Abstract
Primary aldosteronism is the most frequent cause of secondary arterial hypertension. As adrenal imaging has been shown to have only limited value for differential diagnosis, adrenal vein sampling (AVS) has been implemented as a gold standard in the guidelines. However, AVS is a not well-standardized technique, and success rates vary in a wide range. Successful AVS procedures presuppose careful preparation and operational efficiency in an interdisciplinary team. Besides ruling out malignancy, multidetector-row helical computed tomography facilitates the localization of the adrenal veins. Rapid cortisol measurement has been shown to increase cannulation rates. The values of cosyntropin stimulation and bilateral simultaneous versus sequential catheterization remain unclear, but consistency is important. AVS should be performed in specialized centers by a limited number of radiologists in order to ensure success rates of at least 70%. Standardization of cutoff values should be accomplished through a consensus statement for consistent decision-making in patient care.
Medscape: Continuing Medical Education Online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Expert Reviews Ltd. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/expertneurothera; (4) view/print certificate.
Release date: 17 October 2012; Expiration date: 17 October 2013
Learning objectives
Upon completion of this activity, participants will be able to:
• Analyze the epidemiology and diagnosis of primary aldosteronism
• Describe adequate patient selection and preparation for AVS
• Evaluate means to improve the success of AVS
• Interpret the results of AVS effectively
Financial & competing interests disclosure
EDITOR
Elisa Manzotti
Publisher, Future Science Group, London, UK
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME Author
Charles P. Vega, MD
Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine, CA, USA
Disclosure: Charles P. Vega, MD, has disclosed no relevant financial relationships.
Authors and Credentials
Evelyn Fischer, MD
Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Ziemssenstr. 1, 80336 München, Germany
Disclosure: Evelyn Fischer, MD, has disclosed no relevant financial relationships.
Christoph Degenhart, MD
Institut für Klinische Radiologie, Klinikum der Ludwig-Maximilians-Universität München, Germany
Disclosure: Christoph Degenhart, MD, has disclosed no relevant financial relationships.
Martin Reincke, MD
Medizinische Klinik und Poliklinik IV, Klinikum der Universität München, Ziemssenstr. 1, 80336 München, Germany
Disclosure: Martin Reincke, MD, has disclosed the following relevant financial relationships: This work is based on the German Conn's Registry – Else
Kröner-Fresenius Hyperaldosteronism Registry supported by a grant of the Else Kröner-Fresenius-Stiftung to Martin Reincke.
Despite being dismissed as a rare endocrine condition for many decades, primary aldosteronism (PA) is nowadays widely accepted as the most frequent cause of secondary arterial hypertension, certainly due in part to the widespread introduction of the aldosterone to renin ratio as a screening instrument Citation[1,2]. With a prevalence of approximately 5% of hypertensive subjects in primary care and 10% of hypertensives in referral centers, screening strategies should be extended in designated risk populations Citation[3]. With a growing awareness about the cardiovascular and renal comorbidities of sustained aldosterone hypersecretion, and the concomitant possibility of reversing these consequences through an early detection and therapy, the diagnosis of PA should not be delayed Citation[4–7]. Besides rare familial forms, unilateral adrenal hyperplasia and aldosterone-producing carcinoma, the two main causes of PA are aldosterone-producing adenoma (APA) and idiopathic bilateral adrenal hyperplasia (IHA), accounting for more than 95% of the cases. As treatment strategies diverge substantially for these two entities, subtype differentiation is a crucial step towards a tailored therapy of the affected patients. Whereas unilateral adrenalectomy offers the possibility of hypertension cure in APA, IHA is treated very efficiently with mineralocorticoid (MR)-antagonists. High-resolution computed tomography (CT) and MRI have only limited sensitivity and specificity for subtype differentiation Citation[8–12]. Most of APAs are smaller than 10–15 mm; in addition, incidentalomas, which are present in 2–10% of adults, can not be distinguished accurately from APA Citation[13]. A meta-analysis from 38 studies in a total of 950 patients showed that cross-sectional imaging results disagreed with those of adrenal vein sampling (AVS) in a considerable proportion (38%) of the cases Citation[14]. More recent data suggest even higher disagreements between imaging and AVS in up to 73% of cases Citation[15]. The Endocrine Society Practice Guidelines therefore recommend, besides the mandatory use of adrenal imaging to rule out malignancy, the performance of AVS for the differentiation of the subtypes Citation[2]. However, this ‘gold standard’ is a technically challenging and cost-intensive technique, and success rates for selective bilateral catheterization of the adrenal veins vary in a wide range between 31 and 98%, depending on the center and the expertise of the interventional radiologists Citation[15]. Risk of adrenal vein rupture is small, albeit not negligible. In addition, since its introduction in the 1960s, the procedure is not well standardized with regards to the necessity of adrenocorticotropic hormone (ACTH 1–24) stimulation, selectivity and lateralization index cutoffs Citation[16–18]. As a consequence, many centers perform anatomical imaging with CT/MRI as the only diagnostic procedure for surgical planning. Different techniques such as rapid cortisol measurement, simultaneous sampling of both adrenals and ACTH 1–24 stimulation during AVS have been evaluated to optimize the results Citation[15,19–22]. The purpose of this review is to summarize the different strategies for improving the success rates of AVS.
