Abstract
Growing concerns about the spread of multidrug-resistant tuberculosis (MDR-TB) and the emergence of extensively drug-resistant TB have triggered substantial interest in the development and application of rapid tests for the detection of drug-resistant TB. Molecular assays to detect gene mutations that signal drug resistance are widely recognized as being most suited for rapid diagnosis. Among molecular assays, line probe assays have shown great promise. Currently, two line probe assays are commercially available: the INNO-LiPA® Rif. TB kit (Innogenetics NV, Gent, Belgium) and the GenoType® MTBDRplus assay (Hain Lifescience GmbH, Nehren, Germany). Evidence from a systematic review suggests that INNO-LiPA is a highly sensitive and specific test for the detection of rifampicin resistance in culture isolates. The test, however, appeared to have relatively lower sensitivity when used directly on clinical specimens. Another meta-analysis showed that the GenoType MTBDR assays had excellent accuracy for rifampicin resistance, even when used directly on clinical specimens. While specificity was excellent for isoniazid, sensitivity estimates were modest and variable. Based on evidence and expert opinion, the WHO recently endorsed the use of molecular line probe assays for rapid screening of patients at risk of MDR-TB. Special initiatives have been announced to make these assays accessible and affordable for countries with high MDR-TB prevalence. With strong evidence and new policy directives, the stage is now set for the use of rapid tests for MDR-TB diagnosis. Whether molecular tools, such as line probe assays, will actually make a clinical and public-health impact remains to be determined.
Growing threat of drug-resistant tuberculosis
Tuberculosis (TB) continues to be one of the most important infectious disease threats to human health Citation[1]. Multidrug-resistant TB (MDR-TB) is TB that is resistant to at least two of the most important first-line antimycobacterial drugs – isoniazid (INH) and rifampicin (RIF). Extensively drug-resistant TB (XDR-TB) is a relatively rare but potentially lethal type of MDR-TB. XDR-TB is defined as TB that is resistant to INH and RIF, plus resistant to any fluoroquinolone and at least one of three injectable second-line drugs (amikacin, kanamycin or capreomycin) Citation[2].
It has been estimated that over 400,000 new cases of MDR-TB occur each year and, although their exact incidence rates are currently unknown, XDR-TB cases have been detected in every country where there is the capacity to detect them Citation[2,3]. Growing concerns regarding the spread of MDR-TB and alarm over the emergence of XDR-TB have sparked a great deal of interest in the development and application of rapid diagnostic tests for the detection of drug-resistant Mycobacterium tuberculosis disease, especially in settings with high HIV prevalence Citation[4–7]. Early detection of MDR-TB and XDR-TB is critical to initiate appropriate treatment, reduce morbidity and mortality, and prevent further transmission of drug-resistant strains of TB.
Line probe assays: what is the evidence?
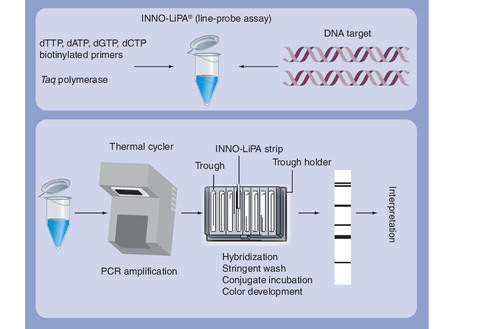
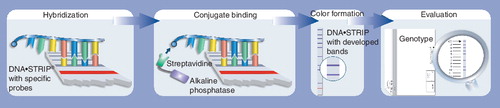
Molecular assays to detect gene mutations that signal drug resistance are widely recognized as being most suited for rapid diagnosis, especially since these assays can be directly used on clinical specimens, such as sputum Citation[4,5]. Among the molecular assays, line probe assays have shown great promise Citation[4]. Line probe assays are a family of novel DNA strip-based tests that use nucleic acid amplification techniques (e.g., PCR) and reverse hybridization methods for the rapid detection of mutations associated with drug resistance. A major advantage of line probe assays is that they can be directly used on clinical specimens, such as sputum.
