Abstract
There were only a few options 3 years ago to treat metastatic renal cell carcinoma (mRCC), a disease with a very poor prognosis. With the approval of targeted therapies for mRCC since December 2005, this situation has changed dramatically. Currently, oncologists can choose between several promising options to improve the longevity and quality of their patients’ lives. A widely accepted treatment scheme for targeted therapies in mRCC does not yet exist. Based on a selective literature search, drawing on studies with six targeted therapies for mRCC, and including data from the latest American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) Annual Meetings, this review introduces the available therapies, evaluates patient-specific criteria for their application and suggests an algorithm for a patient-based treatment scheme. Clinical experiences with sequential therapies are summarized and potential combination therapies discussed. In conclusion, the crucial criteria of the treatment scheme we propose are the tumor burden and the disease pace, as well as the quality of life of a patient. These define whether tumor control or tumor remission should be the primary therapeutic goal. This scheme suggests which kind of therapeutic sequence to pursue to optimize patient care in mRCC.
EGFR: EGF receptor; HIF: Hypoxia inducible factor; PDGFR: PDGF receptor; Raf: Rapidly growing fibrosarcoma; VEGFR: VEGF receptor; VHL: Von Hippel–Lindau protein complex.
Adapted from Citation[15] (erlotinib is not included in this review).
![Figure 1. Selected targeted agents in metastatic renal cell carcinoma.EGFR: EGF receptor; HIF: Hypoxia inducible factor; PDGFR: PDGF receptor; Raf: Rapidly growing fibrosarcoma; VEGFR: VEGF receptor; VHL: Von Hippel–Lindau protein complex.Adapted from Citation[15] (erlotinib is not included in this review).](/cms/asset/89b70a52-4eaa-4b9b-a8f7-b46a4287aca9/iery_a_11219482_f0001_b.jpg)
A therapy decision must balance tumor burden and quality of life as the most crucial, yet contrary points.
Modifed with permission from Citation[64].
![Figure 3. Course of therapy according to tumor burden.Modifed with permission from Citation[64].](/cms/asset/44bf7aab-7079-4534-90cd-9db346098ece/iery_a_11219482_f0003_b.jpg)
†For highly selected patients.
‡Including the sequential use of several tyrosine kinase inhibitors, depending on tumor burden, disease pace and quality of life.
PS: Performance status; VEGFR: VEGF receptor.
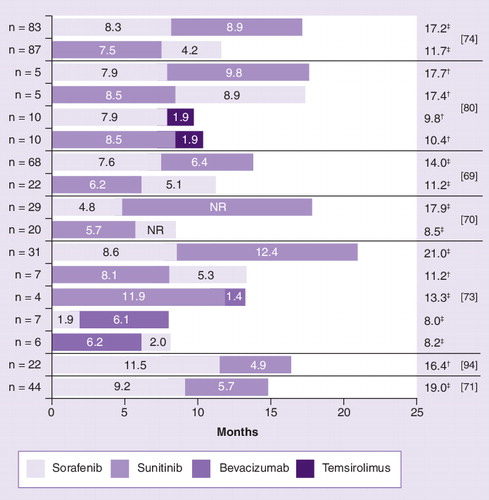
In the case of Richter et al., second-line progression-free survival for temsirolimus did not distinguish between sorafenib or sunitinib as prior therapy.
†Sum of medians.
‡Median.
NR: Not reported.
Renal cancer was expected to account for 4.2% of all new cancers, and the mortality was 12.980 in the USA in 2009 Citation[1]. Of these cancers, 85% arise from the renal epithelium and are classified as renal cell carcinoma (RCC). Surgical intervention is the primary treatment for RCC. The application of radical nephrectomy to the affected kidney as the standard therapy has largely been replaced by partial nephrectomy or enucleation surgery, whenever technically possible, in order to prevent chronic kidney disease and associated cardiovascular morbidity. However, recurrence after resection occurs in a third of patients Citation[2]. A total of 25% of patients already suffer from advanced disease upon presentation Citation[2].
