA (mm2) and pressure (mmHg) within the balloon; in other words, it is a measurement of the tightness and compliance of the EGJ. In untreated achalasia patients, distensibility is expected to be low since the EGJ is tight and the pressure required to further inflate the balloon high compared with healthy individuals. After treatment, the EGJ is expected to be more distensible since continuity of the LES is disrupted. Previous studies have shown that distensibility measurements are useful to objectify symptoms associated with an abnormal EGJ function. In 2010, Kwiatek et al. [Citation6] reported the initial experience with EGJ distensibility measurements in healthy volunteers and in patients with gastroesophageal reflux disease (GERD). They found that patients that exhibited GERD symptoms had a two- to threefold increased EGJ distensibility compared with healthy volunteers (p = 0.01). Interestingly, no correlation was found between endoscopically assessed EGJ morphology and distensibility measurements (p = 0.17), suggesting that distensibility measurements may be complementary to endoscopy when evaluating GERD patients.
Rohof et al. [Citation3] reported on EGJ distensibility measurements in achalasia patients and healthy volunteers. They found that EGJ distensibility increased significantly after treatment in previously untreated achalasia patients; 2.9 (interquartile range [IQR] 1.2–7.4) mm2/mmHg versus 0.6 (IQR 0.37–0.94) mm2/mmHg (p = 0.02), respectively. Moreover, the correlation between an increase in distensibility and decrease in symptom score was excellent (Spearman’s rank test r = 0.89; p = 0.005). Results in five previously treated patients were comparable; median EGJ distensibility increased after pneumodilation (three patients) and laparoscopic Heller myotomy (LHM) (two patients) after a median of 6 months follow-up (5.6 [IQR 3.7–6.6] mm2/mmHg vs. 0.79 [IQR 0.58–1.2] mm2/mmHg; p = 0.03). Interestingly, while residual LES pressure was not predictive of the height of stasis on a timed barium esophagram (r = 0.25; p = 0.26), EGJ distensibility showed a good correlation with the height of stasis (r = 0.72; p < 0.001). Therefore, the authors concluded that EGJ distensibility is a better predictor of esophageal emptying and long-term success of achalasia treatment than manometry [Citation3]. Pandolfino et al. [Citation7] reported similar results. They compared EGJ distensibility measurements with timed barium esophagram, high-resolution manometry and Eckardt score in treated and untreated achalasia patients. The correlation between the Eckardt score and EGJ distensibility was confirmed (r = 0.41; p < 0.05) [Citation8]. Moreover, the EGJ distensibility was low in several patients with symptoms (Eckardt score ≥3) while LES pressure and even timed barium esophagram were normal. These findings suggest that distensibility measurement devices may identify patients that require additional treatment when other measurement tools fail to do so. Later studies have shown comparable results [Citation9]. Verlaan et al. [Citation10] compared distensibility measurements before and after peroral endoscopic myotomy (POEM) in a small study. A significant increase in distensibility was seen after POEM compared with distensibility beforehand; 6.7 mm2/mmHg versus 1.0 mm2/mmHg (p = 0.02), respectively. These results were in line with a reduction in the Eckardt score; 1 after POEM versus 8 before POEM. Rieder et al. [Citation11] showed that distensibility measurements during POEM are useful to evaluate the effect of myotomy and hence to determine whether to dissect more tissue. Comparable applications were studied with LHM [Citation12], Nissen fundoplication [Citation13,Citation14] and gastric banding [Citation15].
The above-mentioned studies were all conducted with the endoscopic functional luminal imaging probe (EndoFLIP; Crospon Ltd., Galway, Ireland). It is important to note that reference data from publications with the EndoFLIP should not be equated with data of EsoFLIP measurements, even though the EsoFLIP dilation balloon uses the same measurement technology. Unlike the EndoFLIP balloon, the EsoFLIP balloon is not infinitely compliant. While the esophageal lumen shapes the rather soft balloon of the EndoFLIP, the inverse is true for the EsoFLIP where the esophageal lumen is shaped by the stiffer EsoFLIP balloon (during dilation). This requires the development of new outcome data for typical pre- and postdilation pressures as measured with the dilation balloon in achalasia patients. As with EndoFLIP measurements, we used a balloon inflation volume of 30 mL for pre- and postdilation measurements in a feasibility study [Citation16]. This volume produces a distending pressure of approximately 70 mmHg, whereas with EndoFLIP the same inflation volume produces a pressure of approximately 15 mmHg, the difference being the result of a greater balloon stiffness of the dilation balloon [Citation7,Citation16]. More reference data are required before the EsoFLIP data can be used to predict outcomes in clinical practice. Ideally, a prospective comparison between intra-balloon pressure measurements using the EsoFLIP and EndoFLIP balloon in various patient groups should be performed. With these data, it may be possible to develop conversion tables, which would allow comparison of previously obtained data with the EndoFLIP with those obtained with the EsoFLIP balloon.