Measures for improving success rates of AVS
Patient selection
The first step towards successful AVS is a rational patient selection. To avoid unnecessary procedures, AVS should only be performed in patients with confirmed PA Citation[2]. The main purpose for performing AVS is the possibility to distinguish between unilateral and bilateral disease, with the option for surgical hypertension cure or improvement in 74–100% of the cases in APA Citation[23]. Patients with IHA can be managed pharmacologically with MR-antagonist treatment with a similar long-term prognosis. The unselective MR antagonist spironolactone has been the drug of first choice for many years. As its dose-dependent progestational and antiandrogenic adverse effects limit the use of spironolactone, especially in male patients, the selective MR-antagonist eplerenone has been shown to be an effective and safe alternative for the treatment of IHA Citation[24,25]. Conversely, unilateral or bilateral adrenalectomy leads to a cure rate of only 15–20% in these patients Citation[24,26]. There is no study comparing the direct outcome of adrenalectomy or pharmacological treatment in patients with APA, but long-term results seem to be similar for clinical surrogate markers such as blood pressure decline and normalization of potassium levels as well as for reversal of end-organ complications Citation[27–29]. Therefore, MR-antagonist treatment can be a therapeutic option for APA patients not eligible for general anesthesia or adrenalectomy because of age, clinical comorbidities or refusal for operation. As a consequence, the Endocrine Society Practice Guidelines recommend that AVS should be offered to all PA patients who are candidates for adrenalectomy in case of unilateral diagnosis and who seek surgical care Citation[2]. The recent AVIS studies analyzing a total of 2604 AVS procedures showed that only 77% of all patients with confirmed PA undergo AVS with a range of 19–100% Citation[18]. Some authors follow the approach to catheterize only those patients with a high clinical probability for APA or in cases of equivocal imaging findings Citation[11,30]. Mulatero et al. tried to establish the clinical and biological criteria to reliably distinguish APA from IHA based on blood pressure, potassium levels and aldosterone to renin ratio Citation[31]. They postulate that the probability of a micro-APA is very low when the adrenals are described as normal by an expert radiologist. In their opinion, therapy with MR antagonists could be initiated without any further diagnostic procedures in such cases Citation[31]. On the other hand, patients younger than 40 years of age, who display a very low chance of being affected by a nonfunctioning nodule, could be sent to unilateral adrenalectomy without AVS in case of a single APA bigger than 1 cm and a normal contralateral gland on adrenal imaging Citation[11,31,32]. Identical postoperative results were reported for patients receiving either AVS and CT scan or only CT scan for differential diagnosis prior adrenalectomy Citation[33,34]. However, a recent study emphasized the importance of AVS before an adrenalectomy whatever the nodule size found by CT, because AVS potentially restored a correct diagnosis and avoided inappropriate surgery in 36% of patients with a unilateral APA and 37% of patients with a macronodule only assessed by CT Citation[35]. Prospective studies analyzing the definitive role of AVS on outcome parameters, such as remission and improvement of hypertension, have not been published Citation[8,11,36].
Preparations before starting AVS
It is known that antihypertensive medication has an impact on the renin–angiotensin–aldosterone system and can thus lead to false-positive or false-negative screening tests in PA Citation[37]. Consequently, antihypertensive medication might influence aldosterone concentration during AVS and as such the lateralization index. This can potentially lead to pitfalls in the differential diagnosis of PA. Systematic studies analyzing the influence of different classes of antihypertensive drugs on AVS results have not been performed. Therefore, recommendations will be based on extrapolation of those studies addressing screening or confirmatory testing. Along this line, it is reasonable that all drugs antagonizing renin suppression should be discontinued preferentially 4 weeks before AVS. This includes MR antagonists, potassium-sparing diuretics such as amiloride or triamterene, loop diuretics or other medications increasing renin secretion in the adrenal glands (i.e., angiotensin-converting-enzyme inhibitors, angiotensin-II receptor blockers and renin inhibitors). Those drugs will obscure the lateralization in unilateral disease through renin-induced aldosterone secretion from the contralateral, initially suppressed adrenal gland, incorrectly assuming idiopathic (bilateral) adrenal hyperplasia. Although the best option would be to test the patient either free of medications, or only on medications with a minimal influence on the renin–angiotensin–aldosterone system (extended release verapamil and peripheral α1-adrenergic receptor antagonists) hypertension has to be medically controlled as clinically required Citation[38,39]. The authors do not suggest β-blockers to be withdrawn because β-blocker-induced suppression of renin levels appears to be uncritical in this setting.
As hypokalemia can lead to reduced aldosterone secretion, potassium levels should be in a normal range during AVS Citation[40]. As exogenous glucocorticoids suppress adrenal cortisol secretion, it is mandatory to stop steroids prior AVS. Whether time of day or fasting state influences AVS results has not been analyzed. Pre-AVS laboratory testing should include potassium, coagulation tests, blood count and creatinine. Contraindications concerning contrast agents (thyroid autonomy, renal insufficiency, pregnancy, metformin intake and allergies) must be taken into account when sending the patient to AVS.
Interdisciplinary procedure
In an analysis of AVS procedure in the German Conn’s Registry, the authors compared success rates for AVS in five German PA centers before and after the introduction of procedures designed to improve rates of successful cannulation. Among other factors, success rates of AVS improved in those centers in which the interdisciplinary team during AVS included the treating endocrinologists Citation[15]. This allows prompt feedback and direct rewarding to the radiologist in case of successful cannulation. The authors postulated that presence of an interdisciplinary team during the procedure could lead to a higher identification with this rather difficult procedure. The interdisciplinary discussion of different strategies may also be helpful in improving AVS quality.
