Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) was first recognized in 2012 and since then has resulted in cases in 23 countries in four continents. The majority of these cases were reported from the Kingdom of Saudi Arabia. The disease caused a spectrum of illness, from asymptomatic to severe and possibly fatal disease. Recent studies showed that the transmission of MERS-CoV among family contacts remains relatively low. Currently, there are no approved vaccines or therapeutics for MERS-CoV.
The first Middle East respiratory syndrome coronavirus (MERS-CoV) case was a 60-year-old Saudi businessman, previously healthy, non-smoker. He was admitted on 10 June 2012 to a local hospital in Bisha, Kingdom of Saudi Arabia (KSA) with 1-week history of cough, fever and shortness of breath Citation[1,2]. Community-acquired pneumonia was confirmed on admission. He subsequently left that hospital and was admitted to a hospital in Jeddah on 13 June 2012. His course was significant for the development of adult respiratory distress syndrome on day 2 and acute renal injury requiring hemodialysis on day 3. The patient had rapidly deteriorating clinical course resulting in death on 24 June 2012 Citation[1,2]. A new β coronavirus was isolated from the patient at the Department of Viroscience of the Erasmus Medical Centre in Rotterdam, the Netherlands, 3 months later; the virus was first called human coronavirus Erasmus Medical Center virus Citation[3] then renamed MERS-CoV by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses Citation[4]. Since the initial case of MERS-CoV infection, a total of 909 laboratory-confirmed cases of infection with MERS-CoV, including 331 (36.4%) deaths were reported to the WHO Citation[5].
Epidemiology
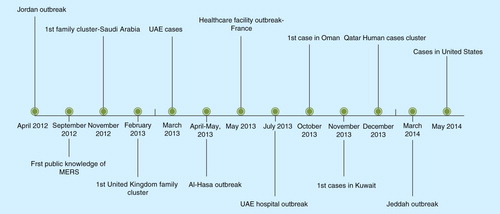
Globally, as of November 2014, the virus has been reported in 23 countries in four continents. The number of countries reporting MERS-CoV in the Middle East is 10 (Saudi Arabia, United Arab Emirates, Qatar, Jordan, Oman, Kuwait, Egypt, Yemen, Lebanon, Iran), eight countries in Europe (Turkey, Austria, UK, Germany, France, Italy, Greece, The Netherlands) and other countries including Tunisia, Algeria, Malaysia, the Philippines and the USA Citation[6]. In the KSA, from 1 June 2012 to 18 September 2014, there were a total of 748 MERS cases reported Citation[7]. All cases outside the Middle East were imported following travel to the Middle East. Timeline of the major event in MERS-CoV infection is shown in . The majority of these cases were among males (61% males vs 39% among females) and 27% were between 46 and 60 years of age . The male to female ratio was initially 2.8:1 to 3:1 Citation[8–10]. The change in the ratio is likely related to the initial involvement of hemodialysis unit with predominance of males. In 2014, a large cluster of cases occurred in Jeddah, KSA. Between February and 26 April 2014, there were a total of 128 MERS-CoV patients in Jeddah treated in 14 hospitals. One hospital had 45 cases and other hospitals had fewer patients Citation[11]. Screening of contacts showed that 7 of 554 household contacts (1.3%) were positive by polymerase chain reaction for MERS-CoV Citation[11]. The outbreak was caused by biologically unchanged viruses in connection with nosocomial transmission Citation[12]. The mortality rate was higher during the Al-Hasa outbreak in 2012 than the latest outbreak in Jeddah (65 vs 40%) Citation[9,10,6]. MERS-CoV has three patterns of transmission: sporadic community cases with presumed non-human exposure Citation[13], family clusters resulting from contact with infected family index case Citation[14–17] and healthcare acquired infections between patients and from patients to healthcare workers Citation[9,12,18–23]. Thus, any investigation of MERS-CoV cases should aim to investigate those contacts in the community and the healthcare setting. The first MERS-CoV case was that of a 60-year-old Saudi businessman who was admitted to a local hospital in Bisha and then he traveled to Jeddah Citation[2]. An extensive contact investigation of this case included 53 contacts: household contacts (3 wives, 10 sons, 11 daughters, 12 grandchildren and a housemaid), healthcare worker contacts (11 nurses and 3 physicians) and 2 shepherds, as well as investigation of the camels (n = 5) that he owned . All obtained samples were negative for MERS-CoV by PCR.