Expert commentary
The dilation technique used with the EsoFLIP balloon is similar to other dilation balloons; however, the fact that the EsoFLIP balloon performs integrated measurements of the CSA offers potential advantages.
First, the ease of EGJ luminal opening (distensibility) can be measured directly before and after dilation to assess the effect of dilation therapy, without the need for additional procedures. As the balloon is deflated after dilation, the degree of recoil of the EGJ lumen can be measured; if the lumen recoils strongly toward the pre-dilation diameter and, more importantly, the pre-dilation distensibility, this may likely indicate that the performed dilation has not been sufficient. Moreover, in centers where treatment is performed in two or three sessions (usually with incremental balloon diameters), the distensibility measurement prior to the second dilation may prove particularly useful to assess recoil of the EGJ over time, thus possibly predicting the need for a more intensive follow-up and/or a third dilation with a larger-sized balloon (e.g. 40 mm).
Second, as can be seen in , the waist of the EGJ is visualized before, during and after dilation, avoiding the need for fluoroscopy. This has the advantage that radiation exposure to patients and staff is reduced. Furthermore, it has logistic advantages since dilations no longer need to be performed in a room or even center with fluoroscopy equipment available. Recently, Lirio et al. [Citation17] reported on the first hydraulic dilation in a patient with eosinophilic esophagitis and a benign stricture. The authors used a 20 mm dilation balloon without fluoroscopy in this patient, which went smoothly and without complications, as was our experience and that of others [Citation18].
Third, a pre-dilation diameter and distensibility measurement may be useful to predict the risk of procedure-related complications. As is well known from the experience with Savary dilators for benign esophageal strictures, encountering resistance while inserting the dilator is a reason to advance only one or two more dilators with incremental diameters in an attempt to avoid perforation [Citation19]. With pneumodilation in achalasia, such tactile feedback is not available and thus incremental balloon sizes are used on different days to safely dilate the EGJ. Knowledge of the EGJ distensibility before dilation might be useful to decide upon the appropriate balloon size; if the EGJ is tight (i.e. low distensibility), a smaller balloon should be chosen, and if the EGJ is relatively compliant (i.e. ‘high’ distensibility), it may well be safe to use a larger balloon. This could be taken into consideration during dilation procedures, further personalizing dilation regimes based on EGJ distensibility to optimize effectiveness. However, clinical trials are necessary to evaluate such a regime.
Fourth, the EsoFLIP balloon may be a cost-effective choice. The disposable 30 mm EsoFLIP is offered at a price of approximately €295, which is comparable to the commonly used Rigiflex balloon (Boston Scientific, Watertown, MA). The EsoFLIP balloon requires an EndoFLIP system for inflation. However, if future trials show that the decision for additional dilation therapy can indeed be individualized, unnecessary dilations with considerable procedural and equipment costs may be avoided; consequently, EsoFLIP dilations may prove to be a cost-effective option. Furthermore, as fluoroscopy can be avoided procedural costs will be further reduced.
Initial experience
Our group conducted the first preclinical study with the EsoFLIP in achalasia in 2013 [Citation4]. We recently also reported the results of the first human study in newly diagnosed idiopathic achalasia patients [Citation16]. In this pilot study, 10 patients were dilated twice with a 30 mm balloon, with a follow-up of 3 months. Technical success was achieved in all patients, and no severe adverse events were reported. EGJ distensibility before the first and directly after the second dilation was significantly different; 1.1 (IQR 0.6–1.3) versus 7.0 (IQR 5.5–17.8), respectively (p = 0.005). A low EGJ distensibility and small CSA at the day of the second dilation, directly before the procedure, were associated with recurrent dysphagia with a p = 0.04 and 0.002, respectively. Although these findings will need to be confirmed, our findings suggest that EGJ distensibility measurements during dilation may predict clinical success. Dilation with the 30 mm balloon achieved clinical success in 9 of the 10 patients that were treated. During follow-up, two patients reported recurrent dysphagia after 3 months, suggesting that dilation with just a 30 mm balloon is not sufficient in a subgroup of patients, which has been reported before [Citation20].
