Abstract
Teriflunomide, a once-daily, oral disease-modifying therapy, is a valuable new treatment option for the management of patients with relapsing–remitting multiple sclerosis. This article reviews key efficacy and safety data arising from pivotal teriflunomide studies that demonstrate the utility in treating both treatment-naïve patients and those previously treated with another disease-modifying therapy who, for a variety of reasons, may require an alternative treatment.
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the CNS Citation[1] and one of the most common neurological diseases in young adults Citation[2]. Although MS is heterogeneous among individuals, it follows a relapsing–remitting course in the majority of patients (∼85%; relapsing–remitting MS [RRMS]), which often transitions over time to a secondary progressive phase, characterized by a reduction in the number of attacks and accumulating disability. In a subset of patients (∼10%), a progressive course occurs from the outset Citation[2].
Clinical trials of disease-modifying therapies (DMTs) in RRMS have generally focused on evaluating treatment benefits on annualized relapse rate (ARR) as a primary end point, largely driven by regulatory requirements for drug approval, despite the fact that relapses can resolve completely without residual neurological deficits. Treatments are often informally compared based on relative reductions in ARR, although the relationship between relapses and long-term disability progression is not fully understood Citation[3,4].
Irreversible disability is the critical long-term consequence of MS, affecting physical and cognitive functioning, with a major adverse impact on health-related quality of life Citation[5]. In addition, disability is associated with substantial personal, health and societal costs Citation[5]. Although reduction in frequency and severity of relapse is important, the primary critical goal of MS therapy should be to slow or reverse disability progression in order to maintain or even improve patients’ neurological functioning. However, the evidence for consistent and positive effects of available DMTs on disability progression remains mixed.
Although DMTs have been available for many years, the majority of agents have, until recently, required parenteral administration. Teriflunomide (Aubagio®, Genzyme, Cambridge, MA, USA) is one of the new oral therapies approved for the treatment of RRMS.
Overview of the market
DMTs for MS have been available for over 20 years. The first products to be licensed, the interferons (IFNs) Citation[6–8] and glatiramer acetate (GA) Citation[9], are injectable preparations that are effective in reducing the frequency of relapses and the development of new MRI lesions in patients with RRMS and early MS, and, at least for the IFNs, the risk of disability progression in patients with RRMS. While IFNs are associated with flu-like symptoms, and both IFNs and GA are associated with injection-site reactions that may impact treatment adherence, long-term follow-up studies suggest that these agents have very favorable safety profiles. However, significant proportions of patients continue to exhibit disease activity while on treatment.
Pegylation of drugs can extend their half-life and potentially reduce dosing frequency. Pegylated IFNβ-1a, recently approved by the USFDA, is dosed every 2–4 weeks and has shown significant reductions versus placebo for ARR and disability progression, with a safety profile consistent with non-pegylated IFNs Citation[10]. A high-dose formulation of GA with reduced injection frequency (3-times weekly), also approved in the USA Citation[11], may prove to be a more convenient treatment than the previous regimen of daily injections and could lead to improved adherence Citation[12].
The introduction of three new oral therapies has provided patients with MS with additional treatment options. These agents may offer enhanced efficacy and appear to have satisfactory safety and tolerability. Many patients prefer the convenience and greater comfort of oral treatment compared with regular injections.
The first oral agent to be approved for the treatment of RRMS was fingolimod, a sphingosine 1-phosphate (S1P) receptor modulator. In two pivotal clinical trials Citation[13–16], fingolimod was associated with relative reductions in ARR of over 50% and with significant reduction in the risk of disability progression in one trial of approximately 30% Citation[13,14]. Significant benefits on MRI outcomes were observed in both studies. The EMA has recommended increased cardiovascular monitoring following administration of the first dose of fingolimod after reports of increased risk of events, including bradycardia and atrioventricular block Citation[17].
Ceralifimod and ponesimod are newer oral, once-daily S1P receptor antagonists, currently being evaluated in patients with RRMS, which have shown encouraging results on ARR and T1 gadolinium (Gd)-enhancing lesions (ceralifimod, Citation[18,19]) or T2 lesions (ponesimod, Citation[20,21]) compared with placebo. A clinical trial is also underway to investigate the efficacy and safety of siponimod, another S1P modulator, in patients with secondary progressive MS Citation[22].
Dimethyl fumarate, a twice-daily oral treatment for relapsing forms of MS, has been shown to have beneficial effects in preclinical models of neuroinflammation, neurodegeneration and toxic oxidative stress Citation[23,24]. Dimethyl fumarate significantly reduced ARR (up to 53%) and MRI activity compared with placebo in two Phase III clinical trials Citation[25–28] and significantly reduced the risk of disability progression by 38% in one but not both studies. Commonly reported adverse events are flushing and gastrointestinal events, usually diminishing after the first month of treatment Citation[29].
Natalizumab, a monoclonal antibody targeting the adhesion molecule VLA4 thereby preventing lymphocyte migration across the blood–brain barrier into the CNS, is associated with reductions in relapse rates of up to 68% Citation[30,31]. However, the incidence of progressive multifocal leukoencephalopathy, a serious and sometimes fatal brain infection caused by JC virus, in patients treated with natalizumab remains a concern. Natalizumab is usually reserved for patients with more aggressive disease, and it is recommended that patients are screened for JC virus antibodies and frequently monitored for progressive multifocal leukoencephalopathy Citation[32].
Alemtuzumab is a monoclonal antibody initially administered by five daily infusions and then repeated for 3 days a year later. It is currently approved in the EU and other countries for the treatment of patients with active RRMS, but is not presently approved in the USA. Alemtuzumab selectively targets CD52 and depletes circulating T and B cells. In clinical trials, alemtuzumab significantly reduced relapse rate (in treatment-naïve patients in CARE-MS I Citation[33,34] and in patients with disease activity despite prior therapy in CARE-MS II Citation[35,36]) and the risk of disability progression (CARE-MS II only) compared with subcutaneous (sc) IFNβ-1a 3-times weekly and improved most measures of disease activity on MRI. In a follow-up of the CARE-MS studies Citation[37], the majority of patients were free of detectable MS disease activity 3 years after initiating treatment with alemtuzumab, although most patients received no treatment in Year 3 Citation[38]. The use of alemtuzumab has been associated with autoimmune disorders, including thyroiditis, immune thrombocytopenic purpura and nephropathies; thus close monitoring for these events is required Citation[39].
Newer monoclonal antibodies with encouraging preliminary data include daclizumab, ocrelizumab and ofatumumab. Daclizumab is administered every 4 weeks and works by blocking the IL-2 receptor on immune cells and expanding CD56 natural killer cells. In the Phase IIb SELECT study Citation[40], daclizumab significantly reduced ARR, the risk of disability progression and the appearance of new Gd-enhancing lesions at both the low and high doses tested Citation[41]. Key safety concerns to date include serious infections and serious cutaneous events. Ocrelizumab and ofatumumab are monoclonal antibodies directed against CD20, albeit with different pharmacologic properties. A Phase II study of ocrelizumab, which is a humanized version of rituximab, has shown significant reductions in ARR and lesions on MRI when compared with placebo and demonstrated a favorable short-term safety profile Citation[42,43]. In a Phase II dose-finding study Citation[43], ofatumumab showed no safety signals in patients with RRMS and a >99% reduction in new lesion activity on MRI after 24 weeks of treatment Citation[44]. Results of the Phase II MIRROR Citation[45] study with ofatumumab demonstrated significant reductions in brain lesions at 24 weeks Citation[46], and this suppression of lesions was maintained in a 24-week extension phase during which patients did not receive treatment Citation[47].