Standard operational procedure
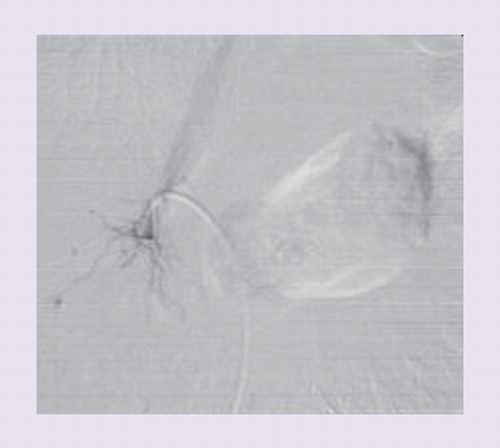
Another measure to improve AVS success rate in the German Conn’s Registry was the introduction of a defined standard operation procedure (SOP) protocol for AVS Citation[15]. It defines preinterventional measures (such as medication, potassium levels and anticoagulation), the intra-interventional procedure and postinterventional measures (discharge of the patient and interpretation of results). The center-specific SOP was introduced in 2008 and includes written directions for all of the participating staff members including radiologists, endocrinologists, treating physicians and laboratory personnel Citation[19]. The exact implementation of every step of the SOP is essential for a functioning AVS team. Besides the exact order of the required blood samples, the protocol requires the preparation and prelabeling of all test tubes before starting AVS to avoid confusion or mix-up of the tubes in the radiology suite or in the laboratory. The SOP gives instructions about placement of the sheath and angiography catheter via the femoral vein. Positioning of the catheter is assessed by venous angiograms before and after selective blood withdrawal. A simultaneous sample from the sheath is collected with each of the selective samples. Samples from the infra- and supra-renal section of inferior vena cava (IVC) are taken in pair with a sample from the sheath. Validated and accurate laboratory assays for cortisol and aldosterone ensure the quality of AVS as well as standardized selectivity and lateralization cutoffs. A specific report developed by the laboratory prevents any misinterpretation of data Citation[41]. Overall, success rate increased from 31 to 61% in German centers using a SOP. In the authors’ center, success rates increased from 57 up to 75% and more recently up to 93%.
Multidetector-row helical CT mapping
Corresponding to the Endocrine Society Practice Guidelines, all patients with biochemically confirmed PA should undergo CT of the adrenal region to rule out malignancy and to localize adenomas Citation[2]. In addition to the characterization of adrenal masses, multidetector-row helical CT (MDCT) is holding another potential; on thin-slice images (2–3 mm) obtained with modern MDCT-scanners the adrenal veins can be identified in many cases. The position of the adrenal vein (particularly on the right side) can be determined in relation to the ribs and vertebral bodies. Therefore, when performing AVS, the adrenal vein can be catheterized precisely. Daunt estimated that in more than half of patients, the right adrenal veins can be detected Citation[42]. Matsuura et al. found that the right adrenal vein was detected in 76% of patients suffering from thoracoabdominal vascular disease; however, these findings were not corroborated by angiographic results Citation[43]. Systematic surveys to MDCT-mapping are not yet available, but the authors’ own unpublished data suggests that MDCT-mapping is a valuable means in AVS.
Rapid cortisol assay during AVS
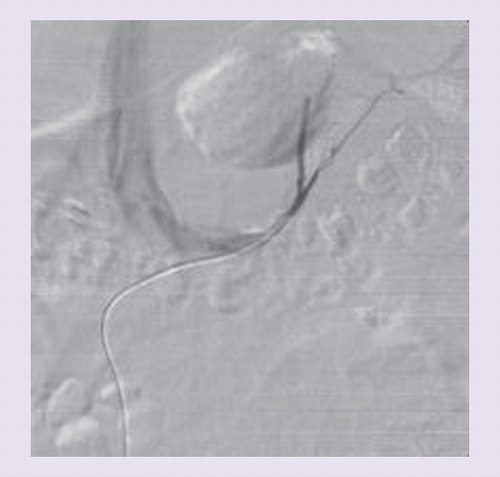
As mentioned earlier, the adrenal veins are difficult to catheterize due to their anatomical variations (e.g., anomalous confluence of adrenal and hepatic vein), their small size and the limited blood flow of approximately 0.5 ml/min Citation[41,44]. Displacement of the catheter during AVS can occur without notice of the radiologist. In contrast to the left adrenal vein that usually drains directly into the left renal vein, the right adrenal vein is only very short and empties directly into the IVC in a steep angle and is therefore often missed & . A considerable amount of blood from accessory hepatic or subphrenic veins, from the IVC or from renal veins is admixed to the adrenal blood and leads to a dilution and variation of actual aldosterone concentrations, depending on the exact position of the catheter tip Citation[19,41,45,46]. As a consequence, aldosterone values are adjusted by dividing them with the cortisol value from the same probe (AC = cortisol - corrected aldosterone ratio). This allows the elimination of variable dilution of the adrenal blood. The ‘selectivity index’ (SI) represents the ratio of cortisol concentrations in the adrenal vein and in the IVC (SI = CAdrenal/CIVC) and allows the information about the correct position of the catheter tip in the adrenal vein. The higher the SI, the less is the dilution and the more representative is the sample for adrenal venous blood. This information is unfortunately usually available only after hours or even days. After drawing blood from the adrenal veins and the IVC, uncertainty remains about the actual selectivity of the catheterization. The patient leaves the radiology suite and the hospital and is contacted later on about the technical success or failure of the AVS procedure. To overcome this problem, Mengozzi et al. first showed the feasibility of a rapid on-site cortisol measurement during AVS in five patients in 2007 Citation[21]. In 2009, Auchus et al. showed that the use of rapid cortisol assays (RCAs) allowed immediate resampling, thus increasing the success rate of AVS Citation[20]. In 2011, the authors analyzed the effect of RCA on the outcome of AVS in the authors’ center that had a low success rate in a historic control group . The authors demonstrated that the overall improvement of the selectivity rate from 55 to 85% was mainly due to a better cannulation of the right adrenal vein by resampling and through a more delayed training effect of the radiologist (as discussed later in this article). The authors were able to achieve a time to results in 25–30 min, providing nearly immediate information as to whether the AVS study was successful or not. The lag time is used in the authors’ institution to continue the study on the contralateral side. RCA reduces the necessity for repeat studies to a minimum. In fact, only one of 120 patients underwent AVS a second time after introduction of RCA in the center. The authors postulated that RCA represents a useful tool especially in centers with low success rates. Rossi et al. and Reardon et al. recently confirmed these results with an improvement of bilateral selectivity through intraprocedural cortisol measurement from 82 to 93% and 76 to 92%, respectively Citation[47,48].