Currently, two line probe assays are commercially available: the INNO-LiPA® Rif. TB kit (Innogenetics NV, Gent, Belgium) and the GenoType® MTBDRplus assay (Hain Lifescience GmbH, Nehren, Germany) . Since the introduction of these assays, several studies have evaluated the diagnostic accuracy of these tests in various settings. provides a brief summary of available evidence, based on two systematic reviews Citation[8,9].
In a systematic review and meta-analysis, Morgan and colleagues summarized the evidence on the INNO-LiPA assay for detection of RIF resistance Citation[8]. In 14 studies that applied INNO-LiPA to culture isolates, 12 reported sensitivity estimates greater than 95%, and 12 out of 14 studies reported specificity of 100%. Thus, INNO-LiPA was highly accurate in detecting RIF resistance in culture isolates. By contrast, the four studies that applied INNO-LiPA directly to clinical specimens (i.e., sputum) had 100% specificity and sensitivity estimates ranged between 80 and 100%. Thus, the evidence from this systematic review suggested that INNO-LiPA is a highly sensitive and specific test for the detection of RIF resistance in culture isolates. The test, however, appears to have relatively lower sensitivity when used directly on clinical specimens.
More recently, Ling and colleagues performed a systematic review and used meta-analysis methods to summarize the evidence on the GenoType MTBDR and MTBDRplus assays for rapid detection of RIF and INH resistance Citation[9]. They identified 14 comparisons for RIF and 15 comparisons for INH in ten articles that used GenoType MTBDR assays. After meta-analysis, the pooled sensitivity (98.1%; 95% confidence interval [CI]: 95.9–99.1) and specificity (98.7%; 95% CI: 97.3–99.4) estimates for RIF resistance were very high, and consistent across all groups, assay versions (GenoType MTBDR and GenoType MTBDRplus assays) and specimen types (i.e., clinical specimen vs culture isolate). The accuracy for INH was variable, with sensitivity lower (84.3%; 95% CI: 76.6–89.8) and more inconsistent than specificity (99.5%; 95% CI: 97.5–99.9). Thus, the evidence suggested that GenoType MDTBR assays had excellent accuracy for RIF resistance, even when used directly on clinical specimens. While specificity was excellent for INH, sensitivity estimates were modest and variable.
With overall performance characteristics that are superior to conventional culture and drug susceptibility testing, and the possibility for high throughput with potential cost savings, line probe assays have the potential to greatly enhance MDR-TB diagnosis and treatment. However, evidence on accuracy alone is insufficient to make clinical and public health policies. Available evidence from systematic reviews must be considered together with data from field demonstration projects in real-world settings Citation[10]. In fact, field projects are already providing useful data, such as feasibility, affordability, cost and clinical impact Citation[10]. In addition to collecting field data, research is also necessary to develop rapid molecular assays for the diagnosis of XDR-TB. Clearly, the diagnosis of XDR-TB is a tougher challenge, especially since it requires additional data on resistance to several second-line drugs. Unfortunately, second-line drug-resistance testing is not well standardized and gene mutations for second-line drugs are also not well characterized Citation[11].
From evidence to policy
Based on currently available evidence and expert opinion, the WHO recently issued a policy statement on molecular line probe assays for the rapid screening of patients at risk of MDR-TB Citation[101]. In this statement, the WHO endorses the use of line probe assays and provides several guiding principles that need to be considered while adopting the use of these tests in TB programs at the country level (Box 1). The policy statement also outlines the agenda for future research.
On 30 June 2008, a major initiative was announced by the WHO, the Stop TB Partnership, UNITAID and the Foundation for Innovative New Diagnostics (FIND) to improve the diagnosis and treatment of MDR-TB in resource-limited countries Citation[102]. As part of this initiative, over the next few years, 16 countries will begin using rapid methods to diagnose MDR-TB, including line probe assays. These countries will receive the tests at specially negotiated prices through the Stop TB Partnership’s Global Drug Facility, which provides countries with both drugs and diagnostic supplies Citation[102].