Products in earlier stages of development include anti-LINGO-1, a human monoclonal antibody that targets LINGO-1, a protein expressed selectively in the CNS that is known to negatively regulate axonal myelination and axonal regeneration. Rats with experimental autoimmune encephalomyelitis treated with anti-LINGO-1 showed evidence of remyelination Citation[48], which if shown in clinical trials has the potential to reverse some of the neurological deficit associated with MS. Findings from preliminary studies in healthy volunteers Citation[49] and patients with MS Citation[50] indicate that anti-LINGO-1 is well tolerated Citation[51].
Mitoxantrone is an immunosuppressive agent that reduces exacerbations and the number of Gd-enhancing lesions on MRI, and in some countries is one of the few products approved for the treatment of secondary progressive MS Citation[52]. Although effective, mitoxantrone is associated with the risk of serious adverse events, including acute leukemia and cardiotoxicity Citation[53].
Despite significant advances in the development of DMTs in recent years, unmet needs remain. Therapies to prevent or slow the progressive forms of disease are currently unavailable. Neuroregeneration is another focus of investigation in MS treatment. The identification of biomarkers may eventually lead to personalized treatment for patients with MS.
Teriflunomide
Teriflunomide, a new, once-daily oral DMT, is approved for the treatment of RRMS and has demonstrated significant and consistent benefits on a range of clinical and MRI markers of disease activity in a number of clinical studies and patient populations. Together with a well-characterized and manageable safety and tolerability profile confirmed over a long-term period, teriflunomide is another useful treatment option for the management of patients with RRMS.
Chemistry
Teriflunomide, (Z)-2-cyano-3-hydroxy-but-2-enoic acid-(4-trifluoromethylphenyl)-amide Citation[54], is a white to almost white powder that is formulated as film-coated tablets for oral administration. Tablets are available in 14- and 7-mg doses, although the 7-mg dose is only available in the USA Citation[54,55].
Teriflunomide is the principal active metabolite of leflunomide, licensed in 1998 to treat rheumatoid arthritis Citation[56]. Upon oral ingestion, 70% of leflunomide is rapidly converted into teriflunomide.
Proposed mechanism of action
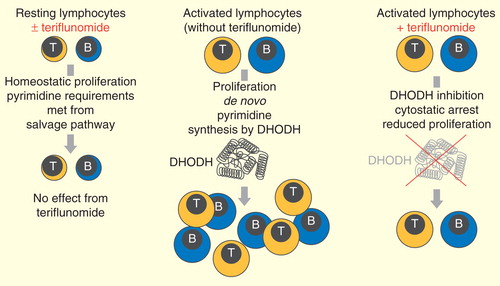
Teriflunomide reversibly inhibits the mitochondrial enzyme, dihydroorotate dehydrogenase (DHODH), required for de novo pyrimidine synthesis in rapidly dividing lymphocytes; it thereby limits the proliferation of activated T and B cells, which are thought to cross the blood–brain barrier and participate in the damaging inflammatory processes within the CNS that are associated with MS Citation[57].
As resting and slowly dividing cells, including lymphocytes and non-lymphoid cells, use a pyrimidine salvage pathway to meet their needs, basic homeostatic functions of these cells appear to be unaffected by teriflunomide, and protective immunity is preserved .
In vitro and in vivo experiments have provided supporting evidence for this mechanism of action. Teriflunomide was shown to inhibit proliferation of stimulated human T and B cells in vitro; this inhibition was effectively reversed by the addition of uridine, confirming DHODH dependency, while teriflunomide was not associated with any significant adverse effect on lymphocyte viability Citation[58].
In the Dark Agouti experimental autoimmune encephalomyelitis rat model, therapeutic administration of teriflunomide improved disease outcomes, delayed disease onset and reduced maximal and cumulative neurological disease scores compared with vehicle-treated animals; reduced demyelination and axonal loss in the spinal cord was also seen when teriflunomide was dosed therapeutically or prophylactically Citation[59]. Furthermore, treatment with teriflunomide was associated with reduced infiltration of T cells, natural killer cells, macrophages and neutrophils into the CNS Citation[60]; protection against oligodendrocyte cell death in the CNS Citation[61] and improvements in sensory and motor neuron functional outcomes Citation[59,62].
Another rodent model of MS, Theiler’s murine encephalomyelitis virus model has also shown that the progression of neurological deficits is mitigated by therapeutic teriflunomide treatment Citation[63].
Pharmacokinetics
Teriflunomide is rapidly absorbed, reaching peak plasma concentrations (tmax) 1–4 h post-dose following repeated oral administration, and absolute oral bioavailability approaches 100% Citation[54,55]. Although steady-state concentrations are reached after approximately 3 months, the pharmacodynamic, clinical and MRI effects (including reductions in relapse rates and combined unique active lesions) of teriflunomide are apparent before these levels are achieved Citation[64]. Teriflunomide is extensively (>99%) bound to plasma proteins and displays linear pharmacokinetics, with a long plasma half-life of approximately 19 days. However, elimination of teriflunomide from the circulation can be accelerated, if needed, with the use of cholestyramine or activated charcoal Citation[54,55,65]. No significant pharmacokinetic differences are apparent according to food intake, mild-to-moderate hepatic impairment, renal impairment, gender or age Citation[54,55].
Clinical efficacy: teriflunomide monotherapy
The efficacy and safety of teriflunomide monotherapy have been evaluated in an extensive clinical trial program in patients with relapsing MS (RMS) and in those with a first clinical episode suggestive of MS. .
Table 1. Overview of teriflunomide clinical trial program.
Phase II study
The Phase II proof-of-concept study Citation[66] was designed to evaluate the safety and efficacy of oral teriflunomide in 179 patients with RMS Citation[67]. Patients were randomized 1:1:1 to receive teriflunomide 14 or 7 mg, or placebo for 36 weeks.
At 36 weeks, patients receiving either teriflunomide 14 or 7 mg exhibited a significant decrease in the number of combined unique active lesions per scan, the study’s primary end point (p < 0.01 for teriflunomide 14 mg and p < 0.03 for 7 mg, compared with placebo). This effect was observed as early as Week 6, reaching significance by Week 12, and was maintained throughout the study. Patients treated with either dose of teriflunomide also had fewer T1 Gd-enhancing lesions (p < 0.02 for teriflunomide 14 mg and p < 0.04 for 7 mg, vs placebo) and new or enlarging T2 lesions (p < 0.03 in the teriflunomide 14-mg group and p < 0.04 in the 7-mg group). A significant 69% relative reduction (p < 0.04) was also seen with the 14-mg dose versus placebo in the proportion of patients with an increase in disability (increased score on the Expanded Disability Status Scale [EDSS] at Week 36 vs baseline).
