SUMMARY
The co-formulation insulin degludec/insulin aspart (IDegAsp) contains insulin degludec (IDeg), a basal insulin, and the rapid-acting insulin aspart (IAsp). Its unique pharmacodynamic profile provides a stable basal insulin action over a 24-h period due to the flat, ultra-long effect of IDeg, combined with prandial control from IAsp, which is unaffected by the basal component. IDegAsp provides a distinct mealtime insulin peak effect and reduces the likelihood of postprandial glucose excursions. The phase 2 and 3 clinical trial program demonstrates that IDegAsp provides effective glycemic control with lower rates of hypoglycemia compared with the current standard of care for insulins. Compared with premixed insulin formulations, IDegAsp allows mealtime flexibility, enabling the time of injection to be adjusted to a different meal(s) on a daily basis to suit changing needs, and has the potential to improve adherence rates. IDegAsp offers a promising new insulin strategy for the treatment of type 2 diabetes.
Introduction
Although the underlying cause of type 2 diabetes mellitus (T2DM) remains controversial, effective early glycemic control, continued long-term, is a central element of the multiple-intervention approach to the management of T2DM [Citation1]. Good glucose control can improve islet β-cell function and improve insulin sensitivity through reduction of glucose toxicity and, perhaps, lipotoxicity[Citation2]. Microvascular complications, including nephropathy [Citation3], retinopathy [Citation4], neuropathy [Citation5], and arterial damage [Citation6], have been shown to be related to glycosylated hemoglobin (HbA1c) [Citation4,Citation7]. Better long-term glycemic control from diagnosis has been shown to decrease the occurrence of microvascular complications in patients with T2DM [Citation8,Citation9]. By contrast, intensive glycemic control in advanced T2DM does not appear to be associated with improved cardiovascular outcomes, at least within 5 years [Citation10]. Currently, at the time of diagnosis, it is not possible to identify with any certainty the people with T2DM who are at risk of future development of micro- or macrovascular complications. Accordingly, position statements recommend lowering HbA1c to <7.0%, except where life expectancy is short, complications of therapies ensue, or if the informed choice indicates otherwise [Citation11].
Use of prandial/basal insulin combination in T2DM
The use of basal insulin in the management of T2DM as the initial insulin treatment is currently well accepted, although this partly relates to insulin therapy generally being started once the insulin secretory defect is well advanced [Citation12]. The use of combination basal and mealtime regimens in T2DM depends on the observation that mealtime glucose excursions can be a problem with basal insulin alone, even at the time when it is started and certainly after some years [Citation13], that continuing islet β-cell decline occurs with time [Citation14], and that some groups of people, notably of Asian origin, tend to have greater mealtime glucose excursions [Citation15]. These observations are complemented by data reviews of the importance of postprandial blood glucose control [Citation16]. However, in the absence of a near-impossible randomized control trial, the evidence supports the importance of mealtime excursions, specifically in the development of arterial complications of diabetes. This has been demonstrated in preclinical studies that report an association between glycemic variability and increased vascular damage as a result of greater production of reactive oxygen species, suggesting that the management of glycemic variability may reduce cardiovascular disease in patients with diabetes [Citation17].
An additional and unresolved issue here is hypoglycemia risk—not least because, when more complete insulin replacement therapy is indicated (greater islet β-cell deficiency), the loss of feedback control of insulin delivery results in more hypoglycemia [Citation18]. Although an in-depth analysis, notably the ACCORD study, has failed to substantiate a role for hypoglycemia in the causation of macrovascular events (with which it is epidemiologically associated) [Citation19], mechanistic evidence continues to suggest the possibility of such a role [Citation20]. However, even without these pathophysiological considerations, hypoglycemia is still a significant problem for patients with T2DM, as fear of it reduces quality of life as well as imposing a substantial cost burden on health-care systems [Citation21,Citation22].
The alternative approaches to mealtime insulin therapy while continuing basal insulin delivery include: moving to a full multiple injection replacement regimen, stepwise addition of mealtime insulin according to the meal(s) with the greatest need, addition of a glucagon-like peptide 1 (GLP-1) receptor agonist (if not already used), or moving to a biphasic (premix) insulin preparation [Citation11]. In general, the addition of separate rapid-acting mealtime insulins (insulin aspart [IAsp], glulisine, lispro) is regarded as the optimal choice for minimizing postprandial glucose excursions and the risk of late postprandial hypoglycemia [Citation11,Citation16].
