Drug-induced acute kidney injury (AKI) is an important problem that is frequently encountered by clinicians Citation[1]. Both prescribed and over-the-counter agents have the potential to injure all renal compartments and induce AKI (Box 1)
Hemodynamic insult
Afferent arteriolar vasoconstriction
– NSAIDs, calcineurin inhibitors
Efferent arteriolar vasodilatation
– ACE-inhibitors, angiotensin receptor blockers
Vascular injury
Thrombotic microangiopathy
– Anti-angiogenesis drugs, gemcitabine, mitomycin C
– Interferon, calcineurin inhibitors, m-TOR inhibitors
– Thienopyridines, quinine, oxymorphone (Opana) ER
Vasculitis
– Propylthiouracil, infliximab
– Cocaine containing levamisole
Atheroemboli
– Anticoagulants
Glomerular injury
Membranous
– NSAIDs, gold, bucillamine, penicillamine
Minimal change
– NSAIDs, lithium, interferon, pamidronate, vaccinations
Focal segmental glomerulosclerosis
– Lithium, pamidronate. interferon, sirolimus
– Androgenic anabolic steroids, heroin
– Vaccinations
Lupus-like glomerulonephritis
– Methyldopa, hydralazine, procainamide, quinidine
Tubular injury
Acute tubular injury/necrosis
– Chemotherapeutic agents (platins, ifosfamide, pemetrexed, zoledronate, etc.)
– Anti-microbial agents (aminoglycosides, polymixins, amphotericin B, tenofovir, etc.)
– Many other medications including iron chelating agents
Acute crystalline nephropathy
– Antiviral agents (acyclovir, indinavir, atazanavir)
– Antibacterial agents (sulfonamides, ciprofloxacin)
– Methotrexate, triamterene, ascorbic acid, sodium-phosphate purgatives
Acute osmotic nephropathy
– Intravenous immunoglobulin (sucrose), hydroxyethyl starch, dextran, mannitol
Interstitial disease
Acute interstitial nephritis
– Anti-microbial agents (B-lactams, sulfonamides, fluoroquinolones, etc.)
– NSAIDs, selective cyclo-oxygenase inhibitors
– Proton pump inhibitors
– Many other medications
Hemodynamic AKI
A number of medications cause hemodynamic AKI; however, NSAIDs, including topical gels are one of the most common and important causes Citation[2]. These drugs reduce afferent arteriolar blood flow by antagonizing vasodilatory prostaglandins in patients with true or effective intravascular volume depletion or underlying chronic kidney disease Citation[2]. In this setting, glomerular filtration rate (GFR) drops and AKI develops, which is often rapidly reversible but sometimes associated with ischemic tubular injury Citation[2].
NSAID-induced hemodynamic AKI is recognizable in the at-risk host ingesting these drugs. Increased serum creatinine within 2–5 days, certain electrolyte disturbances (hyponatremia, hyperkalemia, metabolic acidosis) and bland urine sediment (sometimes hyaline casts and renal tubular epithelial cells) support the diagnosis Citation[2,3]. Depending on other comorbidities, increased peripheral and pulmonary edema (heart failure patients) and increased peripheral edema and ascites (cirrhotics) may also be observed Citation[2,3].
Treatment for this form of AKI mandates drug discontinuation and management of the associated comorbidity Citation[2,3]. Therapy includes intravenous fluids for hypovolemic patients, diuretics and specific cardiac care for those with heart failure, and paracentesis and vasopressors for cirrhotics with hepatorenal physiology Citation[2,3]. Depending on the severity of AKI and associated comorbidity, kidney function generally recovers in 3–5 days. Rarely, in a small percentage of patients, dialysis maybe required.
A second form of hemodynamic AKI may be caused by renin–angiotensin system (RAS) antagonism Citation[4], which decreases GFR by lowering arterial blood pressure and dilating the efferent arteriole. While these drugs are nephro-protective in the setting of chronic kidney disease, they have the potential to induce AKI when administered in the setting of volume depletion and prior to radiocontrast exposure and cardiac surgery Citation[5]. Coadministration of NSAIDs and RAS antagonists risk induction of more severe AKI. Prevention of AKI is best achieved by avoiding these drugs in certain clinical situations; however, AKI is generally rapidly reversible with drug discontinuation unless ischemic acute tubular injury (ATI) develops. Some argue, however, that the functional drop in GFR with RAS antagonists is not pathologic and may protect tubular perfusion by enhancing peritubular blood flow Citation[6].