ACTH 1–24 stimulation
ACTH 1–24 stimulation through continuous cosyntropin infusion (50 µg/h started 30 min before sampling) or bolus (250 µg) during AVS was introduced in 1979 and is currently being evaluated by many authors Citation[2,49,50]. The AVIS study showed that 11 out of 20 analyzed centers, currently use ACTH 1–24 stimulation, which is believed to have three major effects as follows: to minimize stress-induced fluctuations in cortisol and aldosterone secretion during sequential AVS; to increase the cortisol gradients from the adrenal vein to IVC with a better selectivity index; and to enhance the aldosterone secretion from APA Citation[8,18,38]. In addition, ACTH 1–24 stimulation is necessary in case of steroid prophylaxis in patients with a history of allergy to contrast agents in order to overcome glucocorticoid-induced suppression of endogenous ACTH Citation[51]. It is still controversial whether ACTH 1–24 leads to improvement of AVS quality and its role remains undetermined. Some authors did not demonstrate an advantage for AVS interpretation or even concluded that increased selectivity comes at a price of decreased lateralization accuracy through stimulation of the contralateral adrenal gland, especially after the use of ACTH 1–24 bolus injection Citation[22,52]. Cutoff values for AVS selectivity interpretation increase when ACTH 1–24 stimulation is used, with the potential consequence of a decreased percentage of evaluable AVS studies. In consequence, some authors recommend more permissive cortisol ratios Citation[49]. On the other hand, some authors emphasize the importance of awareness regarding successful catheterization of both adrenal veins for further patient management Citation[38]. A recent study of the Torino group compared the effect of stimulation through both continuous infusion or bolus of ACTH 1–24 with unstimulated AVS protocols. They showed that ACTH 1–24 stimulation protocols perform at least as well as unstimulated protocols and led to an increase of overall selectivity from 49 to 87%. Hence, in their opinion, ACTH 1–24 stimulation may be particularly useful in centers with a low rate of cannulation Citation[51]. In addition, they did not find an impairment of lateralization index with the possibility of false-negative results as described earlier by Seccia et al. Citation[22]. Finally, the study confirmed previous results showing the importance of using strict criteria for selectivity and lateralization indices to achieve diagnostic reproducibility.
Bilaterally simultaneous or sequential catheterization
There is no general consensus on the particular workflow in AVS. Even major steps of the procedure are not performed uniformly at different institutions, although there might be an impact on the aldosterone and cortisol concentrations measured in the withdrawn samples of adrenal vein blood. One question that needs to be addressed more specifically is whether to perform simultaneous bilateral catheterization of the adrenal veins or to cannulate the adrenal veins sequentially. The rationale for bilateral simultaneous AVS is that aldosterone is secreted in a pulsatile order, especially in response to stress-induced ACTH release. At least in theory, there are chances to create artificial gradients between the adrenals, especially when sequential sampling is used. Many groups advocate the use of continuous ACTH 1–24 during AVS to minimize stress-induced fluctuations in aldosterone secretion during sequential AVS Citation[2]. Most centers are using sequential sampling procedures, probably owing to the fact that simultaneous bilateral AVS is somewhat more cumbersome for the radiologist because displacement of an already positioned catheter may occur accidently when the second catheter is manipulated Citation[22]. The catheter may also dislocate due to breathing excursions and pulsation of nearby aorta or the left renal artery over time. In particular, bilateral or two punctures on one side are required, increasing the risk for puncture-related complications and theoretically, there is a higher risk for adrenal vein thrombosis, as when the first catheter is positioned it may obstruct the vessel until also the second vein is successfully catheterized. The AVIS study showed that almost two thirds of the centers use sequential technique with cosyntropin stimulation, whereas only a few centers use ACTH 1–24 stimulation with bilateral simultaneous catheterization Citation[18]. There is little data concerning this issue. Carr et al. compared uni- and bi-lateral sampling techniques in a small group of patients and concluded that sequential bilateral catheterization does not compromise the reliability of the intervention Citation[53]. However, in their study, different techniques of stimulation with ACTH 1–24 were used, possibly confounding results; while sampling in the ‘simultaneous sampling’ group (n = 11) was performed before and after ACTH 1–24 stimulation, sequential sampling (n = 11) was done under continuous ACTH 1–24 infusion.