Premixed formulations provide some basal and mealtime insulin coverage from all injections and contain a fixed proportion of soluble, rapid-acting insulin analog with a protaminated analog comprising the remainder, which has an intermediate action profile [Citation23,Citation24]. They remain the standard of care in many global regions [Citation25]. Reasons for this include a perceived decrease in injection burden and a reduced need for glucose self-measurement, which may improve adherence to therapy compared with full multiple injection regimens [Citation26]. The pharmacokinetic profile of the protamine-based basal component of existing biphasic insulins does not give a physiologically flat profile. In addition, existing biphasic insulins may not provide the duration of action for a physiological basal insulin replacement [Citation27,Citation28] and are more complex to titrate than basal insulin analogs used alone. Additional disadvantages include the fixed ratio of meal to basal insulin dose, and the inability of some basal insulin analogs (insulin detemir [IDet] and insulin glargine [IGlar]) to be co-formulated with mealtime insulin due to alterations of their pharmacokinetic/pharmacodynamic profiles, making them unsuitable for optimum glycemic control [Citation29–Citation31].
The hypoglycemia risk of protamine-based premixes derives partly from pharmacological variability, resulting in hyper- and hypoinsulinemia [Citation28]. There is, therefore, a strong clinical need for the development of a combination insulin that can provide sustained and stable basal insulin coverage together with optimal prandial insulin kinetics in a single injection, without either compromising the other. The aim is to enable a simplified use of the basal and bolus insulin in a combined regimen, providing flexible mealtime administration and reduced rates of hypoglycemia in comparison with existing premix insulins.
Characteristics of insulin degludec/insulin aspart
Chemistry and the mode of protraction
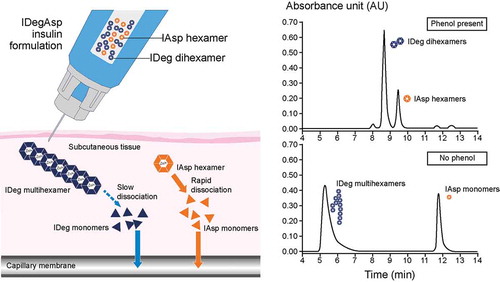
Insulin degludec/insulin aspart (IDegAsp) is a soluble co-formulation of the novel basal analog IDeg (70%), which has an ultra-long duration of action, and IAsp (30%), a rapid-acting prandial insulin () [Citation32]. IDegAsp aims to provide stable physiological basal and prandial insulin coverage (). IDeg has a unique mechanism of protraction: in the presence of phenol and zinc, it forms a soluble and stable dihexamer but, after injection, as phenol diffuses away, it reorganizes to form multihexamer chains that will have a long residence time at the injection depot [Citation33]. With gradual diffusion of zinc, these chains gradually disassemble to release monomers from the terminal ends of multihexamers [Citation33]. For the IAsp component of IDegAsp, IAsp hexamers dissociate into monomers, which are rapidly absorbed into the circulation [Citation33]. Size-exclusion chromatography studies indicate that IDeg and IAsp remain separate entities when in solution together, without the alteration of their individual absorption profiles after injection or any evidence of physical or chemical interaction between the two insulins [Citation34]. The ultra-long duration of IDeg is attributed to the formation of insulin multihexamer chains at the subcutaneous injection site, from which there is a continuous and sustained release of IDeg monomers, providing stable coverage of basal insulin needs [Citation35,Citation36]. Prandial insulin requirements are met by the IAsp component, which quickly dissociates subcutaneously to monomers, which can then be rapidly absorbed into the circulation [Citation34] ().
Table 1. Key pharmacological and clinical characteristics of IDegAsp.
Figure 1. The mechanism of action of the insulin degludec/insulin aspart co-formulation (IDegAsp). In solution, the insulin degludec (IDeg) component forms soluble dihexamers at neutral pH, whereas insulin aspart (IAsp) remains as distinct hexamers. On subcutaneous injection, IDeg dihexamers immediately self-associate into stable multihexamers, from which IDeg monomers dissociate slowly and continuously. The IAsp hexamers promptly dissociate to monomers that are rapidly absorbed into the circulation. Adapted with permission from: Havelund S, Ribel U, Hubálek F, et al. Investigation of the physico-chemical properties that enable co-formulation of basal insulin degludec with fast-acting insulin aspart. Pharm Res 2015;32(7):2250–2258. © The Authors 2015.
Pharmacokinetics/pharmacodynamics of IDegAsp
IDegAsp shows distinct prandial and sustained basal glucose-lowering effects at steady state in clamp studies after 6 days of administration (estimated by the area under the glucose infusion rate curve during a dosing interval at steady state) [Citation35]. The basal IDeg component of IDegAsp has a subcutaneous absorption half-life of >25 h [Citation37], and thus a duration of action (assuming adequate dosage) that exceeds 42 h [Citation33,Citation36] and is reported to have four times lower day-to-day variability than IGlar [Citation38]. The pharmacokinetic profile for IAsp appears 14 min after injection, with a peak concentration after 72 min [Citation32], followed by a decline in action over 4 h. After this, the stable basal glucose-lowering effect due to the basal IDeg component takes over [Citation37].
