Obstructive sleep apnea (OSA) is characterized by repetitive obstruction of the upper airway that leads to reduced or absent airflow, known as hypopnea or apnea. These events result in oxygen desaturation and arousal from sleep. The patients show episodes of loud snoring during the night, and daytime manifestations include excessive sleepiness, poor concentration and fatigue. This predisposes affected individuals to work-related and car accidents, for which the risk is sevenfold higher in OSA patients than normal subjects. In an Australian report, overall costs of sleep disorders were estimated at Aus$7494 million/year Citation[1]. The prevalence of OSA is 2–4% of the general population and highest in men in their fifth decade (4–8%). Factors that increase the risk for sleep apnea include male sex, obesity, age and race, whereby obesity, particular visceral obesity, is the strongest risk factor Citation[2]. Furthermore, patients with OSA have a higher incidence of pulmonary and systemic hypertension, cardiac arrhythmia, and higher morbidity and mortality due to cardiovascular and cerebrovascular disease.
There is a growing amount of data to suggest that sleep apnea is also associated with disturbances in glucose metabolism, such as fasting hyperglycemia, insulin resistance and diabetes mellitus. Large epidemiological studies from Sweden, Denmark and the USA have shown that snoring as surrogate parameter for OSA was independently associated with abnormalities in glucose metabolism Citation[3–5]. Furthermore, recent evaluations showed a higher incidence of snoring in patients with diabetes mellitus and in patients with the metabolic syndrome Citation[6]. The limitation of these studies was the fact that snoring is a self-reported symptom and that correlations do not allow differentiation between cause and effect.
Two studies using polysomnography showed that the apnea–hypoxia index (number of apneas and hypopneas per hour of sleep) was associated with glucose intolerance and insulin resistance Citation[7,8]. In the large multicenter Sleep Heart Study Citation[9], the apnea hypoxia index was associated with the Homeostasis Model Assessment (HOMA) index for insulin resistance and 2 h glucose concentrations after an oral glucose load. This association was independent of body mass index (BMI) and waist circumference as a surrogate measure of visceral adiposity.
Interestingly, leptin-deficient, obese mice periodically kept under hypoxic conditions (reduction of air oxygen from 21 to 5% for 30 sec every 30 sec during a several-day protocol) became more hyperinsulinemic than lean control animals kept under the same hypoxia regimen conditions Citation[10]. The authors concluded that in the absence of leptin, hypoxia can accelerate the progression of insulin resistance and glucose intolerance associated with obesity. Taken together, the majority of evidence suggests that sleep apnea is associated with insulin resistance independently of obesity, but it appears that obesity plays a permissive role. Since visceral fat, but not subcutaneous fat, was correlated with sleep apnea in another study, it appears that this particular depot is of greatest importance Citation[11,12].
The central question now is whether both insulin resistance and OSA are simply consequences of obesity (i.e., guilty by correlation), or whether OSA can directly induce insulin resistance. The problem of correlation versus causation can be somewhat circumvented by examining studies in which an experimental intervention of one parameter was introduced. Continuous positive airways pressure (CPAP) is used to alleviate OSA. Consequently, a number of studies have addressed the question of whether CPAP can also improve insulin sensitivity in patients with OSA. Recently, 12 studies examining glucose metabolism and insulin sensitivity before and after CPAP treatment have been systematically reviewed and appeared to provide an inconsistent picture at first glance, since some studies did find an effect while some did not Citation[2]. However, only five out of the 12 used the euglycemic hyperinsulinemic clamp technique, the ‘gold standard’ for measuring insulin sensitivity in vivo. Moreover, in two studies the subject number was very small and one study was an extension of previous work of the same laboratory. This leaves two interpretable studies, which both found significant improvements in insulin sensitivity after 3 and 4 months, respectively Citation[13,14]. With all caution, this suggests that OSA is causally related with insulin resistance.
Furthermore, in these studies obesity appeared to be an important modifier of the effect of CPAP since lean patients had a more rapid improvement of insulin sensitivity than obese patients. This is in accordance with a paper by Ip and colleagues, reporting that obesity is the main predictor for changes in insulin sensitivity Citation[7]. It is likely that extreme obesity will overrun any effect that CPAP might possibly have on insulin resistance.