Training effect of radiologist
It is hardly surprising that like most other skills, AVS also needs some practice. While the catheterization of the left adrenal vein can be easily performed in almost all cases, the catheterization of the right adrenal vein is substantially more difficult for a number of reasons. The right adrenal vein is very short and entering the IVC in caudocranial direction. Thus it may cause difficulties to stabilize the catheter in the small vein. Furthermore, there are some other small veins entering the IVC nearby the right adrenal vein, for example, accessory hepatic veins or veins of the renal capsule that may cause confusion in phlebography. Whereas standard cortisol assay is completed only after the AVS procedure, the RCA delivers results immediately. The advantage is twofold: first, in case of negative sampling, resampling can be performed as explained earlier and second, there is a direct training effect for the interventional radiologist. By instant feedback regarding the withdrawn samples, she/he can directly learn whether a venous structure was correctly considered to be the adrenal vein or not. This assumption is corroborated by the analysis of AVS in the authors’ department: resampling was required in 67% of the first 12 patients, repeated sampling was only required in 25% of the subsequent 12 patients. Simultaneously, the proportion of successful sampling increased from 67 to 100% Citation[19]. Furthermore, it needs some experience to choose the appropriate catheter for the particular anatomy. Generally, Cobra Catheters (mostly C2) are suitable for both adrenal veins. In case the IVC is very narrow, a C1-configuration may be more appropriate. For more dedicated use, MK1B configuration was recommended to catheterize the left adrenal vein and reverse configurations (e.g., Sidewinder) may be suitable for the right adrenal Citation[42]. The AVIS study showed that the median number of radiologists performing AVS in the evaluated centers was 2 (range 1–7). The rate of major complications, namely adrenal vein rupture, was only between 0.51 and 0.61% and therefore lower than previously thought, demonstrating the safety of AVS in experienced hands. Regression analysis showed that adrenal vein rupture was significantly associated with the number of AVS performed by each radiologist and the number of AVS performed per center Citation[18].
Interpretation of results
As discussed earlier, SI is the key value to determine whether the blood obtained during the AVS procedure represents adrenal venous blood or rather diluted blood from hepatic or renal veins Citation[45]. The cutoff values for SI were set arbitrarily or on local opinion and are not based on randomized prospective studies. When analyzing the literature, it becomes obvious that agreement on AVS criteria are lacking; published cutoff levels for SI vary from 1.1 to >10, depending on whether cosyntropin stimulation is used or not Citation[8,31,45,54]. Low SI cutoffs bear the danger to oversee the inherent error of laboratory assays of cortisol. By definition, 99.7% of measured values are within the range of three standard deviations from the mean. Furthermore, cortisol can fluctuate more than 10% even with ACTH 1–24 infusion. Therefore, in our and others opinion, a SI of at least 2.0 reliably guarantee the differentiation of adrenal and nonadrenal samples.
The lateralization index (LI) is defined as the ratio of the higher (dominant) over the lower (nondominant) AC ratio (LI = ACDominantAdrenal/ACNondominantAdrenal) and determination of which adrenal gland(s) is (are) responsible for the autonomous hypersecretion of aldosterone. The cutoffs for LI are as empiric and diverge as much as those for SI from ≥2 to ≥5 Citation[1,8,55]. Prospective studies that could determine the exact cutoff value for LI by analyzing the outcome of surgery as gold standard for all adrenalectomized patients on the basis that AVS will probably never be performed because of ethical considerations Citation[38,41,54]. In addition, approximately two thirds of referral centers require the suppression of the contralateral gland (ACNondominantAdrenal < ACIVC), which is the case in 93% of patients with surgically confirmed APA, to diagnose lateralization Citation[8,18,56]. The contralateral suppression index can be helpful especially in equivocal cases, for example when the right adrenal vein could not be catheterized successfully. When the AC of the left adrenal gland is much lower than that from the IVC, aldosterone secretion must be coming from the right adrenal gland. On the other hand, when the AC of the left adrenal is much higher than that from the IVC, lateralization should be coming from the left side; however, this cannot be said with absolute certainty Citation[41]. More reliable secondary criteria are needed for these equivocal cases.
The choice of SI and LI can have considerable consequences on the diagnostic conclusion and therefore for the further management of the investigated patients Citation[57]. The AVIS study showed wide variations of interpretation strategies of AVS results throughout major referral centers, with some centers even using absolute aldosterone concentrations without correcting the dilution factor with the help of cortisol Citation[18]. In the authors’ AVS studies, the authors use a SI ≥2 (without ACTH 1–24 stimulation) and require a LI ≥4 for the diagnosis of lateralization Citation[19]. Lower SI leads to a higher proportion of studies regarded as successful. This will provide more patients with a definitive subtype diagnosis for the costs of a less reliable subtype diagnosis. Mulatero et al. analyzed the impact of three different diagnostic criteria during AVS on reproducibility of subtype diagnosis in patients who had undergone two separate AVS procedures because the first study had been regarded as unsuccessful according to locally used cutoffs [57]. It became obvious that raising the SI from 1.1 to 3.0 lowered the proportion of studies regarded as being selective from 91 to 34%. The more permissive SI had a diagnostic reproducibility of only 35% and revealed the diagnosis of unilateral PA six-times more often (61 vs 9%) than the most stringent criteria. The authors could demonstrate that more stringent criteria for successful cannulation and lateralization led to higher diagnostic reproducibility, with 100% achieved only in studies using a SI ≥2.75 Citation[57]. Similar results had been reported earlier in a study by Ceral et al. Citation[58]. Finally, in a recent study analyzing KCNJ5 mutations in PA in large European population, the authors were able to demonstrate heterogeneity of the prevalence of mutations found in a total of 380 APAs across the investigated centers. Interestingly, the frequency of KCNJ5 mutations was found to be higher in patients adrenalectomized based on more conservative criteria in AVS Citation[59].