The duration of action of IDeg in IDegAsp (>42 h) [Citation36] permits people with T2DM to change the time of administration when taken once daily, as long as it is dosed with the largest meal [Citation32]. Once steady state is achieved, the glycemic effects of IDegAsp are dependent on the total daily dose administered and not the frequency of injection [Citation35]. In a model that simulated the pharmacodynamic response to twice-daily IDegAsp, the glucose-lowering effect of this regimen was equivalent to that seen with once-daily dosing, provided that the total daily insulin dose was the same [Citation35]. However, with twice-daily IDegAsp administration, two distinct mealtime glucose-lowering effects were seen, these being proportionally lower than the peak observed following once-daily IDegAsp administration of the same total dose [Citation35]. With IDegAsp, steady-state glucose-lowering of the basal component is reached in 2–3 days [Citation35]. Phase 1 studies using a 30-h euglycemic glucose clamp in people with type 1 diabetes mellitus (T1DM) demonstrated that IDegAsp has a dose-proportional glucose-lowering effect at steady state, and that the distinct basal and prandial effects of the IDeg and IAsp components are preserved [Citation35]. It has been reported that IDegAsp has dose-proportional glucose-lowering effects for both the basal and mealtime components [Citation39]. Accordingly, dose titration will affect both components equally in clinical practice. Furthermore, unlike current premixes, the basal component is unchanging during the period of action of the mealtime component, removing uncertainty over contributions to insulin action in the period of 2–5 h after injection.
There were no clinically relevant differences in the pharmacodynamics of IDegAsp in older people or younger adults with T1DM [Citation40]. As insulin is primarily cleared through its receptor, the pharmacokinetic and pharmacodynamic data indicate that IDegAsp can be used in people with renal or hepatic impairment. However, as people with renal and liver disease are subject to changes in insulin sensitivity, careful glucose monitoring is important on an individual basis [Citation32].
Efficacy and safety of IDegAsp in T2DM
In the identified phase 2 and phase 3 studies, the primary end point was the difference in HbA1c change between treatment arms from baseline to end of study, modeled by analysis of variance, and usually presented as mean difference with 95% confidence limits (). Change is given as HbA1c percent-units, not to be confused with percent change. A similar calculation was performed in the reports for fasting plasma glucose (FPG). Hypoglycemia can be reported as percent population affected (one or more events over the study duration), event rate (usually per person-year), and the rate difference reported as statistically modeled rate ratio (RR) (95% confidence interval (CI)) (), where 1.00 implies identical rate.
Table 2. Summary of phase 2 and phase 3 clinical trials of IDegAsp in type 2 diabetes. Review of published data regarding clinical efficacy and safety of IDegAsp. Literature was searched through the electronic medical databases and findings from key studies of the IDegAsp clinical trial program were chosen. Clinical data indicate that IDegAsp was a well-tolerated insulin combination that provides similar overall glycemic control with a reduced risk of hypoglycemia compared with current insulin preparations.
Phase 2 clinical trials
A proof-of-concept, 16-week, open-label, treat-to-target trial compared IDegAsp with IGlar (both once daily predinner, and in combination with metformin), in 178 insulin-naïve people with T2DM () [Citation41]. IDegAsp was well tolerated and provided similar overall glycemic control (as HbA1c) to IGlar (IDegAsp: 7.0% vs. IGlar: 7.1%), but with better dinner-time postprandial glycemic control and lower rates of hypoglycemia [Citation41]. The trend of lower hypoglycemia rates in phase 2 trials was later observed in phase 3 trials (). A supplementary continuous glucose monitoring study, performed in the last 3 days before the 16-week end point, found a 21% reduction in hypoglycemic nocturnal glucose excursions (area under the curve) with IDegAsp than with IGlar in people with T2DM [Citation42].
Subsequently, a phase 2, open-label, parallel-group, randomized, controlled, 16-week, treat-to-target trial was undertaken to compare the safety and efficacy of IDegAsp with biphasic insulin aspart 30 (BIAsp 30), both twice daily and in combination with metformin in 182 insulin-naïve people with T2DM () [Citation43]. In this study, IDegAsp resulted in HbA1c reduction rates that were comparable with BIAsp 30, but with significantly lower FPG levels (difference −0.99 [95% CI −1.68, −0.29] mmol/L) and a significantly lower rate of any-time confirmed hypoglycemia (RR 0.42 [95% CI 0.23, 0.75]). For nocturnal hypoglycemia, the trial was underpowered (RR 0.66 [95% CI 0.22, 1.93]), and the CIs were widely spaced, meaning that it was not possible to draw any firm conclusions [Citation43].
No severe hypoglycemic event was recorded in either phase 2 trial on any insulin.
Phase 3 clinical trials
The efficacy of IDegAsp in insulin-naïve and in insulin-experienced people in the phase 3 program of randomized, open-label, treat-to-target clinical studies is summarized in .