Disease activity as measured by MRI remained low in patients with up to 12 years of teriflunomide exposure in a long-term open-label extension of the Phase II study Citation[68,69], in which placebo-treated patients in the core study were re-randomized to teriflunomide 7 or 14 mg; ARR and disability progression were also minimal in both teriflunomide-treatment groups over the course of the extension with up to 8.5 years of exposure Citation[70].
Phase III clinical trials
TEMSO
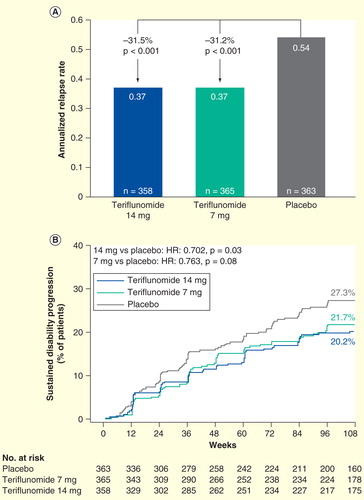
The TEMSO trial Citation[71] was the first Phase III study to evaluate the efficacy of teriflunomide in reducing the frequency of relapses and progression of physical disability in patients with RMS. 1088 patients aged 18–55 years, with EDSS scores ≤5.5 and at least one relapse in the previous year or at least two relapses in the previous 2 years, were assigned 1:1:1 to receive once-daily doses of teriflunomide 14 or 7 mg, or placebo for 108 weeks Citation[72].
Patients treated with teriflunomide showed significant reductions in ARR versus placebo (0.37 for both treatment arms, compared with 0.54 for placebo, representing 31.5% and 31.2% relative risk reductions for the 14- and 7-mg groups, respectively; p < 0.001) , as well as a longer time to first relapse. A greater proportion of teriflunomide-treated patients remained relapse free, and the risk of disability progression sustained for 12 weeks was also lower in both teriflunomide groups, with a significant reduction of 29.8% (p = 0.03) for teriflunomide 14 mg compared with placebo, and a non-significant reduction of 23.7% (p = 0.08) for the 7-mg dose versus placebo .
With regard to MRI measures of disease, teriflunomide 14 and 7 mg showed significantly smaller changes in total lesion volume (p < 0.001 and p = 0.03, respectively), fewer T1 Gd-enhancing lesions per MRI scan (p < 0.001 for both comparisons) and fewer unique active lesions per scan (p < 0.001 for both comparisons) compared with placebo Citation[72,73].
Table 2. MRI outcomes from the TEMSO trial†.
In the long-term extension of the TEMSO study Citation[74], benefits of teriflunomide in patients treated for up to 9 years were observed with lower relapse rates than those recorded in the core study Citation[75]. There was also a low risk of disability progression, and EDSS scores remained relatively stable for all groups Citation[75].
Relapses with incomplete recovery leading to neurological deficits (sequelae) are more severe manifestations of the disease and can contribute to disability progression. In addition, more severe attacks can result in hospitalization and increased healthcare costs. Post hoc analysis of TEMSO evaluated the effects of teriflunomide on a range of relapse outcomes and healthcare resource use Citation[76]. These analyses found that compared with placebo, teriflunomide 14 mg significantly reduced ARRs with sequelae, defined by an EDSS/functional score increase at 30 days post-relapse, by 36% (p = 0.0011), relapse leading to hospitalization by 59% (p < 0.0001), relapse requiring intravenous (iv.) corticosteroids by 34% (p = 0.0003), and relapse with sequelae, as determined at the end of the relapse by the investigator, by 53% (p < 0.0001) Citation[76]. There were also significant reductions for teriflunomide 7 mg compared with placebo in ARRs with sequelae as defined by EDSS/functional score increase and relapse requiring iv. corticosteroids Citation[76]. There were no significant differences in the intensity of relapse between either dose of teriflunomide and placebo Citation[76].
Pre-specified subgroup analyses found that the beneficial effect observed for teriflunomide on ARR was consistent across subgroups defined by baseline demographics (gender, race and age), disease characteristics (baseline EDSS strata, relapse history and MS subtype), MRI parameters (Gd-enhancing lesions and total lesion volume) and prior use of other DMTs Citation[77]. The effect of teriflunomide on the risk of disability progression was also consistent across subgroups. However, there was a non-significant trend in both treatment groups toward a larger difference in the reduction of sustained disease progression for the EDSS >3.5 subgroup compared with the ≤3.5 subgroup (p = 0.07 and p = 0.09 for teriflunomide 14 and 7 mg, respectively) Citation[77].
TOWER
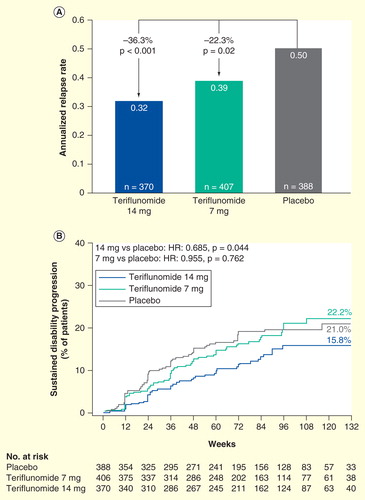
TOWER Citation[78] was the second pivotal study to evaluate the effects of teriflunomide on ARR and disability progression in patients with RMS (n = 1169) Citation[79]. Treatment duration was variable, ending 48 weeks after the last patient was randomized. In view of the positive data already provided from the Phase II and TEMSO studies, MRI parameters were not assessed in TOWER.
Results from TOWER were consistent with TEMSO, with significant reductions in ARR (36.3%, p < 0.001 and 22.3%, p = 0.02) observed for teriflunomide 14 and 7 mg, respectively, compared with placebo . Furthermore, the risk of sustained disability progression was also decreased by 31.5% (p = 0.04) in patients who received teriflunomide 14 mg compared with those receiving placebo Citation[79].
Figure 3. Annualized relapse rate and sustained accumulation of disability in TOWER. Modified intent-to-treat population. The annualized relapse rate (protocol defined relapses occurring between randomization and last dose of study drug or placebo received) was based on a Poisson regression model with robust error variance including factors for treatment, baseline Expanded Disability Status Scale (EDSS) strata, and region. (A) Sustained accumulation of disability (B) was defined as an increase of at least 1 point in EDSS score from baseline (or at least 0.5 points for patients with baseline EDSS score of >5.5 points) that persisted for at least 12 weeks.
The TOWER study also reported a positive effect of teriflunomide on quality-of-life outcomes, with a greater increase in fatigue in patients treated with placebo compared with teriflunomide 14 mg, as measured by the Fatigue Impact Scale, and a significant benefit with teriflunomide 14 mg compared with placebo in the Short Form-36 mental health summary score Citation[79].
A post hoc analysis demonstrated that teriflunomide 14 mg significantly reduced ARRs with sequelae (as determined by both EDSS/functional score, p = 0.0021, or by the investigator, p = 0.0004), relapses requiring iv. corticosteroids (p = 0.0002), or relapses leading to hospitalization (p = 0.0155) and intense relapses, utilizing the definition employed by Panitch and colleagues from the EVIDENCE study Citation[80] (p = 0.0015) Citation[81].