Table 1. Total number of cases in countries reporting Middle East respiratory syndrome coronavirus in 2014.
Figure 2. Number (and percent) of MERS-CoV cases per age group as reported from the Kingdom of Saudi Arabia from 1 June 2012 to 18 September 2014.
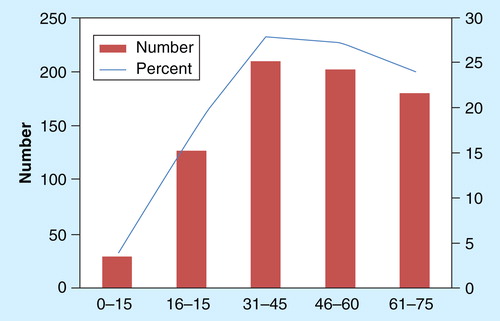
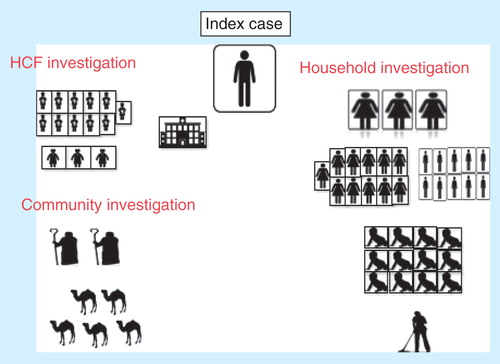
Figure 3. Investigation of MERS-CoV cases has multiple lines of investigation of the contacts of index case and includes contacts in the community, healthcare setting and household contact. Healthcare facility (HCF) investigation includes the investigation of all exposed healthcare workers and other exposed patients. Community investigation included the investigation of family contacts and the evaluation of any animal exposure, such as camels. Household investigation includes the investigation of all family members irrespective of the age group. The figure below shows the extent of the investigation of the first MERS-CoV case that originated in Bisha that included 53 contacts: household contacts (3 wives, 10 sons, 11 daughters, 12 grandchildren, and a housemaid), healthcare worker contacts (11 nurses and 3 physicians), and 2 shepherds, as well as investigation of the camels (N = 5) that he owned.
Clinical features & diagnosis
MERS-CoV infection causes a spectrum of illness ranging from asymptomatic to mild disease to severe and fatal disease. A full review of the clinical picture of MERS was recently published Citation[8,24–26]. MERS-COV causes two distinct forms of disease, severe bilateral pneumonia with multi-organ failure and mild/asymptomatic disease. Severe disease is usually seen in primary index cases, immunocompromised and people with underlying comorbidities and older patients. However, few severe cases were seen in healthy and young patients as well Citation[27]. Mild or asymptomatic disease is seen in secondary cases, usually in young patients, and in the previously healthy individuals. shows the outcome of index cases in 14 different clusters. The all primary cases were males, with a mean age of 54 (range, 24–83) years. There were a total of 64 secondary cases.
Table 2. The outcome of index cases in different clusters.