In a 26-week, open-label, multinational, parallel-group, treat-to-target trial, IDegAsp given once daily before one main meal and IGlar given in the morning or at bedtime, were compared in an insulin-naïve Japanese population who were inadequately controlled on oral glucose-lowering drugs. In this trial, despite identical mean insulin doses, IDegAsp was superior to IGlar in lowering HbA1c, having a clinically relevant treatment difference of −0.28 (95% CI −0.46, −0.10), with similar FPG (). Central estimates for any-time and nocturnal hypoglycemia were lower but not statistically significantly different () [Citation44]. Body-weight change and adverse-event profiles were similar. Together, the results suggest that the co-formulation, at the same daily dose, gave better overall outcomes than basal IGlar alone, perhaps largely due to improvement in post-dinner plasma glucose control (−3.2 [95% CI −4.1, −2.3] mmol/L), the time when 81% of participants had their IDegAsp injection.
When insulin-naïve people were randomized to IDegAsp or BIAsp 30 twice daily, HbA1c change was similar (and non-inferior) at 26 weeks [Citation45]. However, IDegAsp showed superior reduction in FPG, and 9-point self-monitored plasma glucose (SMPG) profiles revealed that SMPG values were lower for IDegAsp than BIAsp 30 before breakfast, 90 min after breakfast, and before breakfast the following day. IDegAsp also demonstrated less hypoglycemia nocturnally ().
Use of IDegAsp compared with BIAsp 30 (both twice daily) in people already using insulin was explored in 26-week, randomized, open-label, treat-to-target trials, one in a global and one in an Asian population [Citation46,Citation47]. For glucose-lowering (difference in change from baseline in HbA1c after 26 weeks), the results were similar, with similar and non-inferior HbA1c levels between treatments in both trials and better FPG in both trials (); this was on a lower daily insulin dose with IDegAsp (−11% and −21% compared with BIAsp 30). For the global trial, hypoglycemia rates were clearly better on IDegAsp than BIAsp 30, driven by very much lower nocturnal rates (). The use of premixed insulin is more common in Asia, and trial results in this population may reflect the experience of the patient and physician in administering this regimen in an optimal manner [Citation47]. For the Asian population, treatment with IDegAsp led to numerically fewer episodes of nocturnal hypoglycemia, although there was not enough power to confirm this statistically, whereas any-time hypoglycemia was unchanged. Vaag and colleagues performed a combined analysis of the two trials; the rates of overall confirmed, nocturnal confirmed, and severe hypoglycemic events were lower with IDegAsp, particularly during the maintenance period () [Citation47]. Similar findings were reported when this combined analysis was restricted to participants who achieved HbA1c <7.0% (<53 mmol/mol) by the end of the trial, indicating achievement of optimal glycemic targets as recommended by clinical guidance [Citation11,Citation48].
The use of IDegAsp given twice daily was compared with IDeg once daily plus mealtime IAsp (2–4 injections daily) as alternative approaches to optimization of basal insulin therapy using a 26-week, open-label clinical trial [Citation49]. Although HbA1c was reduced in both trial arms (to 7.0% and 6.8%, respectively) and the proportion of patients achieving the HbA1c target of <7% was similar between both groups (56.5% vs. 59.6%, respectively) [Citation50], the daily total dose at the end of the trial was 1.11 U/kg with IDegAsp versus 1.34 U/kg with IDeg once daily plus mealtime IAsp. IDegAsp was not shown to be non-inferior to IDeg + IAsp (upper CI bound +0.41%-units). Although IDegAsp was associated with numerically lower rates of overall and nocturnal hypoglycemia, this was not statistically significant (). However, people on IDegAsp gained significantly less weight, perhaps associated with the lower mean total daily insulin dose, the treatment difference being large for a 26-week trial (estimated treatment difference [ETD]: −1.0 [95% CI −2.0, −0.1] kg, p < 0.05) [Citation49]. Comparison of overall changes from baseline score for social functioning using a health-related quality of life questionnaire (SF-36 v2) showed that IDegAsp was associated with better social functioning than IDeg once daily plus mealtime IAsp (ETD 2.2 [95% CI 0.3, 4.1]; p < 0.05) [Citation50].
No severe hypoglycemic event was recorded in the IDegAsp versus IGlar trial [Citation44]. The previously insulin-naïve trial against BIAsp 30 recorded low rates of severe hypoglycemia in the IDegAsp and comparator arms [Citation45]. As noted for confirmed hypoglycemia (see above), the global and Asia trials in people previously treated with insulin were heterogeneous for hypoglycemic events, with very small numbers of severe hypoglycemic episodes occurring in the Asia trial (4 people on IDegAsp, 2 people on BIAsp 30) [Citation47]. The global study had four times higher prevalence—7 people affected on IDegAsp versus 16 people on BIAsp 30 [Citation46]. However, overall, the numbers affected are too small to be statistically meaningful.
Adverse-event profile
In the trials mentioned above, there were no signals that excessive incidence of any particular adverse event or serious adverse event occurred for IDegAsp. Post-marketing, IAsp has had massive exposure without concern. In contrast, IDeg has had limited documented exposure and, although its risk–benefit profile was assessed as being satisfactory in the EU and many other markets, the US FDA is understood to have requested more data on cardiovascular safety [Citation51]. Resubmission with new data was announced by the manufacturer in 2015 [Citation51].