Expert commentary
The purpose of this review was to give an overview of the procedures available to improve success rates in AVS, the so-called gold standard in the differentiation of the two major subtypes of PA. However, such a gold standard becomes fragile regarding the high variability AVS procedures and cutoffs used in different centers regarded themselves as centers of excellence. As observed by Stewart and Allolio, standardization in regard of SI, LI, ACTH 1–24 stimulation or bilaterally simultaneous or sequential catheterization in AVS is missing Citation[16]. However, while being regarded as the last instance before sending a patient to definitive therapy in means of adrenalectomy, standardization should be the smallest denominator for this procedure. The need for AVS should be determined in a randomized controlled trial comparing AVS and adrenal imaging as differentiation instruments. Such a study is currently being performed and we are awaiting its results with eagerness. Besides this, AVS is a cost- and personnel-intensive procedure; different algorithms and noninvasive techniques must be, and currently are being, developed (e.g., 18-oxocortisol measurement, chromogranin A, [11] C-metomidate PET-CT) Citation[60–63]. The establishment of national and even international registers has shown to be an immense benefit for patient care and research collaborations, but may also help to find new guidelines for quality management. The development of an inter-center SOP should be the first step towards this goal. At least, AVS represents an invasive procedure, potentially compromising the patient, and therefore requires great expertise and experience. Hence, we suggest that AVS should only be performed in institutions with a sufficient number of cases and reproducible selectivity rates of at least 70%.
Five-year view
We believe that standardization of guidelines in AVS will be achieved soon by consensus statement. Outcome of AVS studies might be further improved through implementation of quality management strategies over all instances. The development of biomarkers for the identification of IAH and nuclear medicine tools to localize functioning aldosterone secreting tumors should be further pursued in order to reduce the number of necessary AVS studies.
Table 1. Effect of rapid cortisol measurement on technical success rate of adrenal vein sampling in five studies.
Key issues
• Adrenal vein sampling (AVS), which represents the key procedure for subtype diagnosis of primary aldosteronism, is not a well standardized technique and has limited success rates.
• Optimal patient selection and preparation avoids unnecessary procedures.
• Interdisciplinary team work and exact implementation of standard operation procedure improves security and reproducibility of AVS.
• Multidetector-row helical CT mapping and rapid cortisol assays are helpful tools in improving success rates of AVS.
• The value of cosyntropin stimulation and the use of bilateral versus sequential catheterization remains unclear but consistency of methods should be a matter of course.
• AVS should be performed in specialized centers by a limited number of dedicated radiologists in order to ensure quality and success rates of at least 70%.
• Standardization of cutoff values through consensus statement should be accomplished for consistent decision-making in patient care.
Acknowledgments
The authors gratefully acknowledge the support of the whole team of the Endocrine Laboratory and the Munich PA-Group.
References
- Mulatero P, Stowasser M, Loh KC et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J. Clin. Endocrinol. Metab. 89(3), 1045–1050 (2004).
- Funder JW, Carey RM, Fardella C et al.; Endocrine Society. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 93(9), 3266–3281 (2008).
- Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies – a review of the current literature. Horm. Metab. Res. 44(3), 157–162 (2012).
- Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 45(8), 1243–1248 (2005).
- Catena C, Colussi G, Nadalini E et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch. Intern. Med. 168(1), 80–85 (2008).
- Born-Frontsberg E, Reincke M, Rump LC et al.; Participants of the German Conn’s Registry. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn’s Registry. J. Clin. Endocrinol. Metab. 94(4), 1125–1130 (2009).
- Reincke M, Meisinger C, Holle R et al.; Participants of the German Conn’s Registry. Is primary aldosteronism associated with diabetes mellitus? Results of the German Conn’s Registry. Horm. Metab. Res. 42(6), 435–439 (2010).
- Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery 136(6), 1227–1235 (2004).
- Mulatero P, Bertello C, Verhovez A et al. Differential diagnosis of primary aldosteronism subtypes. Curr. Hypertens. Rep. 11(3), 217–223 (2009).
- White ML, Gauger PG, Doherty GM et al. The role of radiologic studies in the evaluation and management of primary hyperaldosteronism. Surgery 144(6), 926–933; discussion 933 (2008).
- Young WF. Primary aldosteronism: renaissance of a syndrome. Clin. Endocrinol. 66(5), 607–618 (2007).
- Nwariaku FE, Miller BS, Auchus R et al. Primary hyperaldosteronism: effect of adrenal vein sampling on surgical outcome. Arch. Surg. 141(5), 497–502; discussion 502 (2006).
- Mantero F, Terzolo M, Arnaldi G et al; Study Group on Adrenal Tumors of the Italian Society of Endocrinology. A survey on adrenal incidentaloma in Italy. J. Clin. Endocrinol. Metab. 85(2), 637–644 (2000).
- Kempers MJ, Lenders JW, van Outheusden L et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann. Intern. Med. 151(5), 329–337 (2009).