The initial healthcare associated cluster in Al-Hasa was identified based on severe cases and a high mortality rate of 65% Citation[9]. Subsequent analysis of 47 cases also showed a high mortality rate Citation[10]. Asymptomatic contacts were not included initially in the laboratory screening, but were included later on, and this change in screening resulted in 29% of cases to be asymptomatic patients. The case fatality rate decreased from 65 to 30% Citation[8–10,28–32], as more asymptomatic cases were included in the screening protocols. This increase in percentage is related to the change in the laboratory screening of patients with severe MERS initially to include asymptomatic contacts as well. Initial MERS-CoV cases were reported in the hemodialysis patients Citation[9]. The presence of acute renal injury was documented in 12 patients Citation[33]. Acute renal injury occurred after a median time to onset of 11 days Citation[33]. The excretion of MERS-CoV in urine may be a source of transmission, especially in those with unrecognizable exposure. The diagnosis of MERS-CoV infection relies on the identification of MERS-CoV by PCR. MERS-CoV PCR has been standardized and works extremely well with respiratory samples in experienced laboratories. If MERS-CoV infection is suspected and initial testing is negative, then repeat testing is indicated. The performance of the test is best with lower respiratory tract samples than upper respiratory specimens Citation[34]. There are multiple serology kits being developed and validated with good results Citation[35]. Serologic evaluation of 130 blood donors sampled in 2012 in Jeddah and 226 slaughterhouse workers in October 2012 (after the virus was identified) in Jeddah and Makkah, KSA, showed only eight reactive serology against recombinant spike proteins of human coronaviruses other than MERS-CoV Citation[36]. In another study, none of 268 tested samples, May 2010–2011 and December 2012, was positive for neutralizing MERS-CoV antibodies Citation[37]. Another study from KSA in 2013 showed that seroprevalence among household contacts of MERS-CoV infected patients was 4% of 280 contacts Citation[38].
Genomic analysis
Genomic analysis of the MERS-CoV from patients with known dates and locations helps answer specific questions: how fast the virus changes, when the virus began circulating in its current form, the virus adaptation to humans and those geographical patterns may help locate an animal source. Two papers from KSA reported more than 60 genome sequences. The first report described 21 sequences combined with other nine published MERS-CoV genomes Citation[18]. Phylogenetic analysis showed that there are three distinct MERS-CoV genotypes in Riyadh and the zoonotic reservoir was geographically dispersed. It was also shown that Al-Hasa cluster Citation[39] and the community outbreak in Hafr-Al-Batin Citation[16] was caused by more than one introduction of the virus. The second paper estimated the rate of MERS-CoV evolution to be 1.12 × 10–3 substitutions/site per year (95% CI of 8.76 × 10–4 to 1.37 × 10–3) Citation[39]. The time of the Most Recent Common Ancestor was estimated to be March 2012 (95% CI, December 2011; June 2012) Citation[39]. In another estimate, it is thought Most Recent Common Ancestor is the end of 2010 Citation[40]. Phylogenetic analysis showed that the source is geographically dispersed Citation[18,39].
Animal (putative) reservoirs & risk factors for zoonotic transmission
The analysis of an African bat virus related to MERS-CoV showed evidence that this virus was the ancestor of camel MERS-CoV and that camels may act as a mixing vessel for MERS-CoV Citation[41]. The contribution of camels to the epidemiology of MERS-CoV was recently reviewed Citation[8,24–26]. Of all the studies, between 0 and 100% of tested camel sera (depending on country of origin) were positive for MERS-CoV antibodies Citation[8,24–26] depending on the country of the study Citation[42–52]. MERS-CoV was also isolated from camels by PCR in few instances Citation[44,53,54]. Experimental infection of three camels with human isolates of MERS-CoV was recently published Citation[55]. The infection caused transient upper respiratory tract infection and camels shed viable MERS-CoV in large quantities up to 7 days and viral PCR was positive for 35 days Citation[55]. Juvenile camels had a lower seroprevalence of MERS-CoV antibodies than adult (>2 years) camels, indicating that camels get infection later in life Citation[8,24–26]. Since not all primary cases have a link to camels, another source of infection may yet be revealed. In a recent report of 70 human MERS-CoV infections, only 1 (1.7%) had contacts with camels Citation[30]. In a previous summary of 161 cases, 49 (30.4%) had contact with animals, 7 (4.3%) had contact with camels and another 7 (4.3%) had contact with sheep Citation[29]. Recent reports showed that a patient was in contact with a diseased camel Citation[56], and one patient treated a sick camel and that two MERS-CoV from the patient and the camel were identical Citation[53,54].
Transmission of MERS-CoV among family contacts
The transmission of MERS-CoV among family contacts seems to be limited. Initial data suggested that the transmission of MERS-CoV among family members was 11–19% based on screening of a small number of contacts of 28–42 family members Citation[14–16]. Screening of 462 family members revealed a positivity rate of only 3% by PCR Citation[57]. Further screening of household contacts utilizing both PCR and serology revealed a positivity of 12 out of 280 contacts, showing a positivity rate of only 4% Citation[38].