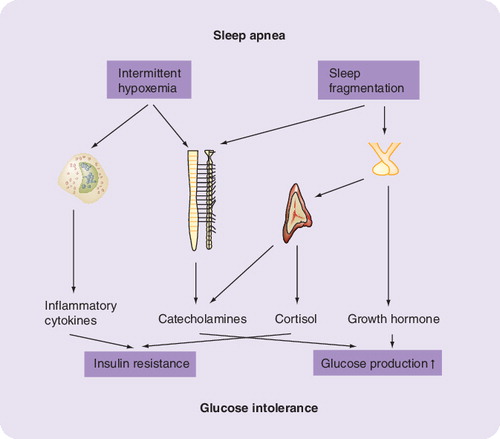
What is the current understanding of the relationship between OSA and insulin resistance? A number of not necessarily diverging theories have been put forward. Mechanisms including activation of the hypothalamus–pituitary–adrenal (HPA) axis, increased sympathetic nerve activity and chronic subclinical inflammation interacting in a complex manner are currently under discussion.
An episode of OSA is characterized by transient periods of activation of the sympathetic nervous system. The proposed mechanism is that a stimulus such as hypoxemia and hypercapnia triggers a stress response by activating chemoreceptors in the carotid bodies, aorta and medulla of the brain stem, resulting in the release of hormones from the HPA axis, such as epinephrine, norepinephrine and cortisol. This is supported by increased cortisol levels in patients with sleep apnea Citation[2] and the observation that sleep deprivation increases cortisol levels by up to 45% Citation[15]. Cortisol has been shown to interfere with the insulin signaling cascade at various steps but also stimulates lipolysis and free fatty acid release, which in turn induces insulin resistance. In addition, glucocorticoids directly stimulate endogenous glucose production, which contributes to the development of (especially fasting) hyperglycemia Citation[16,17].
Furthermore, Fletcher and colleagues reported increased urinary catecholamine concentrations in patients with OSA, which decreased significantly after removal of the airway obstruction by tracheostomy Citation[18]. The systemic effect of increased catecholamines is hyperglycemia and hyperinsulinemia, which leads to insulin resistance and, ultimately, to Type 2 diabetes. Metabolic consequences of elevated catecholamines include increased glycogenolysis in liver and increased gluconeogenesis in liver and kidney Citation[19]. Moreover, surgical removal of pheochromocytoma, resulting in normalization of increased plasma catecholamine concentrations, led to an improvement in insulin-stimulated glucose disposal during a euglycemic hyperinsulinemic clamp Citation[20]. This can be interpreted as the sum effect on stimulating skeletal muscle glucose uptake and decreasing endogenous glucose production. Interestingly, in this study effects on patients both with and without Type 2 diabetes were observed.
CPAP treatment of patients with OSA may decrease sympathetic nerve activity and urinary catecholamine excretion, but this issue is still under investigation Citation[21]. Studies of CPAP effects on cortisol levels have also produced conflicting results. Therefore, controlled clinical studies are needed to extricate the exact relationships between HPA abnormalities in sleep apnea, the effect of treatment and the predominant impact on insulin sensitivity.
It is still unclear in which way the somatotropic axis may be involved. Insulin-like growth factor (IGF)-1 levels have been found to be decreased in patients with OSA, indicating a decrease in pituitary growth hormone release. Since growth hormone infusion has been shown to stimulate endogenous glucose production, the potential role of this hormone as a mediator between OSA and glucose intolerance remains entirely unclear Citation[22].
Another interesting hypothesis linking insulin resistance to OSA is the wide field of inflammation. Subclinical inflammation is more and more regarded as a marker for elevated cardiovascular risk. Some studies have shown that C-reactive protein (CRP) as a surrogate parameter of subclinical inflammation is a strong predictor of cardiovascular disease. Patients with OSA have elevated CRP, tumor necrosis factor (TNF)-α Citation[11,23] and interleukin (IL)-6 levels Citation[2]. High IL-6 levels are found in patients with Type 2 diabetes and levels correlate with insulin resistance. Although TNF-α has been shown to link inflammation with insulin resistance in animals and cell models, this is less clear for humans Citation[24]. Since CPAP treatment decreases circulating levels of CRP and IL-6 Citation[25], it is possible that subclinical inflammation is an additional mechanistic link between OSA and IR. This is supported by the observation that hypoxia induces inflammation-associated transcription factors such as nuclear factor (NF)κB and HIF1a Citation[26].