Thrombotic microangiopathy
Thrombotic microangiopathy (TMA) is form of vascular injury that is commonly associated with AKI. A number of medications cause this lesion, including more recently the anti-angiogenesis drugs Citation[7,8]. These agents are widely utilized to treat several cancers and include monoclonal anti-VEGF antibodies, circulating VEGF decoy-receptor molecule and VEGF receptor tyrosine-kinase inhibitors Citation[7,8]. These drugs combat malignancies by targeting the VEGF signaling pathway by which cancers proliferate and disseminate through unregulated tumor angiogenesis Citation[7,8].
Anti-angiogenesis drugs promote adverse renal effects by disturbing the normal housekeeping role and tissue repair of VEGF-associated angiogenesis in the kidneys. VEGF production by podocytes preserves glomerular integrity through paracrine binding of endothelial and epithelial receptors, respectively Citation[9]. This crosstalk between podocytes and endothelial cells maintains the structure and function of the glomerular filtering unit Citation[9]. In experimental models, progressive inhibition of VEGF expression is associated with glomerular endotheliosis and TMA Citation[9].
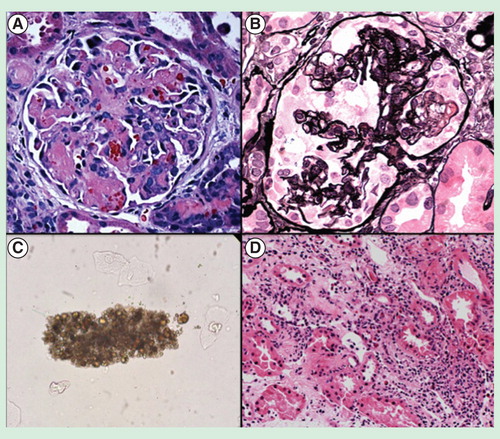
Figure 1. Drug-induced AKI. (A) Glomerulus demonstrating thrombotic microangiopathy from bevacizumab, an anti-VEGF drug. (B) Glomerulus showing collapsing focal segmental glomerulosclerosis from interferon therapy. (C) Urine microscopy demonstrating a methotrexate crystal cast, indicative of crystalline-associated AKI. (D) Acute interstitial nephritis marked by an inflammatory cell infiltrate and edema within the interstitium of the kidney. This lesion occurred from omeprazole exposure.
Anti-angiogenesis drug-associated TMA most often present with proteinuria and hypertension, with varying levels of AKI often supervening Citation[8–11]. This clinical pattern of kidney injury has been described in over 100 patients Citation[12]. In contrast to some other forms of TMA, microangiopathic hemolytic anemia and thrombocytopenia are only observed in ∼ 50% of patients, despite the findings of TMA in the kidney Citation[10,12]. Hypertension occurs in >80% of cases, while proteinuria is nearly universal Citation[11,12]. Mild to severe AKI requiring dialysis may be observed.
Treatment hinges upon blood pressure control and proteinuria reduction, while progressively worsening AKI necessitates drug withdrawal Citation[10,12]. As the development of hypertension often correlates with effective anti-tumor activity Citation[12], nephrologists have to balance drug withdrawal for nephrotoxicity against effective cancer therapy. Thus, hypertension and proteinuria should prompt antihypertensive therapy, whereas AKI is an indication to interrupt drug therapy Citation[10,12]. Drug withdrawal reverses hypertension and may be associated with some improvement of AKI.
Minimal change disease/focal segmental glomerulosclerosis
Visceral epithelial cell (podocyte) injury by medications can result in several glomerular lesions including minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS). IFNs, consisting of IFN-α, IFN-β and IFN-γ, are cytokines that are synthesized by various cells (leukocytes, T-cells, etc.) and are employed to treat multiple medical conditions Citation[13]. All three are administered via either the subcutaneous or intramuscular route and are associated with adverse constitutional symptoms and abnormal laboratory results. Both nephrotic syndrome and AKI are the clinical complications observed with IFN-associated MCD and FSGS Citation[13].