Transmission of MERS-CoV within the healthcare facility
In KSA, from 1 June 2012 to 18 September 2014, a total of 748 MERS cases were reported, and 27% were among healthcare workers Citation[7]. Studies of the transmission of MERS-CoV in healthcare setting revealed a low transmission rate. The investigation of healthcare worker contacts of the first MERS case in KSA showed that none of the 48 case-contact and 48 non case-contacts was positive for MERS-CoV by HKU5.2N nucleocapsid enzyme immunoassay Citation[58]. Upper respiratory symptoms developed among 27% of case-contact compared to 33% of non-case-contacts Citation[58]. Of the 48 case-contact, 87.5% had surgical mask and 33% had N-95 mask Citation[58]. Contact investigation of healthcare workers (HCWs) of MERS-CoV patients also revealed no transmission in two other studies Citation[59,60]. Transmission of MERS-CoV to HCWs was confirmed in other studies Citation[9]. In an investigation of >200 HCW, two laboratory-confirmed cases were identified Citation[9].
The retrospectively identified Jordanian outbreak took place in April 2012 and involved 13 people (including 10 healthcare workers) Citation[21]. The investigation revealed two laboratory-confirmed and 11 probable cases Citation[21]. Further serologic investigation of this outbreak showed that 7 of 124 (6%) contacts were positive Citation[20]. Of the exposed healthcare workers, 6 (10%) were positive and none recalled developing respiratory symptoms Citation[20]. During Al-Hasa outbreak, between 1 April and 23 May 2013, a total of 23 cases with MERS-CoV were identified, and all confirmed cases and 11 probable cases were part of a single cluster involving four healthcare facilities Citation[9]. The most recent outbreak in Jeddah (February–April 2014) involved 128 MERS-CoV patients in 14 hospitals Citation[11], where the infection was amplified in the healthcare setting Citation[12,61].
Controversies exist regarding the best infection control measures for MERS-CoV. WHO advocates contact and droplet precautions Citation[62], whereas the US and the European CDC calls for additional airborne infection isolation precautions Citation[63,64]. This recommendation is also favored by WHO when dealing with aerosol-generating procedures Citation[62]. The Al-Hasa outbreak involved a hemodialysis unit, and the outbreak was controlled with the use of contact and droplet precautions Citation[9].
Molecular mechanism by which MERS-CoV enters target cells
A number of cell lines are susceptible to MERS-CoV and include human-derived Calu-3, HFL, Caco-2, Huh-7, HEK and His-1 cell lines Citation[65]. Dipeptidyl peptidase 4 was found to be a critical factor for MERS-CoV entry into cells Citation[66]. It seems that MERS-CoV prefers human dipeptidyl peptidase 4 over bat dipeptidyl peptidase 4 Citation[67]. In addition, cellular proteases such as Type II transmembrane serine protease and members of the cathepsin family are believed to be activators of the viral spike (S) glycoprotein Citation[68]. MERS-CoV entry into host cells depends on the viral spike protein and MERS-CoV spike protein is a substrate for proprotein convertases Citation[69]. MERS-CoV spike protein site is subjected to proteolytic process by furin during protein biosynthesis, Citation[70],. The use of the viral S protein allows MERS-CoV to enter cells within endosomes or at the plasma membrane depending on cell type Citation[71]. Camostat, a serine protease inhibitor, is able to block MERS-CoV cell entry in vitro Citation[72]. Furthermore, inhibition of MERS-CoV is blocked by interferon-inducible transmembrane proteins Citation[73].