Expert commentary
The co-formulation of IDeg and IAsp appears to be a welcome advance in the convenience of administration of basal-plus mealtime insulin in people with diabetes. Although the premix series of insulins was able to adopt, first, human insulin and then rapid-insulin analogs when they became available, the basal component remained technologically based on protamine complexes originating in the 1940s. Undoubtedly, such co-formulations would have been developed with IGlar and IDet had that been possible technologically, but there are fundamental reasons why that was not the case. As it is, the new co-formulation is able to benefit from further advances in basal insulin therapy that comes with IDeg, notably a genuinely flat insulin delivery from once-daily injection after steady state has been established [Citation35], with flexibility around the timing of day-to-day injections [Citation32].
The technological data on the two components remaining functionally separate in the injection and after injection are convincing, as is the demonstration of a typical IAsp mealtime profile of action superimposed on the steady-state action of IDeg [Citation35]. A consequence should be that the timing of injection(s) (and thus the meal(s)) can be varied from day to day, although overlap of IAsp profiles will be anticipated if meals/injections are taken closer than 4 h apart.
With steady state well beyond 24 h, the pharmacodynamic characteristics of IDegAsp should mean that doses can be taken once or twice daily, as tested in clinical trials (). The strategy of using a limited number of mealtime injections with basal insulin in T2DM has been well investigated in recent years [Citation52–Citation54]. In the current study series, once-daily IDegAsp gave, as expected, superior performance to IGlar alone in insulin-naïve people, without detriment in terms of hypoglycemia despite the improved post-main-meal glucose control [Citation44]. Here, the finding by continuous glucose monitoring of lower glycemic excursions during the night appears to be consistent with the clamp findings of low within-day variability compared with IGlar [Citation42].
The finding of improved glycosylated HbA1c (by 0.28%-units in the Onishi and co-authors study) is of some methodological interest, as the treat-to-target glucose levels design usually results in similar overall glucose control while driving changes in hypoglycemia [Citation44,Citation52]. However, as usual, the treatment target was FPG, whereas the IAsp would be reducing postprandial glucose excursions, thus driving a disconnect between differences in FPG and HbA1c [Citation44].
At the time of starting insulin, many people with T2DM still have significant glucose excursions at mealtimes, perhaps one reason why achieving mean HbA1c much less than 7.0% (<53 mmol/mol) has proved to be problematic unless other strategies such as coadministration of a GLP-1 receptor agonist are used [Citation12,Citation55]. In these circumstances, it appears that the use of IDegAsp once daily or twice daily could become the treatment of choice. Once-daily IDegAsp versus twice-daily IDegAsp has not been tested in insulin-naïve individuals yet, but the impressive gains in prebreakfast glucose and hypoglycemia when it is administered twice daily do imply that it is now the optimal strategy where twice-daily injection is desired—in particular, where a twice-daily premix would otherwise be used. When taken twice daily, IDegAsp may represent a simpler alternative to basal plus mealtime insulin therapy in people who require optimization of basal insulin regimens, especially where adherence to more complex regimens is challenging [Citation49]. The choice of once-daily or twice-daily therapy in insulin-naïve individuals could then be a matter of individual choice, but would also be dependent on the degree of islet β-cell failure. In any case, as with premix [Citation23], a stepwise approach from one to two injections according to need seems sensible, to provide prandial cover for two meals. In these circumstances, the total daily dose will need to remain unchanged (assuming that basal blood glucose control is optimal) at first, which will mean that the meal dose is then divided between the two injections. The meal doses can then be adjusted individually and may depend on disease duration and progression, although, as with current premixes the co-formulation means that changing one component has consequences for the other. As noted above, the number and timing of injections are not relevant to the IDeg effect or profile provided that the total daily dose is the same.
The two phase 3 clinical trials of twice-daily IDegAsp versus BIAsp 30 in prior insulin-treated people [Citation45,Citation46] gave dissimilar findings for hypoglycemia despite similar findings for HbA1c, and similar improvements in FPG (>1.0 mmol/L) over BIAsp 30 (), which, as mentioned above, may be a reflection of local practices in diabetes management [Citation47]. The improvement in nocturnal hypoglycemia in the global trial is impressive (73% reduction in rate), with high statistical confidence (RR 0.27 [95% CI 0.18, 0.41], p < 0.0001). Furthermore, the difference in nocturnal hypoglycemia rate (IDegAsp: 0.7 vs. BIAsp 30: 2.5 episodes per patient-year of exposure [PYE]) is smaller than the difference in any-time rate (9.7 vs. 14.0 episodes per PYE) [Citation46]. By contrast, in the Asia trial, a much smaller difference in the central estimate of reduction in hypoglycemia was not statistically significant, largely because the absolute rate was lower than in the global study in the control group for both nocturnal and any-time hypoglycemia [Citation47]. One-to-one unit dose substitution when converting from basal insulin to IDegAsp would generally seem conservative, but could guard against overdosage of the basal insulin component if that has been over-titrated previously when used alone.