- Vonend O, Ockenfels N, Gao X et al.; German Conn’s Registry. Adrenal venous sampling: evaluation of the German Conn’s registry. Hypertension 57(5), 990–995 (2011).
- Stewart PM, Allolio B. Adrenal vein sampling for primary aldosteronism: time for a reality check. Clin. Endocrinol. 72(2), 146–148 (2010).
- Melby JC, Spark RF, Dale SL, Egdahl RH, Kahn PC. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein cateterization. N. Engl. J. Med. 277(20), 1050–1056 (1967).
- Rossi GP, Barisa M, Allolio B et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J. Clin. Endocrinol. Metab. 97(5), 1606–1614 (2012).
- Betz MJ, Degenhart C, Fischer E et al. Adrenal vein sampling using rapid cortisol assays in primary aldosteronism is useful in centers with low success rates. Eur. J. Endocrinol. 165(2), 301–306 (2011).
- Auchus RJ, Michaelis C, Wians FH Jr et al. Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann. Surg. 249(2), 318–321 (2009).
- Mengozzi G, Rossato D, Bertello C et al. Rapid cortisol assay during adrenal vein sampling in patients with primary aldosteronism. Clin. Chem. 53(11), 1968–1971 (2007).
- Seccia TM, Miotto D, De Toni R et al. Adrenocorticotropic hormone stimulation during adrenal vein sampling for identifying surgically curable subtypes of primary aldosteronism: comparison of 3 different protocols. Hypertension 53(5), 761–766 (2009).
- Steichen O, Zinzindohoué F, Plouin PF, Amar L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm. Metab. Res. 44(3), 221–227 (2012).
- Karagiannis A, Tziomalos K, Papageorgiou A et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin. Pharmacother. 9(4), 509–515 (2008).
- Karagiannis A. Treatment of primary aldosteronism: where are we now? Rev. Endocr. Metab. Disord. 12(1), 15–20 (2011).
- Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J. Clin. Endocrinol. Metab. 94(7), 2437–2445 (2009).
- Ghose RP, Hall PM, Bravo EL. Medical management of aldosterone-producing adenomas. Ann. Intern. Med. 131(2), 105–108 (1999).
- Catena C, Colussi G, Lapenna R et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension 50(5), 911–918 (2007).
- Sechi LA, Novello M, Lapenna R et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA 295(22), 2638–2645 (2006).
- Weinberger MH, Fineberg NS. The diagnosis of primary aldosteronism and separation of two major subtypes. Arch. Intern. Med. 153(18), 2125–2129 (1993).
- Mulatero P, Bertello C, Rossato D et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J. Clin. Endocrinol. Metab. 93(4), 1366–1371 (2008).
- Mulatero P, Monticone S, Veglio F. Diagnosis and treatment of primary aldosteronism. Rev. Endocr. Metab. Disord. 12(1), 3–9 (2011).
- Letavernier E, Peyrard S, Amar L, Zinzindohoué F, Fiquet B, Plouin PF. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J. Hypertens. 26(9), 1816–1823 (2008).
- Zarnegar R, Bloom AI, Lee J et al. Is adrenal venous sampling necessary in all patients with hyperaldosteronism before adrenalectomy? J. Vasc. Interv. Radiol. 19(1), 66–71 (2008).
- Sarlon-Bartoli G, Michel N, Taieb D et al. Adrenal venous sampling is crucial before an adrenalectomy whatever the adrenal-nodule size on computed tomography. J. Hypertens. 29(6), 1196–1202 (2011).
- Tan YY, Ogilvie JB, Triponez F et al. Selective use of adrenal venous sampling in the lateralization of aldosterone-producing adenomas. World J. Surg. 30(5), 879–885; discussion 886 (2006).
- Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm. Metab. Res. 44(3), 170–176 (2012).
- Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin. Endocrinol. 70(1), 14–17 (2009).
- Fischer E, Beuschlein F, Bidlingmaier M, Reincke M. Commentary on the Endocrine Society Practice Guidelines: consequences of adjustment of antihypertensive medication in screening of primary aldosteronism. Rev. Endocr. Metab. Disord. 12(1), 43–48 (2011).
- Cain JP, Tuck ML, Williams GH, Dluhy RG, Rosenoff SH. The regulation of aldosterone secretion in primary aldosteronism. Am. J. Med. 53(5), 627–637 (1972).
- Auchus RJ, Wians FH Jr, Anderson ME et al. What we still do not know about adrenal vein sampling for primary aldosteronism. Horm. Metab. Res. 42(6), 411–415 (2010).
- Daunt N. Adrenal vein sampling: how to make it quick, easy, and successful. Radiographics 25(Suppl. 1), S143–S158 (2005).
- Matsuura T, Takase K, Ota H et al. Radiologic anatomy of the right adrenal vein: preliminary experience with MDCT. AJR. Am. J. Roentgenol. 191(2), 402–408 (2008).
- Sèbe P, Peyromaure M, Raynaud A, Delmas V. Anatomical variations in the drainage of the principal adrenal veins: the results of 88 venograms. Surg. Radiol. Anat. 24(3–4), 222–225 (2002).
- Solar M, Ceral J, Krajina A et al. Adrenal venous sampling: where is the aldosterone disappearing to? Cardiovasc. Intervent. Radiol. 33(4), 760–765 (2010).
- Miotto D, De Toni R, Pitter G et al. Impact of accessory hepatic veins on adrenal vein sampling for identification of surgically curable primary aldosteronism. Hypertension 54(4), 885–889 (2009).