Currently available antiviral agents & vaccines
Currently, there are no proven therapies for MERS-CoV infection. Useful antiviral agents for (MERS-CoV) infection include neutralizing antibody, convalescent plasma, polyclonal human immunoglobulin, Equine F(ab’)2 antibody fragments, anti-S monoclonal antibodies and interferons Citation[74]. Other medications could be repurposed for use against MERS-CoV, including ribavirin, protease inhibitors (lopinavir, nelfinavir), cyclophilin inhibitors (cyclosporine, alisporivir), chloroquine, mycophenolic acid, nitazoxanide, recombinant human mannose-binding lectin and siRNA to key MERS-CoV genes Citation[74]. Approved antiviral agents can be repurposed for use against emerging viral infections to shorten the time from virus discovery to treatment availability Citation[75]. The use of interferon and ribavirin was tried in five patients with MERS-CoV infection. The median time from admission to therapy with these two agents was 19 days, and thus any benefit effect might have not been evident Citation[76]. A larger number of 20 patients received ribavirin and interferon treatment at a median of 3 days Citation[77]. The therapy resulted in improved 14-day survival of 70% (14 of 20 patients) in the treatment group, compared to 29% (7 of 24 of patients) in the comparison group (p = 0·004) with no survival advantage at 28 days (30 vs 17%, p = 0·54) Citation[77].
In an experimental model of MERS-CoV infection in mice, a recombinant receptor-binding domain (rRBD) of MERS-CoV spike (S) glycoprotein in association with Fc of human IgG (RBD-Fc) induced neutralizing antibodies Citation[78,79]. Intranasal and subcutaneous MERS-CoV RBD-Fc vaccination resulted in similar systemic humoral immune responses Citation[80]. A higher systemic cellular immune response and local mucosal immune responses were achieved with intranasal vaccination Citation[80]. Other studies showed similar findings of antigenic property of a rRBD protein of MERS-CoV spike (S) Citation[78,81–83]. Moreover, three adjuvants–alum, IFA, CpG and poly (I:C)–in rRBD subunit vaccines were effective in producing RBD-specific cellular and humoral immune responses Citation[84]. Since the N-terminus RBD of (S) glycoprotein is conserved in currently circulating MERS-CoV strains, this terminus is considered a possible vaccine candidate Citation[85].
Unanswered questions & future directions
Person-to-person transmission of MERS-CoV is definite. However, the exact route of transmission needs to be better defined as it is still not clear. What contribution asymptomatic patients have to the transmission cycle of infection and how long patients remain infectious still needs to be defined? In an evaluation of the pattern of transmission of MERS-CoV, it was speculated that the current epidemiology of the disease may be secondary to human to human transmission with a large proportion of undetected cases Citation[59]. Since screening of a large number of household contacts revealed relatively low rate of positive cases Citation[36], the contribution of those cases to the transmission might have been overlooked Citation[86]. In an analysis of the viral shedding of MERS-CoV, about one-third of the contacts shed the virus for 30 days Citation[87]. Further studies and research are needed to develop effective vaccines for MERS-CoV infection.
Expert commentary & five-year view
In the following months or years, it is important to understand key elements in the transmission of MERS-CoV and therapy. It is also important to identify the exact source and the role of camels in the transmission. The exact means of transmission from camels to humans needs to be elucidated. The contribution of any intermediate hosts should be further studied. The factors associated with the risk of transmission from animal to humans are essential to understand to further prevent the disease. It is essential to comprehend what happened that led to camel–human transmission. Understanding the risk factors for such transmission within the community would further enhance the ability to prevent animal to human transmission of MERS-CoV. The factors leading to transmissions within healthcare facilities should be further delineated. The best strategies to prevent the transmission within healthcare facilities are of paramount importance to prevent the transmission and amplification of the infection in these settings. Enhancing preventive measures within healthcare facilities would contribute to the understanding of the contribution of asymptomatic individuals in the propagation of the disease. The development of effective vaccines and therapies is of paramount importance to further limit the disease. Using new or repurposed medications for the therapy and potentially as a prophylaxis is highly welcomed to curtail the spread of disease. Identifying the risk factors for progression to severe disease and those factors important to render infection short will be facilitated by elucidating the pathogenesis of MERS-CoV infection.
Middle East respiratory syndrome coronavirus (MERS-CoV) continues to cause disease with amplification in healthcare setting.
The best effective therapy for MERS remains unknown.
There are three main patterns of transmission of MERS-CoV.
Phylogeographic analyses suggested that the zoonotic reservoir was geographically disperse.
The recognition of asymptomatic cases decreased the case fatality rate.