As reported in the pre-specified TEMSO subgroup analyses, there were no significant treatment-by-subgroup interactions in ARR observed in TOWER, and similar results were seen for disability progression. However, for disability progression, an interaction by gender (p = 0.017) and the number of relapses within the past 1 (p = 0.018) and 2 years (p = 0.045) was seen in the teriflunomide 14-mg group Citation[82]; such an observation was not noted in the pre-specified subgroup analysis of the TEMSO study.
An integrated subgroup analysis carried out on pooled data from TEMSO and TOWER confirmed results from the individual studies. In patients with high disease activity (patients with ≥2 relapses in the year before study entry), teriflunomide 14 mg significantly reduced ARR and the risk of disability progression confirmed for 12 weeks Citation[83].
Data from the TOWER extension have not yet been reported.
TENERE
The TENERE Phase III study Citation[84] compared teriflunomide with sc IFNβ-1a in 324 patients with RMS Citation[85]. No significant difference between teriflunomide and sc IFNβ-1a was seen in the primary end point of time to treatment failure, defined as the first occurrence of confirmed relapse or permanent discontinuation for any cause. There was also no difference in ARR between sc IFNβ-1a and teriflunomide 14 mg (0.22 vs 0.26; p = 0.6), although ARR was significantly higher with teriflunomide 7 mg (0.41; p = 0.03 vs IFNβ-1a).
Treatment satisfaction, as measured by the Treatment Satisfaction Questionnaire for Medication version 1.4 (TSQM v1.4) Citation[86], was one of the secondary outcomes of the study and was significantly improved in the domains of global satisfaction (p = 0.02 for either dose), side effects (p < 0.0001 for either dose) and convenience (p < 0.0001 for either dose) with teriflunomide compared with sc IFNβ-1a Citation[85].
Fatigue Impact Scale scores indicated more frequent fatigue in the IFNβ-1a group, though differences were only significant with teriflunomide 7 mg (p = 0.03), and the rate of permanent discontinuation due to adverse events was lower in both teriflunomide groups compared with the sc IFNβ-1a group (10.9 and 8.2% of patients for teriflunomide 14 and 7 mg, respectively, compared with 21.8% in the sc IFNβ-1a group) Citation[85].
TOPIC
The TOPIC study Citation[87] was a Phase III study with teriflunomide versus placebo conducted in patients with a first clinical episode suggestive of MS, defined as a new neurological event consistent with demyelination, starting within 90 days of randomization, and two or more T2-weighted MRI lesions ≥3 mm in diameter Citation[88]. Because of revisions to diagnostic criteria enabling earlier diagnosis of MS, the study was stopped ahead of schedule and patients were given the option of entering the extension phase, where they received teriflunomide 14 or 7 mg.
The study demonstrated significant benefits of both doses of teriflunomide on risk of relapse determining conversion to clinically definite MS (reductions of 42.6%; p = 0.0087; and 37.2%; p = 0.0271; with teriflunomide 14 and 7 mg compared with placebo, respectively), as well as the risk of a new clinical relapse or MRI lesion (reductions of 34.9%; p = 0.0003, and 31.4%; p = 0.0020, with teriflunomide 14 and 7 mg vs placebo, respectively).
Patients treated with teriflunomide 14 mg also demonstrated significant improvements in total lesion volume and number and volume of Gd-enhancing T1 lesions per scan, as measured by MRI.
Clinical efficacy: adjunctive therapy studies
Two Phase II studies have evaluated teriflunomide 14 or 7 mg as an add-on therapy to other DMTs. The first of these studies Citation[89] evaluated teriflunomide added to ongoing stable-dosed IFNβ in patients with RMS Citation[90]. At 24 weeks, teriflunomide 14 and 7 mg added to IFNβ demonstrated significant (84.4%; p = 0.0001 and 82.6%; p = 0.0009, respectively) relative risk reductions in the number of Gd-enhancing T1 lesions in comparison with IFNβ alone. In addition, teriflunomide 14 mg showed a significant 64.7% (p = 0.0072) reduction in Gd-enhancing T1 lesion volume. Similar reductions were seen at 48 weeks in patients who completed the 24-week extension Citation[91]. There was also a non-significant trend toward a reduction in ARR at 24 and 48 weeks, with 58% (p = 0.28) and 57.9% (p = 0.10) relative risk reductions in the 14-mg group relative to placebo.
Reflecting observations made in TEMSO and TOWER, a post hoc subgroup analysis suggested that, even as an add-on therapy, there was a more pronounced treatment effect with teriflunomide in patients with more active disease at baseline Citation[90].
A second study, and its extension Citation[91,92] examined the safety and tolerability of teriflunomide as an add-on therapy to a stable dose of GA Citation[93]. A general pattern of lower MRI activity in the teriflunomide groups was observed: teriflunomide 14 mg showed a significant 73% (p = 0.0395) reduction in total Gd-enhancing T1 lesion volume and a non-significant 47% reduction in the number of Gd-enhancing T1 lesions per scan at 48 weeks. Conversely, the reduction of 64% (p = 0.031) in the number of Gd-enhancing T1 lesions per scan with teriflunomide 7 mg was significant, while the 40% reduction in T1 lesion volume was non-significant Citation[93].
A Phase III adjunct study, TERACLES Citation[94], demonstrated an incremental benefit of teriflunomide 14 mg in combination with IFNβ on ARR, and a significant improvement for teriflunomide 14 mg and IFNβ on MRI activity (70.8% reduction [p = 0.006] in Gd-enhancing T1 lesions per MRI scan compared with placebo and IFNβ) Citation[95]. The study was terminated early, as it was felt that recruitment challenges owing to the availability of alternative newer therapies that avoided injectable agents would bias the population and make for a less informative study. However, results do suggest that an immediate transition between IFNβ and teriflunomide without increased safety concerns is possible.
Vaccination studies
Two studies were conducted to evaluate the effect of teriflunomide compared with placebo on immune responses to recall and neoantigens. TERIVA Citation[96] investigated the ability of patients treated with teriflunomide to mount effective immune responses to a recall antigen (seasonal influenza vaccination) Citation[97]. In the second study, immune responses to a neoantigen (rabies vaccination) were assessed in healthy volunteers administered teriflunomide Citation[98]. The results of both studies suggest that teriflunomide does not interfere with protective immunity, further supporting an immunomodulatory rather than immunosuppressive mechanism of action.
Safety & tolerability
The safety and tolerability profile of teriflunomide is well characterized and has been shown to be consistent across studies and similar between doses Citation[67,72,79,88]. In placebo-controlled clinical studies, teriflunomide was generally well tolerated, with predominately mild-to-moderate adverse events, most of which did not require treatment and resolved while continuing teriflunomide therapy Citation[67,72,79,88].