Lastly, optimization of insulin therapy with IDegAsp was tested against the addition of basal-plus insulin (multiple mealtime aspart) approach. Caution is needed in the interpretation of this trial because, as a result of an unfamiliar insulin or a smaller number of injections per day in the IDegAsp arm, the daily insulin dose was lower [Citation48,Citation49], which might account for the marginal failure (0.41 vs. a criterion of 0.40%-units) to reach non-inferiority for the primary end point of change in HbA1c after 26 weeks [Citation50]. However, there were fewer confirmed and nocturnal hypoglycemia episodes, suggesting that IDegAsp is a reasonable clinical choice in this circumstance for a reduced number of injections, although a further trial is warranted, particularly a longer trial as islet β-cell function declines.
Five-year view
The lower number of injections required to achieve both basal and prandial coverage with IDegAsp makes this regimen simpler than previously available regimens, as it does not require resuspension and, consequently, may help a greater number of people with T2DM to achieve lower FPG levels through optimal dose titration with a reduced risk of hypoglycemia. This needs testing outside the clinical trial environment. Similarly, its convenience and ease of titration should allow people to feel more confident about managing their insulin requirements in daily life, with fewer restrictions, and this could improve adherence rates, compared with other insulin formulations. Continuing insulin therapy, once begun, and missing injections in multiple injection regimens, have long been of concern, and recent data suggest that this remains so [Citation26,Citation56]. Of course, the fixed ratio does still imply some limitations; notably, the mealtime dose cannot be varied through carbohydrate counting without disturbing the basal dose.
The hypoglycemia data with IDegAsp look particularly good. Seven of the phase 2 and 3 trials () have estimates for a marked rate reduction for nocturnal hypoglycemia against a mix of comparators. As noted above, reductions in hypoglycemia are a feature of successful treat-to-glucose-target trials, but treating to a tolerated rate of hypoglycemia is perhaps more usual in clinical practice. This might then mean that improvements in measured glucose control occur, but this would require a long-term treat-to-hypoglycemia trial. Reduced hypoglycemia might also be expected to improve adherence to the IDegAsp insulin regimen [Citation26], although (as yet) no data have been found that are related to fear of hypoglycemia or health-related quality of life. Reduction in nocturnal hypoglycemia can also be expected to reduce the problem of hypoglycemia awareness, something that becomes more common in middle and later age [Citation57] but, again, no data are yet available on this issue for IDegAsp.
The improvements in hypoglycemia can be attributed to a number of factors: the flat overnight profile of the degludec component; the buffering of variations in absorption by, primarily, the formation of multihexamers and the albumin-bound pool of this insulin; and the ease of learning about dose titration of a co-formulation with easily understood components.
In summary, IDegAsp represents a novel approach to insulin treatment in T2DM, offering effective glycemic control with a low risk of hypoglycemia (particularly nocturnal hypoglycemia), flexible dose timing, and a low daily injection burden. Together, these features should improve the quality of life of people with T2DM who need to start or optimize their insulin therapy, where the current regimen of choice might be a basal insulin, a premix, or a multiple daily injection regimen.
Financial & competing interests disclosure
J.S. Christiansen had served on advisory boards and speaker panels for Novo Nordisk. P.D. Home, or institutions with which he is associated, receives funding for his advisory, research and speaking activities from Eli Lilly, Sanofi and Novo Nordisk and with regard to IDegAsp. A. Kumar has received funds for conducting clinical trials for Novo Nordisk, as well as honoraria for delivering lectures and for being on advisory boards for Novo Nordisk. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Writing assistance was utilized in the production of this manuscript and provided by apothecom scopemedical (London, UK) and was funded by Novo Nordisk.
Key issues
Early effective glycemic control of T2DM is desirable in order to make a meaningful impact on reducing both the microvascular and macrovascular complications associated with diabetes disease progression.
Basal insulin is widely recommended for starting insulin therapy, primarily on the premise of once-daily administration, with a relatively low risk of hypoglycemia.
An alternative insulin regimen utilizes premixed insulins, targeting postprandial as well as basal glucose control; however, these have a compromised pharmacokinetic/pharmacodynamic profile compared with adding mealtime to basal insulin analogs.
IDegAsp is the first co-formulation of the ultra-long-acting basal insulin, IDeg, and the rapid-acting insulin analog, IAsp, in a single injection.
Clinical trial data have demonstrated that IDegAsp provides significantly greater reductions in FPG and post-meal glucose control when compared with some existing insulin treatment options, in addition to a lower risk of overall and nocturnal hypoglycemia.