Transmission of MERS-CoV among household contacts seems to be low.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Notes
References
- Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012;367:1814-20
- Memish ZA, Alhakeem R, Stephens GM. Saudi Arabia and the emergence of a novel coronavirus. East Mediterr Health J 2013;19(Suppl 1):S7-11
- van Boheemen S, de Graaf M, Lauber C, et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 2012;3:6
- de Groot RJ, Baker SC, Baric RS, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 2013;87(14):7790-2
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) – update. Disease Outbreak News. 7 November 2014. [Accessed 10 November 2014]
- ECDC. Epidemiological update: Middle East respiratory syndrome coronavirus (MERS-CoV). Available from: www.ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?List=8db7286c-fe2d-476c-9133-18ff4cb1b568&ID=1102
- CCC. Saudi MOH. Available from: www.moh.gov.sa/en/CCC/PressReleases/Pages/Statistics-2014-09-18-002.aspx
- Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus: epidemiology and disease control measures. Infect Drug Resist 2014;7:281-7
- Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 2013;369(5):407-16
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 2013;13(9):752-61
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update as of 9 May 2014. Available from: www.who.int/csr/disease/coronavirus_infections/MERS_CoV_Update_09_May_2014.pdf?ua=1
- Drosten C, Muth D, Corman V, et al. An observational, laboratory-based study of outbreaks of MERS-Coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis 2015;60(3):369-77
- WHO. WHO risk assessment. Middle East respiratory syndrome coronavirus (MERS-CoV). 24 April 2014. Available from: www.who.int/csr/disease/coronavirus_infections/MERS_CoV_RA_20140424.pdf?ua=1
- Memish ZA, Zumla AI, Al-Hakeem RF, et al. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med 2013;368(26):2487-94
- Omrani AS, Matin MA, Haddad Q, et al. A family cluster of Middle East Respiratory Syndrome Coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis 2013;17(9):e668-72
- Memish ZA, Cotten M, Watson SJ, et al. Community case clusters of Middle East respiratory syndrome coronavirus in Hafr Al-Batin, Kingdom of Saudi Arabia: a descriptive genomic study. Int J Infect Dis 2014;23:63-8
- World Health Organization. Middle East Respiratory Syndrome Coronavirus, Joint Kingdom of Saudi Arabia/WHO mission. Available from: www.who.int/csr/disease/coronavirus_infections/MERSCov_WHO_KSA_Mission_Jun13_pdf [Last accessed 14 July 2014]
- Cotten M, Watson SJ, Kellam P, et al. Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study. Lancet 2013;382(9909):1993-2002
- Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in healthcare workers. N Engl J Med 2013;369(9):884-6
- Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis 2014;59(9):1225-33
- Hijawi B, Abdallat M, Sayaydeh A, et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J 2013;19(Suppl 1):S12-18
- WHO. WHO concludes MERS-CoV mission in Saudi Arabia. Available from: www.emro.who.int/media/news/mers-cov-mission-saudi-arabia.html [Last accessed 15 July 2014]
- ECDC. Severe respiratory disease associated with Middle East respiratory syndrome coronavirus (MERS-CoV) Ninth update, 24 April 2014. Available from: www.ecdc.europa.eu/en/publications/Publications/Middle-East-respiratory-syndrome-coronavirus-risk-assessment-25-April-2014.pdf [Last accessed 15 July 2014]
- Al-Tawfiq JA, Zumla A, Memish ZA. Travel implications of emerging coronaviruses: SARS and MERS-CoV. Travel Med Infect Dis 2014;12(5):422-8
- Al-Tawfiq JA, Zumla A, Memish ZA. Coronaviruses: severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus in travelers. Curr Opin Infect Dis 2014;27(5):411-17
- Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus: transmission and phylogenetic evolution. Trends Microbiol 2014;22(10):573-9
- Memish ZA, Al-Tawfiq JA, Assiri A, et al. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J 2014;33(9):904-6
- Penttinen PM, Kaasik-Aaslav K, Friaux A, et al. Taking stock of the first 133 MERS coronavirus cases globally – Is the epidemic changing? Euro Surveill 2013;18:39