In the Phase II core study, the most common adverse events (≥10% in either teriflunomide group) reported more frequently with teriflunomide than with placebo included nasopharyngitis, hair thinning, alanine aminotransferase (ALT) increase, paresthesia, back and limb pain, nausea, diarrhea, urinary tract infection and arthralgia Citation[67]. The incidence of serious adverse events was similar in all treatment groups and included elevated liver enzymes, hepatic dysfunction, neutropenia, rhabdomyolysis and trigeminal neuralgia. The nature of adverse events in the long-term, open-label extension of the Phase II study, with up to 8.5 years of teriflunomide exposure, was similar to that observed in the short-term study Citation[70].
In the Phase III TEMSO study, a comparable incidence of adverse events (90.8, 89.1 and 87.5%), serious adverse events (15.9, 14.1 and 12.8%) and adverse events leading to treatment discontinuation (10.9, 9.8 and 8.1%) were reported in the teriflunomide 14- and 7-mg and placebo treatment groups, respectively, following 108 weeks of treatment Citation[72]. The most common adverse events (≥10% of patients) seen more frequently with teriflunomide, and with a dose effect, were diarrhea, nausea, hair thinning and increased ALT levels. Adverse events related to gastrointestinal symptoms (diarrhea and nausea) and decreased hair density led to treatment discontinuation only rarely (<0.5% for any treatment group due to nausea or diarrhea, and 1.4, 0.5 and 0.0% for teriflunomide 14 and 7 mg, and placebo, respectively, due to hair thinning).
Asymptomatic ALT increases (≥1× the upper limit of normal [ULN]) were observed more frequently in teriflunomide groups than with placebo, although the incidence of increases ≥3× ULN was similar in all treatment groups (14 mg, 6.7%; 7 mg, 6.7%; placebo, 6.3%). One patient in each treatment group was reported to have ALT ≥3× ULN and total bilirubin ≥2× ULN; of these three patients, one each had a diagnosis of hepatitis C, incidental bile duct stenosis or cytomegalovirus infection.
Mean reductions in neutrophil and lymphocyte counts were small in magnitude, but slightly greater with teriflunomide 14 mg than with 7 mg or placebo. However, these reductions generally occurred during the first 3 months of treatment and stabilized over time. Moderate neutropenia (defined as neutrophil counts of <0.9 × 109/l) developed in three patients receiving teriflunomide, but two of these patients’ cases resolved spontaneously on treatment, and the third resolved following treatment discontinuation.
Serious infections were reported in 2.5, 1.6 and 2.2% of patients treated with teriflunomide 14 and 7 mg, and placebo, respectively, and no serious opportunistic infections occurred. Three patients in the placebo group were reported to have malignant neoplasms and one patient in the teriflunomide 14-mg group was reported to have cervical carcinoma. No deaths were reported in the study.
Results from TOWER were consistent with those seen in the Phase II study and TEMSO, with no new or unexpected safety signals, and a comparable number of serious adverse events (∼12%) across the teriflunomide 14 and 7 mg, and placebo groups Citation[79]. Although more patients receiving teriflunomide discontinued treatment than those receiving placebo, this was mainly due to the protocol-defined requirement to discontinue treatment in the event of a confirmed ALT increase >3× ULN or decreased neutrophil counts. Raised ALT concentrations >1× ULN were more frequent in the teriflunomide treatment groups compared with placebo, but more profound increases (>5× ULN) were seen in a low and similar proportion across all groups (14 mg, 3%; 7 mg, 2%; placebo, 4% of patients, respectively).
Cases of peripheral neuropathy confirmed by nerve conduction studies occurred more frequently in the teriflunomide treatment groups (2% of patients in each group) compared with placebo (1%) and were mild to moderate in intensity. These led to treatment discontinuation for two patients in the teriflunomide 7-mg group and three in the 14-mg group.
No increased risk of infection or serious infection was seen with teriflunomide compared with placebo, and infections were not associated with decreases in neutrophil counts Citation[99]. Of the two opportunistic infections seen in the study (concomitant hepatitis C and cytomegalovirus infection in the placebo group and intestinal tuberculosis in the 14-mg group), neither was considered by the investigator to be related to study treatment. Four deaths were recorded, none of which was considered by the investigators to be related to teriflunomide treatment. These included respiratory infection (placebo), traffic accident (teriflunomide 7 mg), suicide and septicemia due to Gram-negative infection complicated by disseminated intravascular coagulopathy (teriflunomide 14 mg).
The nature and incidence of adverse events observed in patients with early MS in the TOPIC study were in accordance with the earlier studies Citation[88]. In addition, an analysis of pooled safety data from 3044 patients with >3070 patient-years of teriflunomide exposure demonstrated safety outcomes that were consistent with those of individual studies, and no new or unexpected safety signals were identified with either dose Citation[100].
Pregnancy
Animal studies have demonstrated evidence of embryo toxicity and teratogenicity with teriflunomide Citation[54,55]. Reliable contraception should be used throughout teriflunomide treatment, and women wishing to become pregnant must discontinue teriflunomide treatment Citation[54,55]. Women who do become pregnant are required to undergo an accelerated elimination procedure with cholestyramine or activated charcoal after treatment discontinuation in order to achieve confirmation of plasma teriflunomide levels <0.02 g/l, thought to represent a minimal risk to the fetus Citation[55].
In the course of the clinical trial program, 83 pregnancies in female patients were reported, of which 70 occurred in women treated with teriflunomide Citation[101]. There were 26 live births to female teriflunomide-treated patients, and all newborns were healthy, with no structural or functional abnormalities. Furthermore, the observed rate of spontaneous abortion in teriflunomide-treated female patients (∼19%) Citation[102] was within the range reported for women without MS Citation[103].
The risk of male-mediated embryo-fetal toxicity is considered low, and evidence from preclinical data has indicated no effect of teriflunomide on male fertility or damage to sperm DNA Citation[104]. From 16 live births reported in partners of teriflunomide-treated men (out of 19 reported pregnancies), all newborns were healthy Citation[101]. Current prescribing information in the USA indicates that men should practice reliable contraception and, if they wish to father a child, discontinue teriflunomide and undergo an accelerated elimination procedure as described above Citation[11].
These results are in agreement with a prospective study by the Organization of Teratology Information Specialists Citation[105] and post-marketing surveillance data (with cumulative exposure exceeding 2.4 million patient-years) that did not demonstrate any teratogenic effects for leflunomide.
Prospective data from pregnancies arising in the post-marketing setting are being collected in ongoing teriflunomide pregnancy registries in the USA, Europe and Australia.
Regulatory affairs
As of June 2014, teriflunomide has been approved in 44 countries. In the USA, both the 7- and 14-mg doses of teriflunomide have been approved for the treatment of relapsing forms of MS. In other countries, only the 14-mg dose has been approved. In the EU, the approved indication is for treatment of adult patients with RRMS.
Conclusion
Teriflunomide has shown consistent effects across multiple markers of disease burden and activity, including ARR and risk of disability progression. In TEMSO, improvements versus placebo were seen for both doses of teriflunomide in several MRI outcomes, including total lesion volume, number of unique new active lesions, number of Gd-enhancing T1 lesions and hyperintense T2 lesion volume, as well as for the 14-mg dose in T1 hypointense lesion volume. Importantly, teriflunomide has demonstrated consistent and statistically significant effects on disability in two pivotal Phase III trials, a unique observation among the available licensed oral therapies. This positive impact on disability progression appears to be maintained over the long term, as seen in the Phase II and TEMSO extension studies, although further long-term data in addition to data from observational studies are required to confirm this.