Expert commentary & five-year view
With strong evidence, new policy directives and involvement of funding agencies, the stage appears to be set for the use of rapid tests for MDR-TB diagnosis. In the next 5 years, the use of rapid molecular assays is expected to increase, especially in countries that are dealing with a dual burden of HIV and MDR-TB. Usually, cost considerations would have greatly limited the use of molecular assays in poor countries. However, with the negotiated pricing and special funding initiatives from agencies such as the WHO, Stop TB Partnership, FIND, UNITAID and industry partners, the situation looks hopeful. In the next 5 years, it will become evident if molecular tools, such as line probe assays, can actually make a clinical and public-health impact in the real world. In the meantime, programmatic field studies and cost–effectiveness data are needed to better understand the real world implications of the new policy Citation[12] and to enable evidence-based MDR-TB diagnosis.
Table 1. Findings from systematic reviews of commercial line probe assays for the diagnosis of drug-resistant tuberculosis.
Box 1. Key elements of the 2008 WHO policy statement on line probe assays.
The use of line probe assays is recommended by the WHO, with the following guiding principles:
• Adoption of line probe assays for rapid detection of multidrug-resistant tuberculosis (MDR-TB) should be decided by Ministries of Health within the context of country plans for appropriate management of MDR-TB patients, including the development of country-specific screening algorithms and timely access to quality-assured second-line anti-TB drugs.
• Line probe assay performance characteristics have been adequately validated in direct testing of sputum smear-positive specimens and on isolates of Mycobacterium tuberculosis complex grown from smear-negative and smear-positive specimens. Direct use of line probe assays on smear-negative clinical specimens is not recommended.
• The use of commercial line probe assays, rather than in-house assays, is recommended to ensure reliability and reproducibility of results, as in-house assays have not been adequately validated or used outside limited research settings. Any new or generic line probe assays should be subject to adequate validation, that is, published laboratory validation studies, adequate data to allow systematic review and meta-analysis (including assessment of data quality), and results from field demonstration projects documenting feasibility and consistent performance equal to conventional methods and commercial line probe assays.
• Adoption of line probe assays does not eliminate the need for conventional culture and drug-susceptibility testing (DST) capability; culture remains necessary for definitive diagnosis of TB in smear-negative patients, while conventional DST is required to diagnose extensively drug-resistant TB (XDR-TB). However, the demand for conventional culture and DST capacity may change, based on the local epidemiological situation and the use of line probe assays in country-specific screening algorithms.
• As current line probe assays only detect resistance to rifampicin and/or isoniazid, countries with documented or suspected cases of XDR-TB should establish or expand conventional culture and DST capacity for quality-assured susceptibility testing of second-line drugs, based on current WHO policy guidance.
• Adoption of line probe assays for the rapid detection of MDR-TB should be phased in, starting at national/central reference laboratories or those with a proven capability to conduct molecular testing. Once this has been accomplished, expansion could be considered within the context of country laboratory strengthening plans, and considering availability of suitable personnel in peripheral centers, quality of specimen transport systems and country capability to provide appropriate treatment and management of MDR-TB patients once diagnosed.
• Adequate and appropriate laboratory infrastructure and equipment should be provided, ensuring that the required precautions for biosafety and prevention of contamination are met.
• Appropriate laboratory staff should be trained to conduct line probe assay procedures, especially those relating to amplification and interpretation of results. Supervision of staff by a senior individual with adequate training and experience in molecular assays is strongly recommended.
• A detailed commercial sales contract and customer support plan should be negotiated with manufacturers, guaranteeing an ample and continuous supply of materials, appropriate shipment conditions, customs clearance, equipment installation, maintenance, repair and replacement, and provision of training and ongoing technical support.
• Stringent laboratory protocols, standard operating procedures for molecular line probe assays and internal quality control mechanisms must be implemented and enforced. A program for external quality assessment of laboratories involved in line probe assays should be developed as a matter of priority.
• Mechanisms for rapid reporting of line probe assays results to clinicians must be established to provide patients with the benefit of an early diagnosis.