- The WHO MERS-CoV Research Group. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr 2013;5
- Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis 2014;29:301-6
- WHO. Middle East respiratory syndrome coronavirus (MERS-CoV) – update, 13 June. 2014. Available from: www.who.int/csr/don/2014_06_13_mers/en/ [Last accessed 16 July 2014]
- WHO. Middle East respiratory syndrome coronavirus (MERS-CoV) – update, 26 June. 2014. Available from: www.who.int/csr/don/2014_06_26/en/ [Last accessed 16 July 2014]
- Eckerle I, Müller MA, Kallies S, et al. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol J 2013;10:359
- Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis 2014;210(10):1590-4
- Zumla A, Al-Tawfiq JA, Enne VI, et al. Rapid point of care diagnostic tests for viral and bacterial respiratory tract infections-needs, advances, and future prospects. Lancet Infect Dis 2014;14(11):1123-35
- Aburizaiza AS, Mattes FM, Azhar EI, et al. Investigation of anti-middle East respiratory syndrome antibodies in blood donors and slaughterhouse workers in Jeddah and Makkah, Saudi Arabia, fall 2012. J Infect Dis 2014;209(2):243-6
- Gierer S, Hofmann-Winkler H, Albuali WH, et al. Lack of MERS coronavirus neutralizing antibodies in humans, eastern province, Saudi Arabia. Emerg Infect Dis 2013;19(12):2034-6
- Drosten C, Meyer B, Müller MA, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med 2014;371(9):828-35
- Cotten M, Watson SJ, Zumla AI, et al. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. MBio 2014;5:1
- Epidemic. Molecular Epidemiology and Evolution of Viral Pathogens. Emergence, Evolution and Epidemiology. Available from: http://epidemic.bio.ed.ac.uk/coronavirus_analysis
- Corman VM, Ithete NL, Richards LR, et al. Rooting the phylogenetic tree of Middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J Virol 2014;88(19):11297-303
- Reusken CB, Haagmans BL, Müller MA, et al. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis 2013;13(10):859-66
- Reusken CBEM, Messadi L, Feyisa A, et al. Geographic distribution of MERS coronavirus among dromedary camels, Africa. Emerg Infect Dis 2014;20(8):1370-4
- Haagmans BL, Al Dhahiry SH, Reusken CB, et al. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis 2014;14(2):140-5
- Perera RA, Wang P, Gomaa MR, et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill 2013;18:36
- Meyer B, Müller MA, Corman VM, et al. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis 2014;20(4):552-9
- Chu DK, Poon LL, Gomaa MM, et al. MERS coronaviruses in dromedary camels, Egypt. Emerg Infect Dis 2014;20(6):1049-53
- Nowotny N, Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Euro Surveill 2014;19:16
- Alexandersen S, Kobinger GP, Soule G, Wernery U. Middle East respiratory syndrome coronavirus antibody reactors among camels in Dubai, United Arab Emirates, in 2005. Transbound Emerg Dis 2014;61(2):105-8
- Reusken CB, Ababneh M, Raj VS, et al. Middle East Respiratory Syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill 2013;18(50):20662
- Corman VM, Jores J, Meyer B, et al. Antibodies against MERS Coronavirus in Dromedary Camels, Kenya, 1992-2013. Emerg Infect Dis 2014;20(8):1319-22
- Müller MA, Corman VM, Jores J, et al. MERS coronavirus neutralizing antibodies in camels, eastern Africa, 1983–1997. Emerg Infect Dis 2014;20(12):2093-5
- Memish ZA, Cotten M, Meyer B, et al. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis 2014;20(6):1012-15
- Azhar EI, El-Kafrawy SA, Farraj SA, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med 2014;370(26):2499-505
- Adney DR, van Doremalen N, Brown VR, et al. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis 2014;20(12):1999-2005
- Drosten C, Seilmaier M, Corman VM, et al. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis 2013;13(9):745e51
- Hall AJ, Tokars JI, Badreddine SA, et al. Healthcare worker contact with MERS patient, Saudi Arabia. Emerg Infect Dis 2014;20(12):2148-51
- Memish ZA, Al-Tawfiq JA, Makhdoom HQ, et al. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect 2014;20(5):469-74