Together with consistent effects on key measures of disease activity (relapses and MRI) and a well-characterized and favorable long-term safety and tolerability profile, teriflunomide represents a valuable first-line oral treatment option for the management of patients with RRMS.
Expert commentary
Teriflunomide has demonstrated consistent beneficial effects in patients with relapsing forms of MS, as well as in patients with a first clinical episode of demyelination suggestive of MS, confirming its efficacy in patients with a broad range of disease activity. Compared with placebo, reductions in relapse rates and the risk of disability progression in patients with RRMS treated with teriflunomide were at least equivalent, and in some cases superior, to the injectable DMTs.
Results of studies with teriflunomide have shown that most adverse events were mild or moderate in nature, and rarely resulted in discontinuation of therapy. Monitoring of liver enzymes is recommended in patients receiving teriflunomide, arising from concerns reported in patients with rheumatoid arthritis treated with leflunomide. However, clinical trial results have shown that, while ALT increases were reported more frequently in teriflunomide-treated patients, most were low-level elevations, and the incidence of serious hepatic events was no higher in teriflunomide groups than in patients receiving placebo. Teriflunomide is contraindicated in pregnancy based on signs of developmental toxicity in offspring of rats and rabbits, and although reliable contraception was mandated in clinical trials, a number of pregnancies did occur. All babies born to parents exposed to teriflunomide were healthy and had no structural or functional abnormalities at birth. The favorable safety profile of teriflunomide is supported by long-term data from patients treated for up to 8.5 years in ongoing extension studies. No evidence of new or unexpected safety signals has arisen in more than 6800 patient-years of cumulative teriflunomide exposure.
The once-daily dosing of teriflunomide could contribute to improved treatment adherence in comparison with DMTs requiring frequent injection or oral therapies requiring twice-daily administration, thereby potentially improving treatment outcomes. Results of the TENERE study showed that, compared with IFNβ, patients treated with teriflunomide had greater treatment satisfaction, as measured by the TSQM v1.4.
Two adjunctive studies combining teriflunomide with either IFNβ or GA, while not powered to assess the clinical efficacy of teriflunomide as an add-on therapy, displayed a general pattern of increased efficacy in the teriflunomide groups compared with groups receiving only IFNβ or GA and suggest that an immediate transition between either IFNβ or GA and teriflunomide without increased safety concerns is possible.
Teriflunomide has demonstrated consistent benefits on efficacy outcomes in a broad range of patients and levels of disease activity. Combined with a well-characterized safety profile, current data suggest that teriflunomide is a valuable treatment option for patients with RRMS.
Five-year view
Existing immunomodulatory therapies are effective in reducing the frequency of relapses and disease activity measured by MRI in patients with relapsing forms of MS, but no treatment has been shown to prevent disease progression in the long term. Although IFNβ-1b and mitoxantrone are licensed in some countries to treat secondary progressive MS, none of the clinical trials in primary progressive MS have shown positive outcomes and no therapies are approved for treating it. Progressive MS is associated with pathological mechanisms that differ from those in RRMS, and future strategies are focused on limiting, and potentially reversing, neurodegeneration. Studies in patients with progressive MS are underway with anti-LINGO-1, an agent that has shown evidence of myelin repair in an animal model, and rHIgM22, which in preclinical studies promoted remyelination by stimulating oligodendrocytes to repair areas of demyelination.
Identification of biomarkers that might predict responses to individual DMTs will hopefully lead to personalized treatment of patients with MS. Additional information to assist in the identification of the best treatment sequencing strategies would be extremely valuable to clinicians as the number of available treatments increase and switching becomes more complex. There is evidence to suggest that aggressive treatment in the earliest stages of MS may postpone disease progression, supporting the hypothesis that the immune system might be ‘reset’.
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the CNS and is one of the most common neurological diseases in young adults.
Teriflunomide is a once-daily, oral disease-modifying therapy approved for the treatment of relapsing forms of MS.
Teriflunomide reversibly inhibits the mitochondrial enzyme dihydroorotate dehydrogenase, required for de novo pyrimidine synthesis in rapidly dividing lymphocytes, thereby limiting the proliferation of activated T and B cells, which are thought to cross the blood–brain barrier and participate in the damaging inflammatory processes that are associated with MS.
Clinical trials have shown a consistent beneficial effect of teriflunomide on reducing the frequency of relapses, the risk of disability progression and markers of disease activity on MRI in patients with relapsing forms of MS. In patients with a first clinical episode consistent with MS, teriflunomide was effective in delaying the conversion to clinically definite MS.
Teriflunomide has a well-characterized safety and tolerability profile. No new or unexpected safety signals were identified in patients with long-term exposure (up to 8.5 years) to teriflunomide.
Acknowledgements
All teriflunomide studies discussed in this review were funded by Genzyme, a Sanofi company.
Financial & competing interest disclosures
AE Miller has received grants and personal fees from Genzyme/Sanofi-Aventis, Biogen Idec, Novartis, and Accorda; grants from Genentech, Roche, and Questcor; and personal fees from GlaxoSmithKline, EMD Serono (Merck Serono), Nuron Biotech, Accordant Health Services, and Teva. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial support for this manuscript was provided by M Lens, of Fishawack Communications (Abingdon, UK), and was funded by Genzyme.