IDegAsp offers an alternative insulin optimization regimen with fewer injections, a reduced need for self-monitoring, and flexibility in the timing of administration. In combination, these qualities may facilitate better treatment adherence, provide an effective and practical intensification strategy with fewer injections and less requirement for monitoring, reduced risk of hypoglycemia, and better compliance. IDegAsp offers a promising new insulin strategy for the treatment of T2DM for clinicians and patients alike.
Acknowledgments
The authors wish to dedicate this article to Professor Jens Sandahl Christiansen who passed away in December 2015.
References
- Skyler JS. Effects of glycemic control on diabetes complications and on the prevention of diabetes. Clin Diabetes. 2004;22(4):162–166.
- DeFronzo RA. Current issues in the treatment of type 2 diabetes. Overview of newer agents: where treatment is going. Am J Med. 2010;123(3 Suppl):S38–48.
- Gross JL, Azevedo MJD, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176.
- Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163.
- Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962.
- Muntner P, Wildman RP, Reynolds K, et al. Relationship between HbA1c level and peripheral arterial disease. Diabetes Care. 2005;28(8):1981–1987.
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124(1_Part_2):136–145.
- Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet. 2014;383(9933):2008–2017.
- Holman RR, Paul SK, Bethel MA, et al. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359(15):1565–1576.
- Hemmingsen B, Lund SS, Gluud C, et al. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ. 2011;343:d6898.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149.
- Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49(3):442–451.
- Garber AJ. Insulin intensification strategies in type 2 diabetes: when one injection is no longer sufficient. Diabetes Obes Metab. 2009;11(Suppl 5):14–18.
- Kushner JA. The role of aging upon β cell turnover. J Clin Invest. 2013;123(3):990–995.
- Wang J-S, Tu S-T, Lee I-T, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27(1):79–84.
- International Diabetes Federation (IDF). IDF Guideline for the management of postmeal glucose; 2015; [ cited 2015 Jun 23]. Available from: http://www.idf.org/webdata/docs/Guideline_PMG_final.pdf
- Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15(10):18381–18406.
- Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9(1):463–473.
- Riddle MC, Rosenstock J, Vlajnic A, et al. Randomized, 1-year comparison of three ways to initiate and advance insulin for type 2 diabetes: twice-daily premixed insulin versus basal insulin with either basal-plus one prandial insulin or basal-bolus up to three prandial injections. Diabetes Obes Metab. 2014;16(5):396–402.
- Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63(5):1738–1747.
- Prázný M. [Mild hypoglycaemia is common in type 2 diabetic patients treated with insulin analogues in the Czech Republic and the patients are concerned about it: results of a GAPP2TM survey (Global Attitudes of Physicians and Patient)]. Vnitr̆ní Lékar̆ství. 2015;61(3):267–273.
- Parekh WA, Ashley D, Chubb B, et al. The “Local Impact of Hypoglycaemia Tool (LIHT)” for estimating the economic impact of hypoglycaemic episodes in national, local and user-defined populations. Presented at the 50th Annual Meeting of the European Association for the Study of Diabetes (EASD); 2014 September 15–19; Vienna, Austria. [Internet]. [ cited 2015 Jul 23]. Available from: http://www.easdvirtualmeeting.org/resources/17320
- Garber AJ, Ligthelm R, Christiansen JS, et al. Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes Obes Metab. 2007;9(5):630–639.
- Christiansen JS, Liebl A, Davidson JA, et al. Mid- and high-ratio premix insulin analogues: potential treatment options for patients with type 2 diabetes in need of greater postprandial blood glucose control. Diabetes Obes Metab. 2010;12(2):105–114.
- International Diabetes Federation (IDF). IDF Global guideline for type 2 diabetes; 2012; [ cited 2015 Jun 23]. Available from: http://www.idf.org/sites/default/files/IDF-Guideline-for-Type-2-Diabetes.pdf
- Peyrot M, Barnett AH, Meneghini LF, et al. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med J Br Diabet Assoc. 2012;29(5):682–689.
- Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–2148.
- Evans M, Schumm-Draeger PM, Vora J, et al. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13(8):677–684.
- Cengiz E, Swan KL, Tamborlane WV, et al. The alteration of aspart insulin pharmacodynamics when mixed with detemir insulin. Diabetes Care. 2012;35(4):690–692.
- Ma Z, Parkner T, Christiansen JS, et al. IDegAsp: a novel soluble insulin analogs combination. Expert Opin Biol Ther. 2012;12(11):1533–1540.
- Heise T, Heinemann L. Rapid and long-acting analogues as an approach to improve insulin therapy: an evidence-based medicine assessment. Curr Pharm Des. 2001;7(14):1303–1325.
- Novo Nordisk. IDegAsp SmPC; 2014; [ cited 2015 June 23]. Available from: http://ec.europa.eu/health/documents/community-register/2013/20130121124986/anx_124986_en.pdf
- Jonassen I, Havelund S, Hoeg-Jensen T, et al. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104–2114.