The specific histologic findings associated with IFN therapy are MCD, FSGS-NOS (not otherwise specified) and collapsing FSGS Citation[13]. In IFN-α-treated patients, MCD was observed in six patients, while FSGS-NOS and collapsing FSGS were noted in nine and nine patients, respectively. Fourteen of these 24 patients were being treated for HCV infection. IFN-β was associated with two reports of MCD and three of collapsing FSGS, while there were two reports of collapsing FSGS and one of FSGS-NOS with IFN-γ therapy Citation[13]. IFN therapy in African–Americans has a strong association with collapsing FSGS Citation[14]. Apolipoprotein L1 (APOL1) gene variants confer a greater risk for the development of FSGS and more rapid progression to ESRD in African–Americans Citation[15]. In fact, six African–American patients who developed collapsing FSGS following INF administration were found to be homozygous for high-risk APOL1 alleles Citation[16].
Therapy in those developing MCD or FSGS with IFN mandates drug discontinuation and likely a course of corticosteroids. In the published reports, steroid therapy has been employed in a significant percentage of patients. The combination of drug withdrawal +/– steroid administration was associated with complete or partial remission in all MCD patients, whereas nearly all patients with FSGS-NOS or collapsing FSGS had some improvement in kidney function, although <50% reached partial or complete remission Citation[13].
Crystalline-induced AKI
A number of medications are known to cause AKI from deposition of crystals within renal tubular lumens Citation[17]. For the most part, a perfect storm of drug insolubility in the urine and host risk factors contributes to this renal disorder Citation[17]. In the case of methotrexate-related crystalline-induced AKI, the high dosage required to treat certain malignancies along with patient characteristics, such as true or effective volume depletion and underlying kidney disease are risk factors for the deposition of drug crystals within the kidneys Citation[17].
Methotrexate is increasingly more insoluble in urine as the pH drops progressively below 7.0 Citation[16]. This anti-folate and its metabolites precipitate within the renal tubular lumens in the setting of low urine pH, decreased urinary flow rates from volume depletion and with high urinary methotrexate concentrations Citation[17]. High-dose methotrexate causes kidney injury through multiple possible mechanisms; however, intratubular crystal precipitation is the most likely Citation[18–20]. Diagnosis is suggested when AKI develops in the clinical scenario described and may be supported by visualization of urinary methotrexate crystals .
If preventive measures with alkali-containing intravenous fluids fail, therapy for methotrexate-associated AKI includes drug discontinuation, higher dose leucovorin therapy (based on serum methotrexate concentrations) and continued alkaline fluid administration if the patient is non-oliguric, achieving a urine pH >7.1, and does not have metabolic alkalosis Citation[21].
In the setting of systemic toxicity and advanced AKI, other measures are required. Hemodialysis for 4–6 h utilizing high-flux membranes will clear methotrexate and lower serum levels; however, a rebound in serum levels (∼20%) occurs that necessitates repeat hemodialysis Citation[21]. Anecdotal data suggest that high-cutoff dialysis may improve methotrexate clearance Citation[22]. As dialysis requires the placement of a large bore, dual lumen catheter, it may not be the best option in patients who have thrombocytopenia and other coagulopathies (not to mention bloodstream infection risk in neutropenic patients). In this circumstance, carboxypeptidase-G may be considered to rapidly and safely lower the methotrexate serum levels Citation[21]. Like dialysis, a rebound in serum levels occurs. As this therapy is quite expensive, it should be reserved as last resort.
Acute interstitial nephritis
Drugs are the most common cause of acute interstitial nephritis (AIN) in developed countries, accounting for >75% Citation[18]. While kidney biopsy registries note AIN as the histologic lesion in ∼2–5%, when one examines those with AKI, approximately 15% are due to AIN (ranging from 10 to 27%) Citation[23]. Antimicrobial agents are most commonly associated with AIN; however, NSAIDs and proton pump inhibitors (PPIs) are also common offenders Citation[23]. In particular, PPIs have become one of the most common causes of AIN, especially in the elderly Citation[24,25].
PPIs are widely prescribed and ingested over-the-counter to treat acid-related gastrointestinal diseases Citation[26]. In 1992, omeprazole was described to cause (AIN). Subsequently numerous case reports/series, database studies and population-based case–control studies noted AIN with many different PPIs Citation[24]. In a large nested cohort, the unadjusted odds ratio for AIN was 5.16 for current versus past PPI use Citation[24]. This effect was most pronounced in the elderly, a finding confirmed in another study Citation[25].