Notes
References
- Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502-17
- Gold R, Wolinsky JS. Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol Scand 2011;124:75-84
- Tremlett H, Yousefi M, Devonshire V, et al. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology 2009;73:1616-23
- Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain 2010;133(Pt 7):1914-29
- World Health Organization. Atlas: multiple sclerosis resources in the world. World Health Organization; Geneva, Switzerland: ed. 2008
- PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 1998;352:1498-504
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-94
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993;43:655-61
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45:1268-76
- Reuss R. PEGylated interferon beta-1a in the treatment of multiple sclerosis – an update. Biologics 2013;7:131-8
- US Prescribing Information: copaxone, Teva Neuroscience, 2014. Available from: www.copaxone.com/Resources/pdfs/PrescribingInformation.pdf [Last accessed 24 September 2014]
- Caporro M, Disanto G, Gobbi C, Zecca C. Two decades of subcutaneous glatiramer acetate injection: current role of the standard dose, and new high-dose low-frequency glatiramer acetate in relapsing-remitting multiple sclerosis treatment. Patient Prefer Adherence 2014;8:1123-34
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402-15
- Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387-401
- Efficacy and safety of fingolimod in patients with relapsing-remitting multiple sclerosis with optional extension phase (TRANSFORMS). Available from: http://clinicaltrials.gov/show/NCT00340834
- Efficacy and safety of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS). Available from: http://clinicaltrials.gov/show/NCT00289978
- Summary of Product Characteristics: gilenya, Novartis, 2014. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf [Last accessed 24 September 2014]
- Selmaj K, Vollmer T, Bar-Or A, on behalf of the DreaMS study investigators. Effect of ONO-4641, a potent, oral, selective sphingosine-1-phosphate receptor-1 and -5 agonist, on MRI outcomes in patients with relapsing remitting multiple sclerosis: subgroup analyses from the phase 2 DreaMS study. Mult Scler 2013;19:74-558. P536
- A Study of the safety and efficacy of ONO-4641 in patients with relapsing-remitting multiple sclerosis (DreaMS). Available from: http://clinicaltrials.gov/show/NCT01081782
- Pozzilli C FO, Olsson T, Freedman MS, et al. Maintenance of efficacy, safety and tolerability of ponesimod in patients with relapsing remitting multiple sclerosis: phase II extension study. Mult Scler 2013;19:74-558. P995
- Ponesimod in patients with relapsing-remitting multiple sclerosis -extension study. Available from: http://clinicaltrials.gov/show/NCT01093326
- Exploring the Efficacy and Safety of Siponimod in Patients With Secondary Progressive Multiple Sclerosis (EXPAND), 2014. Available from: https://clinicaltrials.gov/ct2/show/NCT01665144?term=NCT01665144&rank=1 [Last accessed 24 September 2014]
- Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134(Pt 3):678-92
- Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 2012;341:274-84
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098-107
- Efficacy and safety study of oral BG00012 with active reference in relapsing-remitting multiple sclerosis (CONFIRM). Available from: http://clinicaltrials.gov/show/NCT00451451
- Efficacy and safety of oral BG00012 in relapsing-remitting multiple sclerosis (DEFINE). Available from: http://clinicaltrials.gov/show/NCT00420212
- Summary of Product Characteristics: tecfidera, Biogen Idec Ltd, 2014. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002601/WC500162069.pdf [Last accessed 14 October 2014]
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899-910
- Safety and efficacy of natalizumab in the treatment of multiple sclerosis. Available from: http://clinicaltrials.gov/show/NCT00027300
- Summary of Product Characteristics: tysabri, Biogen Idec Ltd, 2014. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000603/WC500044686.pdf [Last accessed 14 October 2014]
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819-28
- Comparison of alemtuzumab and Rebif® efficacy in multiple sclerosis, study one (CARE-MS I). Available from: http://clinicaltrials.gov/show/NCT00530348
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829-39
- Comparison of alemtuzumab and Rebif® efficacy in multiple sclerosis, study two (CARE-MS II). Available from: http://clinicaltrials.gov/show/NCT00548405
- An extension protocol for multiple sclerosis patients who participated in Genzyme-sponsored studies of alemtuzumab. Available from: http://clinicaltrials.gov/show/NCT00930553
- Havrdova E, Arnold DL, Palmer J, Margolin DH. Disease-free outcomes with alemtuzumab: 3-year follow-up of the CARE-MS studies. Mult Scler J 2014;20(Suppl 1):39
- Summary of product characteristics: lemtrada, Genzyme 2014. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003718/WC500150521.pdf [Last accessed 14 October 2014]
- Safety and efficacy study of daclizumab high yield process (DAC HYP) to treat relapsing-remitting multiple sclerosis (SELECT). Available from: http://clinicaltrials.gov/show/NCT00390221
- Gold R, Giovannoni G, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 2013;381:2167-75
- Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779-87
- A study of the efficacy and safety of ocrelizumab in patients with relapsing-remitting multiple sclerosis. Available from: http://clinicaltrials.gov/show/NCT00676715
- Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 2014;82:573-81
- Ofatumumab subcutaneous administration in subjects with relapsing-remitting multiple sclerosis (MIRROR). Available from: http://clinicaltrials.gov/show/NCT01457924
- Bar-Or A, Grove R, Austin D, et al. The MIRROR Study: a Randomized, Double-blind, Placebo-controlled, Parallel-group, Dose-ranging Study to Investigate the Safety and MRI Efficacy of Subcutaneous Ofatumumab in Subjects with Relapsing-Remitting Multiple Sclerosis (RRMS). Neurology 2014;82(Suppl 10):S23.006
- Sorenson PS, Kavanagh ST, Austin DJ, et al. Follow-up data from the Mirror study: a dose-ranging study of subcutaneous ofatumumab in subjects with relapsing-remitting multiple sclerosis. Mult Scler J 2014;20(Suppl 1):67-284. P048
- Pepinsky RB, Shao Z, Ji B, et al. Exposure levels of anti-LINGO-1 Li81 antibody in the central nervous system and dose-efficacy relationships in rat spinal cord remyelination models after systemic administration. J Pharmacol Exp Ther 2011;339:519-29
- BIIB033 single ascending dose study in healthy volunteer subjects. Available from: http://clinicaltrials.gov/show/NCT01052506
- Safety study of BIIB033 in subjects with multiple sclerosis. Available from: http://clinicaltrials.gov/show/NCT01244139
- Tran J PR, Zhao J, Brosofsky K, et al. Safety, tolerability and pharmacokinetics of the anti-LINGO-1 monoclonal antibody BIIB033 in healthy volunteers and subjects with multiple sclerosis. Neurology 2012;78:P02.021
- Rivera VM, Jeffery DR, Weinstock-Guttman B, et al. Results from the 5-year, phase IV RENEW (Registry to Evaluate Novantrone Effects in Worsening Multiple Sclerosis) study. BMC Neurol 2013;13:80
- Martinelli V, Radaelli M, Straffi L, et al. Mitoxantrone: benefits and risks in multiple sclerosis patients. Neurol Sci 2009;30(Suppl 2):S167-70
- US prescribing information: Aubagio, Sanofi. Available from. http://products.sanofi.us/aubagio/aubagio.pdf [Last accessed 14 October 2014]
- EU Summary of product characteristics: Aubagio, Sanofi-Aventis. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002514/WC500148682.pdf [Last accessed 14 October 2014]
- EU Summary of product characteristics: arava, Sanofi-Aventis. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000235/WC500026289.pdf [Last accessed 14]
- Bar-Or A, Pachner A, Menguy-Vacheron F, et al. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014;74:659-74