Compared with standard pneumodilation, certain aspects of the dilation therapy were different. Positioning of the EsoFLIP dilator was done over a guidewire instead of placement through the working channel of the endoscope. After positioning of the EsoFLIP, the endoscope was placed just above the balloon. Furthermore, the EsoFLIP balloon was inflated with a conductive saline solution instead of air. Using a controlled 1 mL/s inflation rate, this means that the dilation is always performed with gradual rather than rapid inflation. To date, we have not seen any complications of fluid dilation. Median pressure to achieve balloon effacement was remarkably high; 565 mmHg (IQR 478–768) during the first dilation and 608 mmHg (IQR 466–669) during the second dilation. This high pressure should not be compared to the pressure used during pneumodilation; while air is compressible, a fluid is practically incompressible. Ex vivo testing has shown that the diameter of the EsoFLIP does not exceed 31 mm if the pressure is kept under 1500 mmHg [Citation21]. Consequently, forceful injection of saline after full inflation of the balloon causes a considerable pressure spike but does not substantially affect balloon diameter. It is, therefore, not expected that using a higher pressure increases the risk of complications. Only if a typical pneumodilation (with approximately 300 mmHg) would not be sufficient to dilate a very noncompliant EGJ to 30 mm but a typical hydraulic dilation (with approximately 600 mmHg) would the risk of perforation might increase.
Balloon inflation was clearly visible on the EndoFLIP system during each dilation, and gradual inflation of the balloon allowed for correction of migration of the balloon proximally or distally during inflation. Moreover, the shape of the balloon on the EndoFLIP system was identical to the fluoroscopical image that was shown in parallel. Therefore, fluoroscopy seems unnecessary when the EsoFLIP balloon is used. More recently, Lirio et al. [Citation17] and Lenglinger et al. [Citation18] also reported that fluoroscopy is not indicated when using the EsoFLIP balloon.
A shortcoming of the EsoFLIP dilator is that distensibility needs to be calculated after measuring CSA and pressure since the pressure meter is not directly connected to the central unit. Although this is not a major issue, it is inconvenient. Furthermore, as of now, only a 30 mm balloon is available. In the pilot study, three patients required repeat dilations during follow-up, which suggests that a larger version of the dilation balloon is required to achieve optimal benefit of dilation therapy. A 40 mm dilator is currently being developed and is expected to become available soon. It may be possible to dilate the EGJ only to 35 mm with the 40 mm balloon since further fluid infusion can be stopped when the waist of the balloon reaches 35 mm. However, the infusion to 35 mm might weaken the EGJ too severe to stop further dilation, thus resulting in a 40 mm dilation, which would probably increase the risk of perforation. Until the 40 mm balloon (and ideally the 35 mm balloon) is available and the central unit is able to perform distensibility measurements calculations, widespread adoption of this device in clinical practice is unlikely.
Five-year view
EsoFLIP is a new dilation technology. In terms of how the actual dilation is performed, it is not dissimilar to existing technologies. Sensitivity to reducing radiation exposure to patients and staff may drive adoption, particularly in pediatric populations. However, the most important goals of intraprocedural distensibility measurements are to improve effectiveness of dilation therapy, predict clinical success before or directly after dilation, and ideally, to reduce costs of dilation therapy. Over the coming years, different sizes of EsoFLIP catheters will become available for performing dilations in various parts of the alimentary tract. The key research agenda in the coming years will focus on how these intraprocedural measurements may be integrated into clinical predictor rules for success or failure of dilation therapy. Unfortunately, since achalasia is rare, performing trials in achalasia patients with sufficient power might prove challenging.
Conclusion
EGJ distensibility measurements have showed to be predictive of success in achalasia treatment and offer an alternative to the barium height test [Citation3]. Distensibility measurements during dilation therapy may provide a useful real-time prediction of success or failure after dilation and this subject remains the base of ongoing research. However, it is probable that any clinical rule developed for prediction of success will need to include factors beyond diameter and distensibility alone and should take notice of other known predictors of failure. Clearly, as compared to fluoroscopy, a more detailed image with quantitative diameter measurements is provided, and this can assist in documenting the therapeutic intervention. The greatest benefit will accrue if intraprocedural CSA, pressure and distensibility measurements can be integrated into a clinical rule to reliably predict whether a further dilation or surgical intervention might be required. Should this be possible, then a patient-specific dilation regimen using this balloon may prove to be the safest and most (cost-) effective dilation technique in achalasia patients.