PPI-associated AIN is difficult to diagnose as patients rarely have allergic symptoms/signs and may develop AIN many months after exposure, on average 10–11 weeks but as late as 9 months Citation[24–26]. Leukocyturia, hematuria and low-grade proteinuria are common.
Treatment requires drug withdrawal and observation for 3–5 days for improvement. A course of corticosteroids for 4–6 weeks may be required for non-responders and often improves kidney function; however, chronic kidney disease can develop in a significant number of patients Citation[23–26].
Summary
In general, most forms of hospital- and ICU-acquired AKI suffer from a lack of targeted therapies to reverse the underlying injury. However, as briefly reviewed, critical to reversing certain forms of drug-induced AKI is recognition of the clinical problem, discontinuation of the offending agent and, in some cases, administration of intravenous fluids, corticosteroids and other disease-directed therapies.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Notes
References
- Perazella MA. Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 2009;4(7):1275-83
- Perazella MA, Tray K. Selective cyclooxygenase-2 inhibitors: a pattern of nephrotoxicity similar to traditional nonsteroidal anti-inflammatory drugs. Am J Med 2001;111(1):64-7
- Eras J, Perazella MA. NSAIDs and the kidney revisited: are selective cyclooxygenase-2 inhibitors safe? Am J Med Sci 2001;321(3):181-90
- Balasubramanian G, Al-Aly Z, Moiz A, et al. Early nephrologist involvement in hospital-acquired acute kidney injury: a pilot study. Am J Kidney Dis 2011;57(2):228-34
- Coca SG, Garg AX, Swaminathan M, et al. Preoperative angiotensin-converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery. Nephrol Dial Transplant 2013;28(11):2787-99
- Perazella MA, Coca SG. Three feasible strategies to minimize kidney injury in ‘incipient AKI’. Nat Rev Nephrol 2013;9(8):484-90
- Gurevich V, Perazella MA. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med 2009;122:322-8
- Markowitz GS, Bomback AS, Perazella MA. Drug-induced glomerular disease: direct cellular injury. Clin J Am Soc Nephrol 2015. [Epub ahead of print]
- Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 2008;358(11):1129-36
- Izzedine H, Massard C, Spano JP, et al. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur J Cancer 2010;46(2):439-48
- Zhu X, Wu S, Dahut WL, et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 2007;49:186-93
- Izzedine H, Escudier B, Lhomme L, et al. Anti-VEGF associated kidney diseases: a 8-year monocentric observational study. Medicine 2014;93(24):333-9
- Markowitz GS, Nasr SH, Stokes MB, et al. Treatment with interferon-alpha, -beta, or -gamma is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2010;5:607-15
- Feldman D, Hoar RM, Niemann WH, et al. Tubuloreticular inclusions in placental chorionic villi of rhesus monkeys after maternal treatment with interferon. Am J Obstet Gynecol 1986;155:413-24
- Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011;22:2129-37
- Nichols B, Jog P, Lee JH, et al. Innate immunity pathways regulate the nephropathy gene apolipoprotein L1. Kidney Int 2015;87(2):332-42
- Sand TE, Jacobsen S. Effect of urine pH and flow on renal clearance of methotrexate. Eur J Clin Pharmacol 1981;19(6):453-6
- Luciano RL, Perazella MA. Crystalline-induced kidney disease: a case for urine microscopy. Clin Kidney J 2015;8(2):131-6
- Perazella MA. Crystal-induced acute renal failure. Am J Med 1999;106(4):459-65
- Yarlagadda SG, Perazella MA. Drug-induced crystal nephropathy: an update. Exp Opin Drug Safety 2008;7(2):147-58
- Perazella MA, Moeckel GW. Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy. Sem Nephrol 2010;30(6):570-81
- Bertram A, Ivanyi P, Hafer C, et al. High cut-off dialysis as a salvage therapy option in high-dose methotrexate chemotherapy? Ann Hematol 2014;93(6):1053-5
- Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol 2010;6(8):461-70
- Muriithi AK, Leung N, Valeri AM, et al. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int 2015;87(2):458-64
- Blank ML, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int 2014;86(4):837-44
- Brewster UC, Perazella MA. Proton pump inhibitors and the kidney: critical review. Clin Nephrol 2007;68(2):65-72