• The WHO and partners should assist countries with operational plans to introduce line probe assays within the appropriate epidemiological and resource availability context.
Key issues
• An estimated 400,000 new cases of multidrug-resistant tuberculosis (MDR-TB) occur each year and, although their exact incidence rates are currently unknown, extensively drug-resistant TB (XDR-TB) cases have been detected in every country where there is the capacity to detect them.
• Molecular assays to detect gene mutations that signal drug resistance are widely recognized as being most suited for rapid diagnosis of MDR-TB.
• Among the molecular assays, line probe assays have shown great promise. Currently, two line probe assays are commercially available: the INNO-LiPA® Rif. TB kit (Innogenetics NV, Gent, Belgium) and the GenoType® MTBDRplus assay (Hain Lifescience GmbH, Nehren, Germany).
• Evidence from a systematic review showed that INNO-LiPA is a highly sensitive and specific test for the detection of rifampicin resistance in culture isolates. The test, however, appeared to have relatively lower sensitivity when used directly on clinical specimens.
• A recent meta-analysis showed that MDTBR assays had excellent accuracy for rifampicin resistance, even when used directly on clinical specimens. While specificity was excellent for isoniazid, sensitivity estimates were modest and variable.
• Based on currently available evidence and expert opinion, the WHO recently endorsed the use of molecular line probe assays for rapid screening of patients at risk of MDR-TB.
• Special initiatives have been announced to make line probe assays accessible and affordable for resource-limited countries.
Financial & competing interests disclosure
M Pai is an external consultant for the FIND, Geneva, Switzerland, a nonprofit agency that partners with several industries (including Hain LifeScience, Germany) for the development and evaluation of new diagnostic tools for neglected infectious diseases. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Notes
Source: WHO 2008 Citation[101].
References
- World Health Organization. Global tuberculosis control. Surveillance, planning, financing. WHO Report 2007. WHO/HTM/TB/2007.376. World Health Organization, Geneva, Switzerland, 1–242 (2007).
- World Health Organization. The Global MDR-TB and XDR-TB Response Plan, 2007–2008. WHO/HTM/TB/2007.387. World Health Organization, Geneva, Switzerland (2007).
- Zignol M, Hosseini MS, Wright A et al. Global incidence of multidrug-resistant tuberculosis. J. Infect. Dis.194(4), 479–485 (2006).
- Migliori GB, Matteelli A, Cirillo D, Pai M. Diagnosis of multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis: current standards and challenges. Can. J. Infect. Dis. Med. Microbiol.19(2), 169–172 (2008).
- Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: part 2. Active tuberculosis and drug resistance. Expert Rev. Mol. Diagn.6(3), 423–432 (2006).
- Migliori GB, Loddenkemper R, Blasi F, Raviglione MC. 125 years after Robert Koch’s discovery of the tubercle bacillus: the new XDR-TB threat. Is “science” enough to tackle the epidemic? Eur. Respir. J.29(3), 423–427 (2007).
- Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J. Infect. Dis.196(Suppl. 1), S15–S27 (2007).
- Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect. Dis.5, 62 (2005).
- Ling DI, Zwerling A, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur. Respir. J. DOI 10.1183/09031936.00061808 (2008) (Epub ahead of print).
- Barnard M, Albert H, Coetzee G, O’Brien R, Bosman ME. Rapid molecular screening for MDR TB in a high volume public health laboratory in South Africa. Am. J. Respir. Crit. Care Med. (2008).
- Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur. Respir. J.25(3), 564–569 (2005).
- Pai M, Ramsay A, O’Brien R. Evidence-based tuberculosis diagnosis. PLoS Med.5(7), e156 (2008).
Websites
- World Health Organization. Policy statement. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB). World Health Organization, Geneva, Switzerland, 2008 www.who.int/tb/features_archive/policy_statement.pdf
- World Health Organization. Rapid tests for drug-resistant TB to be available in developing countries. World Health Organization, Geneva, Switzerland, 2008 www.who.int/tb/features_archive/mdrtb_rapid_tests/en/index.html