In conclusion, the correlation between OSA and insulin resistance is probably more than just a colinearity phenomenon with obesity. In particular, the improvement in insulin resistance after CPAP treatment strongly suggests a causal role. Indirect evidence supports a scenario in which sleep fragmentation and hypoxemia, as key mechanisms of OSA, trigger activation of the HPA axis and the sympathetic nervous system, which both result in increased endogenous glucose production and insulin resistance. In addition, there is some evidence that hypoxemia induces activation of inflammatory pathways and cytokines in adipose tissue, most probably at the visceral depot, which in turn induces insulin resistance.
References
- Hillmann D, Murphy A, Pezullo L. The economic cost of sleep disorders. Sleep29, 282–283 (2006).
- Punjabi N, Polotsky V. Disorders of glucose metabolism in sleep apnea. J. Appl. Physiol.99, 1998–2007 (2005).
- Grunstein R, Stenlof K, Hedner J, Sjostrom L. Impact of obstructive sleep apnea and sleepiness on metablic and cardiovascular risk factors in the swedish obese subjects (SOS) study. Int. J. Obes. Relat. Metab. Disord.19, 410–419 (1995).
- Jennum P, Schultz-Larsen K, Christensen N. Snoring, sympathetic activity and cardiovascular risk factors in a 70 year old population. Eur. J. Epidemiol.9, 477–482 (1993).
- Enright P, Newmann A, Wahl P, Maniolo T, Haponik E, Boyle P. Prevalence and correlates of snoring and observed apneas in 5201 older adults. Sleep19(531), 539 (1996).
- Leineweber C, Kecklund G, Akerstedt T, Janszky I, Orth-Gomer K. Snoring and the metabolic syndrome in women. Sleep Med.4, 531–536 (2003).
- Ip M, Lam B, Ng M, Lam W, Tsang K, Lam K. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med.165, 670–676 (2002).
- Punjabi N, Sorkin J, Katzel L, Goldlieb A, Schwartz A, Smith P. Sleep disordered breathing and insulin resistance in middleaged and overweight men. Am. J. Respir. Crit. Care Med.(165), 677–682 (2002).
- Punjabi N, Shahar E, Redline S, Gottlieb D, Givelber R, Resnick HE. Sleep disorderd breathing, glucose intolerance, and insulin reisitance: the sleep heart health study. Am. J. Epidemiol.160, 521–530 (2004).
- Polotsky V, Li J, Punjabi N et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J. Physiol.552(1), 253–264 (2003).
- Vgontzas A, Bixler E, Chrousos G. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med. Rev.9, 211–224 (2005).
- Shinohara E, Kihara S, Yamashita S et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnea syndrome in obese subjects. J. Int. Med.241, 11–18 (1997).
- Harsch I, Schahin S, Radespiel-Troger M et al. Continious positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med.169, 156–162 (2004).
- Brooks B, Cistulli P, Borkman M et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J. Clin. Endocrinol. Metab.79, 1681–1685 (1994).
- Leproult R, Copinschi G, Buxton O, vanCauter E. Sleep loss results in an elevation of cortisol levels in the next evening. Sleep20, 865–870 (1997).
- Andrew R, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin. Sci.96, 513–523 (1999).
- Reynolds R, Walker BR. Human insulin resistance: the role of glucocorticoids. Diab. Obes.Metab.5, 5–12 (2003).
- Fletcher E, Miller J, Schaaf J, Fletcher J. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep10, 35–44 (1987).
- Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J. Clin. Invest.96, 2528–2533 (1995).
- Wiesner TD, Bluher M, Windgassen M, Paschke R. Improvement of insulin sensitivity after adrenalectomy in patients with pheochromocytoma. J. Clin. Endocrinol. Metab.88(8), 3632–3636 (2003).
- Esler M, Eikelis N. Is obstructive sleep apnea the cause of sympathetic nervous activation in human obesity? J. Appl. Physiol.100, 11–12 (2006).
- Karlander S, Vranic M, Efendic S. Increased glucose turnover and glucose cycling in acromegalic patients with normal glucose tolerance. Diabetologia29(11), 778–783 (1986).
- McNicholas W, Ryan S. Obstructive sleep apnoea syndrome: translating science to clinical practice. Respirology11, 136–144 (2006).
- Stumvoll M, Goldstein B, van Haeften T. Type 2 diabetes: principles of pathogenesis and therapy. Lancet365, 1333–1346 (2005).
- Yokoe T, Minoguchi K, Matsuo H et al. Elevated levels of C reactive protein and IL-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continiuous positive airway pressure. Circulation107, 1129–1134 (2003).
- Ryan S, Taylor C, McNicholas W. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation112, 2660–2667 (2005).