- Havelund S, Ribel U, Hubálek F, et al. Investigation of the physico-chemical properties that enable co-formulation of basal insulin degludec with fast-acting insulin aspart. Pharm Res. 2015;32(7):2250–2258.
- Heise T, Nosek L, Roepstorff C, et al. Distinct prandial and basal glucose-lowering effects of insulin degludec/insulin aspart (IDegAsp) at steady state in subjects with type 1 diabetes mellitus. Diabetes Ther. 2014;5(1):255–265.
- Kurtzhals P, Heise T, Strauss HM, et al. Multi-hexamer formation is the underlying basis for the ultra-long glucose-lowering effect of insulin degludec. Diabetologia. 2011;54(Suppl):S426.
- Heise T, Hovelmann U, Nosek L, et al. Insulin degludec has a two-fold longer half-life and a more consistent pharmacokinetic profile compared with insulin glargine. Endocr Abstr. 2012;28:P188.
- Heise T, Hermanski L, Nosek L, et al. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14(9):859–864.
- Nosek L, Heise T, Klein O, et al. IDegAsp produces dose-proportional glucose-lowering effect in subjects with type 1 diabetes. Poster presented at EASD; 2013 September 23–27; Barcelona, Spain. Poster 1041
- Pieber TR, Korsatko S, Deller S, et al. The distinct prandial and basal pharmacodynamics of IDegAsp observed in younger adults are preserved in elderly subjects with type 1 diabetes. Diabetes. 2013;62(Suppl 1):A237–933P.
- Heise T, Tack CJ, Cuddihy R, et al. A new-generation ultra-long-acting basal insulin with a bolus boost compared with insulin glargine in insulin-naive people with type 2 diabetes: a randomized, controlled trial. Diabetes Care. 2011;34(3):669–674.
- Liebl A, Davidson J, Mersebach H, et al. A novel insulin combination of insulin degludec and insulin aspart achieves a more stable overnight glucose profile than insulin glargine: results from continuous glucose monitoring in a proof-of-concept trial. J Diabetes Sci Technol. 2013;7(5):1328–1336.
- Niskanen L, Leiter LA, Franek E, et al. Comparison of a soluble co-formulation of insulin degludec/insulin aspart vs biphasic insulin aspart 30 in type 2 diabetes: a randomised trial. Eur J Endocrinol. 2012;167(2):287–294.
- Onishi Y, Ono Y, Rabøl R, et al. Superior glycaemic control with once-daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: a randomized, controlled phase 3 trial. Diabetes Obes Metab. 2013;15(9):826–832.
- Franek E, Haluzík M, Canecki Varžić S, et al. IDegAsp provides superior FPG control and reduced hypoglycemia vs BIAsp 30 in insulin-naïve adults with type 2 diabetes: A randomized phase 3 trial. Poster presented at EASD; 2014 16–19 September. Poster 931.
- Fulcher GR, Christiansen JS, Bantwal G, et al. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin-treated type 2 diabetes: a phase 3a, randomized, treat-to-target trial. Diabetes Care. 2014;37(8):2084–2090.
- Kaneko S, Chow F, Choi DS, et al. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre-/self-mixed insulin: a 26-week, randomised, treat-to-target trial. Diabetes Res Clin Pract. 2015;107(1):139–147.
- Vaag A, Sandahl-Christiansen J, Niskanen L, et al. Lower rates of overall, nocturnal and severe hypoglycemia during maintenance treatment with IDegAsp vs biphasic insulin aspart 30 in patients with type 2 diabetes mellitus: A meta-analysis. Presented at EASD; 2013 September 23–27; Barcelona, Spain. Oral presentation 187
- Cooper JG, Pieber TR, Cariou B, et al. Treatment intensification with IDegAsp BID vs IDeg OD plus IAsp in insulin-treated patients with type 2 diabetes: A randomized, controlled phase 3 trial. Presented at EASD, 2014 September 16–19; Vienna, Austria. Oral presentation 147
- Rodbard HW, Pieber TR, Cariou B, et al. Treatment intensification with IDegAsp BID vs IDeg OD plus IAsp in insulin-treated patients with type 2 diabetes: a randomised, controlled phase 3 trial. Poster presented at IDF-WPR; 2014 November 21–24. Poster PO343
- Novo Nordisk. Insulin degludec and insulin aspart FDA briefing document. Insulin degludec and insulin degludec/insulin aspart treatment to improve glycemic control in patients with diabetes mellitus; [ cited 2015 June 23]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM327017.pdf
- Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 study investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086.
- Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736–1747.
- Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716–1730.
- Gough SCL, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885–893.
- Davies MJ, Gagliardino JJ, Gray LJ, et al. Real-world factors affecting adherence to insulin therapy in patients with type 1 or type 2 diabetes mellitus: a systematic review. Diabet Med J Br Diabet Assoc. 2013;30(5):512–524.
- Bremer JP, Jauch-Chara K, Hallschmid M, et al. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care. 2009;32(8):1513–1517.