Key issues
The EsoFLIP hydraulic dilation technology offers the ability to eliminate fluoroscopy during dilation procedures.
All dilations using the EsoFLIP require saline injection into the balloon. Initial experience suggests that hydraulic (in contrast to air) dilations appear safe for achalasia dilation.
Future studies should focus on assessing whether intraprocedural pre- and postdilation distensibility measurements can be used to make a clinical rule to predict therapeutic success after dilation.
Financial & competing interests disclosure
P.D. Siersema has received an unrestricted grant of Crospon Ltd. to conduct studies with the EsoFLIP. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Boeckxstaens GE. Achalasia. Lancet (London, England). 2014;383(9911):83–93.
- Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology. 2013;145(5):954–965.
- Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143(2):328–335.
- O’Dea J, Siersema PD. Esophageal dilation with integrated balloon imaging: initial evaluation in a porcine model. Therap Adv Gastroenterol. 2013;6(2):109–114.
- Lenglinger J. Impedance Planimetry. In: O. Ekberg, editor. Dysphagia: Diagnosis and treatment. New York (NY): Springer; 2012, p. 329–337.
- Kwiatek MA. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP). Gastrointest Endosc. 2010;72(2):272–278.
- Pandolfino JE. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP™) in achalasia patients. Neurogastroenterol Motil. 2013;25(6):496–501.
- Eckardt VF. Clinical presentations and complications of achalasia. Gastrointest Endosc Clin N Am. 2001;11(2):281–292.
- Ponds FA, Bredenoord AJ, Kessing BF, et al. Tu1197 A Subgroup of Achalasia Patients With Manometrically Normal LES Relaxation Can Be Identified by Measurements of Esophagogastric Junction Distensibility. Gastroenterology. 2013;5(144):S–788.
- Verlaan T. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc. 2013;78(1):39–44.
- Rieder E. Intraoperative assessment of esophagogastric junction distensibility during per oral endoscopic myotomy (POEM) for esophageal motility disorders. Surg Endosc. 2013;27(2):400–405.
- Teitelbaum EN. An extended proximal esophageal myotomy is necessary to normalize EGJ distensibility during Heller myotomy for achalasia, but not POEM. Surg Endosc. 2014;28(10):2840–2847.
- Ilczyszyn A, Botha AJ. Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Dis Esophagus. 2014;27(7):637–644.
- Perretta S, Dallemagne B, McMahon B, et al. Video. Improving functional esophageal surgery with a “smart” bougie: Endoflip. Surg Endosc. 2011;25(9):3109.
- Snow RG, O’Dea J. Does Intraoperative Gastric Band Adjustment to a Targeted Stoma Size Improve Weight Loss? One-year results of a feasibility trial. Bariatric Times. 2012;9:10–12.
- Kappelle WFW, Bogte A, Siersema PD. Hydraulic dilation with a shape-measuring balloon in idiopathic achalasia: a feasibility study. Endoscopy. 2015;47(11):1028–1034.
- Lirio RA, Nazarey P, O’Dea J, et al. Sa1664 The First Case Report of Esoflip for Dilation of a Pediatric Esophageal Stricture. Gastrointest Endosc. 2015;81(5):AB299–AB300.
- Lenglinger J, Kristo I, Scharitzer M, et al. Tu1154 Lower Esophageal Sphincter Dilation Using a Balloon Incorporating Impedance Planimetry Imaging (Esoflip®): Feasibility and Initial Experience in the Treatment of Patients With Achalasia. Gastroenterology. 2015;148(4):S–805.
- Siersema PD. Treatment options for esophageal strictures. Nat Clin Pract Gastroenterol Hepatol. 2008;5(3):142–152.
- Farhoomand K, Connor JT, Richter JE, et al. Predictors of outcome of pneumatic dilation in achalasia. Clin Gastroenterol Hepatol. 2004;2(5):389–394.
- EsoFLIP user’s manual [Internet]. [ cited 2015 Sep 1]. Available from: http://crospon.com/ES_330.pdf.