- Rossi E, Regolisti G, Perazzoli F et al. Intraprocedural cortisol measurement increases adrenal vein sampling success rate in primary aldosteronism. Am. J. Hypertens. 24(12), 1280–1285 (2011).
- Reardon MA, Angle JF, Abi-Jaoudeh N et al. Intraprocedural cortisol levels in the evaluation of proper catheter placement in adrenal venous sampling. J. Vasc. Interv. Radiol. 22(11), 1575–1580 (2011).
- Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J. Hypertens. 26(5), 989–997 (2008).
- Weinberger MH, Grim CE, Hollifield JW et al. Primary aldosteronism: diagnosis, localization, and treatment. Ann. Intern. Med. 90(3), 386–395 (1979).
- Monticone S, Satoh F, Giacchetti G et al. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension 59(4), 840–846 (2012).
- Rossi GP, Ganzaroli C, Miotto D et al. Dynamic testing with high-dose adrenocorticotrophic hormone does not improve lateralization of aldosterone oversecretion in primary aldosteronism patients. J. Hypertens. 24(2), 371–379 (2006).
- Carr CE, Cope C, Cohen DL, Fraker DL, Trerotola SO. Comparison of sequential versus simultaneous methods of adrenal venous sampling. J. Vasc. Interv. Radiol. 15(11), 1245–1250 (2004).
- Rossi GP, Sacchetto A, Chiesura-Corona M et al. Identification of the etiology of primary aldosteronism with adrenal vein sampling in patients with equivocal computed tomography and magnetic resonance findings: results in 104 consecutive cases. J. Clin. Endocrinol. Metab. 86(3), 1083–1090 (2001).
- Stowasser M, Gordon RD, Gunasekera TG et al. High rate of detection of primary aldosteronism, including surgically treatable forms, after ‘non-selective’ screening of hypertensive patients. J. Hypertens. 21(11), 2149–2157 (2003).
- Stowasser M, Gordon RD. Primary aldosteronism – careful investigation is essential and rewarding. Mol. Cell. Endocrinol. 217(1–2), 33–39 (2004).
- Mulatero P, Bertello C, Sukor N et al. Impact of different diagnostic criteria during adrenal vein sampling on reproducibility of subtype diagnosis in patients with primary aldosteronism. Hypertension 55(3), 667–673 (2010).
- Ceral J, Solar M, Krajina A, Ballon M, Suba P, Cap J. Adrenal venous sampling in primary aldosteronism: a low dilution of adrenal venous blood is crucial for a correct interpretation of the results. Eur. J. Endocrinol. 162(1), 101–107 (2010).
- Boulkroun S, Beuschlein F, Rossi GP et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension 59(3), 592–598 (2012).
- Burton TJ, Mackenzie IS, Balan K et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J. Clin. Endocrinol. Metab. 97(1), 100–109 (2012).
- Mulatero P, di Cella SM, Monticone S et al. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J. Clin. Endocrinol. Metab. 97(3), 881–889 (2012).
- Nakamura Y, Satoh F, Morimoto R et al. 18-oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J. Clin. Endocrinol. Metab. 96(8), E1272–E1278 (2011).
- Seccia TM, Miotto D, De Toni R et al. Chromogranin a measurement for assessing the selectivity of adrenal venous sampling in primary aldosteronism. J. Clin. Endocrinol. Metab. 96(5), E825–E829 (2011).
Improving adrenal venous sampling in primary aldosteronism
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/expertneurothera. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, [email protected]. For technical assistance, contact [email protected]. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the AMA PRA CME credit certificate and present it to your national medical association for review.
Activity Evaluation: Where 1 is strongly disagree and 5 is strongly agree
1. You are seeing a 53-year-old woman with treatment-resistant hypertension, and you suspect that she has primary aldosteronism (PA). What should you consider regarding the epidemiology and diagnosis of PA?
□ A It is the third most common diagnosis promoting secondary hypertension
□ B It is usually due to unilateral adrenal hyperplasia
□ C Aldosterone producing adenomas (APAs) are generally easy to distinguish from adrenal incidentalomas on CT imaging
□ D The success rate for bilateral catheterization during adrenal vein sampling (AVS) varies from 31% to 98%
2. What should you consider before referring this patient for AVS?
□ A A confirmed diagnosis of PA is not required prior to AVS
□ B Patients under 60 with a single APA greater than 5 mm can be referred for unilateral adrenalectomy without AVS
□ C All drugs which affect renin expression should be stopped 4 weeks prior to AVS
□ D Beta blockers should be stopped at least 2 weeks prior to AVS
3. Which of the following tools has been demonstrated to be most useful in improving the success rates of AVS?
□ A Adrenocorticotropic hormone (ACTH 1-24) stimulation alone
□ B Simultaneous vs sequential catheterization alone
□ C Multidetector-row helical CT (MDCT) and rapid cortisol assays (RCA) alone
□ D ACTH 1-24 stimulation, simultaneous vs sequential catheterization, and MDCT
4. The patient undergoes AVS, and her cortisol selectivity index (SI) value returns at a value of 0.7. How should you interpret this result?
□ A The SI is a ratio of cortisol levels taken from the left and right adrenal veins
□ B Higher accuracy of AVS is reflected in lower SI values
□ C This result indicates substantial contamination with non-adrenal blood samples
□ D More permissive interpretation of SI results in fewer patients receiving a subtype diagnosis