- Pebody RG, Chand MA, Thomas HL, et al. The United Kingdom public health response to an imported laboratory-confirmed case of a novel coronavirus in September 2012. Euro Surveill 2012;17:20292
- Mailles A, Blanckaert K, Chaud P, et al. First cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill 2013;18:20502
- CCC. Saudi MOH. Available from: www.moh.gov.sa/en/CoronaNew/PressReleases/Pages/default.aspx
- WHO. WHO Statement on the third meeting of the IHR Emergency committee concerning Middle East respiratory syndrome coronavirus (MERS-CoV). Wkly Epidemiol Rec 2013;88:435-6
- ECDC. Available from: www.ecdc.europa.eu/en/press/news/_lay-outs/forms/News_DispForm.aspx?List=8db7286c-fe2d- 476c-9133-18ff4cb1b568&ID=1002
- CDC. Interim infection prevention and control recommendations for hospitalized patients with Middle East respiratory syndrome coronavirus. MERSCoV 15 May 2014. Available from: www.cdc.gov/coronavirus/mers/infectionprevention-control.html#infection-prevention
- Chan JF-W, Chan K-H, Choi GK-Y, et al. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J Infect Dis 2013;207:1743-52
- Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013;495:251-4
- Yang Y, Du L, Liu C, et al. Receptor usage and cell entry of bat coronavirus HKU4 provide insight into bat-to-human transmission of MERS coronavirus. Proc Natl Acad Sci USA 2014;111(34):12516-21
- Welsch K, Winkler M, Meyer B, et al. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2 and is targeted by neutralizing antibodies. J Virol 2013;87:5502-11
- Gierer S, Müller MA, Heurich A, et al. Inhibition of proprotein convertases abrogates processing of the middle eastern respiratory syndrome coronavirus spike protein in infected cells but does not reduce viral infectivity. J Infect Dis 2015;211(6):889-97
- Millet JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci USA 2014;111(42):15214-19
- Qian Z, Dominguez SR, Holmes KV. Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PLoS One 2013;8(10):e76469
- Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol 2013;87(23):12552-61
- Wrensch F, Winkler M, Pöhlmann S. IFITM proteins inhibit entry driven by the MERS-coronavirus spike protein: evidence for cholesterol-independent mechanisms. Viruses 2014;6(9):3683-98
- Zumla A, Memish ZA, Maeurer M, et al. Emerging novel and antimicrobial-resistant respiratory tract infections: new drug development and therapeutic options. Lancet Infect Dis 2014;14(11):1136-49
- Coleman CM, Frieman MB. Treating MERS-CoV during an outbreak. Lancet Infect Dis 2014;14(11):1030-1
- Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis 2014;20:42-6
- Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 2014;14(11):1090-5
- Du L, Kou Z, Ma C, et al. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS–CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLoS One 2013;8:e81587
- Zhao G, Du L, Ma C, et al. A safe and convenient pseudovirus-based inhibition assay to detect neutralizing antibodies and screen for viral entry inhibitors against the novel human coronavirus MERS-CoV. Virol J 2013;10:266
- Ma C, Li Y, Wang L, et al. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: implication for designing novel mucosal MERS vaccines. Vaccine 2014;32:2100-8
- Du L, Zhao G, Kou Z, et al. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol 2013;87:9939-42
- Mou H, Raj VS, van Kuppeveld FJM, et al. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol 2013;87:9379-83
- Yang Y, Deng Y, Wen B, et al. The amino acids 736-761 of the MERS-CoV spike protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents. Viral Immunol 2014;27(10):543-50
- Lan J, Deng Y, Chen H, et al. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS One 2014;9(11):e112602
- Ali MT, Morshed MM, Gazi MA, et al. Computer aided prediction and identification of potential epitopes in the receptor binding domain (RBD) of spike (S) glycoprotein of MERS-CoV. Bioinformation 2014;10(8):533-8
- Gardner LM, MacIntyre CR. Unanswered questions about the Middle East respiratory syndrome coronavirus (MERS-CoV). BMC Res Notes 2014;7:358
- Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis 2014;29:307-8