- Li L, Liu J, Delohery T, et al. The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells in vitro. J Neuroimmunol 2013;265:82-90
- Merrill JE, Hanak S, Pu SF, et al. Teriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the dark agouti rat model of experimental autoimmune encephalomyelitis. J Neurol 2009;256:89-103
- Ringheim GE, Lee L, Laws-Ricker L, et al. Teriflunomide attenuates immunopathological changes in the dark agouti rat model of experimental autoimmune encephalomyelitis. Front Neurol 2013;4:169
- Petty M, Lee L, Ying X, et al. Teriflunomide treatment reduces infiltration of macrophages, T cells and B cells, and increases survival of oligodendrocytes in the spinal cord of the dark agouti rat model of experimental allergic encephalomyelitis. Neurology 2010;74:P05.033
- Iglesias-Bregna D, Hanak S, Ji Z, et al. Effects of prophylactic and therapeutic teriflunomide in transcranial magnetic stimulation-induced motor-evoked potentials in the dark agouti rat model of experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther 2013;347:203-11
- Pachner A, Li L. Teriflunomide ameliorates disability progression in the Theiler’s virus-induced demyelinating disease model of MS. Presented at AAN Annual Meeting, San Diego, CA, USA; 16-23 March 2013 P05.196
- Wolinsky J, Dukovic D, Truffinet P, Kappos L. Estimating the onset of efficacy with teriflunomide in patients with relapsing forms of multiple sclerosis. Neurology 2014;82(Suppl 10):P7.214
- Freedman MS. Teriflunomide in relapsing multiple sclerosis: therapeutic utility. Ther Adv Chronic Dis 2013;4:192-205
- Safety and efficacy of teriflunomide (HMR1726) in multiple sclerosis with relapses. Available from: http://clinicaltrials.gov/show/NCT01487096
- O’Connor PW, Li D, Freedman MS, et al. A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 2006;66:894-900
- Li DBK, Traboulsee AL, Truffinet P, et al. Long-term MRI outcomes from patients treated with teriflunomide: results from a phase 2 extension study. Mult Scler J 2014;20:67-284. P079
- Long term safety and efficacy of teriflunomide (HMR1726) in multiple sclerosis with relapses. Available from: http://clinicaltrials.gov/show/NCT00228163
- Confavreux C, Li DK, Freedman MS, et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult Scler 2012;18:1278-89
- Study of teriflunomide in reducing the frequency of relapses and accumulation of disability in patients with multiple sclerosis (TEMSO). Available from: http://clinicaltrials.gov/show/NCT00134563
- O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011;365:1293-303
- Wolinsky JS, Narayana PA, Nelson F, et al. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler 2013;19:1310-19
- Long term safety and efficacy study of teriflunomide 7 mg or 14 mg in patients with relapsing-remitting multiple sclerosis. Available from: http://clinicaltrials.gov/show/NCT00803049
- Freedman M Wolinsky J, Comi G, et al. Safety and Efficacy of Teriflunomide for up to 9 Years in Relapsing Forms of Multiple Sclerosis: update of the TEMSO Extension Trial. Neurology 2014;82(Suppl 10):P3.150
- O’Connor PW, Lublin FD, Wolinsky JS, et al. Teriflunomide reduces relapse-related neurological sequelae, hospitalizations and steroid use. J Neurol 2013;260:2472-80
- Miller AE, O’Connor P, Wolinsky JS, et al. Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult Scler 2012;18:1625-32
- An efficacy study of teriflunomide in patients with relapsing multiple sclerosis (TOWER). Available from: http://clinicaltrials.gov/ct2/show/NCT00751881
- Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:247-56
- Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE Trial. Neurology 2002;59:1496-506
- Miller AE, Macdonell R, Comi G, et al. Teriflunomide reduces relapses with sequelae and relapses leading to hospitalizations: results from the TOWER study. J Neurol 2014;261:1781-8
- Moses H, Freedman MS, Kappos L, et al. Pre-defined subgroups analyses of TOWER, a placebo-controlled phase III trial of teriflunomide in patients with relapsing multiple sclerosis. Neurology 2013;80:S41.006
- Kappos L, Comi G, Freedman MS, et al. Pooled efficacy data from two phase 3 placebo-controlled trials of oral, once-daily teriflunomide. Mult Scler J 2013;19(Suppl 1):74-558. P618
- A study comparing the effectiveness and safety of teriflunomide and interferon beta-1a in patients with relapsing multiple sclerosis (TENERE). Available from: http://clinicaltrials.gov/show/NCT00883337
- Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler J 2014;20:705-16
- Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2:12
- Phase III study with teriflunomide versus placebo in patients with first clinical symptom of multiple sclerosis (TOPIC). Available from: http://clinicaltrials.gov/show/NCT00622700
- Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:977-86
- Phase II study of teriflunomide as adjunctive therapy to interferon-beta in subjects with multiple sclerosis. Available from: http://clinicaltrials.gov/show/NCT00489489
- Freedman MS, Wolinsky JS, Wamil B, et al. Teriflunomide added to interferon-beta in relapsing multiple sclerosis: a randomized phase II trial. Neurology 2012;78:1877-85
- Long term safety of teriflunomide when added to interferon-beta or glatiramer acetate in patients with multiple sclerosis. Available from: http://clinicaltrials.gov/show/NCT00811395
- Phase II study of teriflunomide as adjunctive therapy to glatiramer acetate in subjects with multiple sclerosis. Available from: http://clinicaltrials.gov/show/NCT00475865
- Freedman MS, Wolinsky J, Wamil B, et al. for the Teriflunomide Multiple Sclerosis Trial Group. Oral teriflunomide plus glatiramer acetate in relapsing multiple sclerosis. Int J MS Care 2011;13(Suppl 3):P17
- Efficacy and safety of teriflunomide in patients with relapsing multiple sclerosis and treated with interferon-beta (TERACLES). Available from: http://clinicaltrials.gov/show/NCT01252355
- Freedman MS, Wolinsky J, Comi G, et al. for the TERACLES Study Group. Safety and efficacy of teriflunomide in patients with relapsing multiple sclerosis treated with interferon beta. 8th World Congress on Controversies in Neurology, 2014. Available from: www.comtecmed.com/cony/2014/Uploads/Editor/Freedman.pdf [Last accessed 14 October 2014]
- Study to investigate the immune response to influenza vaccine in patients with multiple sclerosis on teriflunomide (TERIVA). Available from: http://clinicaltrials.gov/show/NCT01403376
- Bar-Or A, Freedman MS, Kremenchutzky M, et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology 2013;81:552-8
- Bar-Or A, Larouche R, Legrand B, et al. Immune response to neoantigen and recall antigens in healthy subjects receiving teriflunomide. Mult Scler 2013;19(Suppl 1):74-558. P622
- Comi G, Freedman MS, Kappos L, et al. Effect of teriflunomide on lymphocyte and neutrophil counts in patients with relapsing multiple sclerosis: results from the TOWER Study. J Neurol Sci 2013;333:e376
- Leist TP, Freedman MS, Kappos L, et al. Pooled safety analyses from the teriflunomide clinical development program. Mult Scler J 2014;20(Suppl 1):67-284. P097
- Kieseier B, Truffinet P, Jung Henson L. Pregnancy outcomes for female patients and partners of male patients in the teriflunomide clinical development program. Mult Scler J 2014;20(Suppl 1):285-496. P846
- Jung Henson L, Benamor M, Truffinet P, Kieseier B. Updated pregnancy outcomes in patients and partners of patients in the teriflunomide clinical trial program. Neurology 2014;82(Suppl 10):P4.161
- Garcia-Enguidanos A, Calle ME, Valero J, et al. Risk factors in miscarriage: a review. Eur J Obstet Gynecol Reprod Biol 2002;102:111-19
- Davenport L, Czich A, Turpaul S. Teriflunomide: non-clinical evaluation demonstrates no effect on sperm DNA or male fertility. Neurology 2014;82(Suppl 10):P2.233
- Chambers CD, Johnson DL, Robinson LK, et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum 2010;62:1494-503
- Sajja BR, Datta S, He R, et al. Unified approach for multiple sclerosis lesion segmentation on brain MRI. Ann Biomed Eng 2006;34:142-51