Until the turn of the millennium, metastatic RCC (mRCC) was regarded as one of the most refractory cancers; highly resistant to both radiation and systemic therapy, and only susceptible to immune modulation in a small proportion of patients Citation[3]. Fortunately, this therapeutic gridlock has dissolved owing to the advent of targeted therapies, which interfere with specific signal transduction pathways of tumor formation and progression. Although these new drugs will not be a cure in the vast majority of mRCC cases, they provide considerable life span and quality of life improvement for patients undergoing palliative treatment Citation[4].
Sorafenib (Nexavar®; Bayer Pharmaceuticals Corporation, CT, USA) and sunitinib (Sutent®; Pfizer, Inc., NY, USA), two antiangiogenic multikinase inhibitors, were the first targeted therapies for mRCC to be approved by the US FDA (in December 2005 and January 2006, respectively). They were followed by temsirolimus (Torisel®; Wyeth Pharmaceuticals, NJ, USA), a mTOR-inhibitor, in May 2007. In addition, the combination of the monoclonal antibody bevacizumab plus IFN-α (Avastin® plus Roferon®; Hoffmann-La Roche Ltd, NJ, USA) was approved as a treatment for mRCC in January 2008 by the EMA and gained approval from the FDA for this indication in August 2009. Everolimus (Afinitor®; Novartis International Ag, Basel, Switzerland) was approved by the FDA and by the EMA by the end of March 2009 and in August 2009, respectively. With pazopanib (Votrient®, GlaxoSmithKline), the FDA has approved the sixth drug for the treatment of kidney cancer since 2005 in October 2009. Pazopanib is currently awaiting approval by the EMA, and further innovative compounds are under development and expected to be available soon.
This rapid growth of therapeutic options for mRCC constitutes a challenge for the practicing oncologist. Since it may still be true that, owing to limited population studies, only approximately a quarter of treatment decisions in clinical oncology are based on level 1 evidence Citation[5], we urgently need to draw on topical clinical evidence to put the new therapeutic options into a meaningful context. However, to date no comprehensive scheme for mRCC treatment exists. In this review, we attempt to design an algorithm for systemic mRCC treatment, based on the latest results of evidence-based medicine and data from the American Society of Clinical Oncology (ASCO) Annual Meetings – and, last but not least, on our own extensive experience treating patients with mRCC Citation[6].
Immunomodulatory therapies
The fact that occasional spontaneous remissions have been observed suggests that RCC is of an immunogenic nature. However, the assumption that a cytokine-mediated boost of tumor antigenicity or host surveillance would translate into significant clinical benefit for patients with mRCC has not been confirmed. A recent meta-analysis of 58 randomized controlled trials involving 6880 patients with mRCC came to the conclusion that no immunotherapy has overall effectiveness for this indication Citation[7]. However, a small and well-defined fraction of patients can benefit from immune therapy and even hope to be cured Citation[8–10]. High-dose IL-2, the only immune therapy approved by the FDA for mRCC, leads to complete and durable responses in some patients. However, most patients, cannot tolerate it because of severe side effects Citation[2,4]. Nevertheless, approximately 15% of mRCC patients are principally eligible for first-line cytokine treatment, provided that they are relatively young, present with a very good performance status, good organ function and a low risk profile Citation[7,11].
In the largest randomized trial performed in patients with mRCC to date, a total of 1006 patients were randomized over 5 years into one of two groups; a group that received IFN-α alone or a group that received a triple therapy of IFN-α, IL-2 plus 5-fluorouracil Citation[12]. Triple therapy demonstrated an advantage over single IFN-α therapy in terms of response but did not significantly prolong progression-free survival (PFS) or overall survival (OS) Citation[12]. The clinical trial results submitted for the approval of the combination therapy of bevacizumab plus IFN-α for mRCC recently revealed that IFN-α therapy alone could achieve at least tumor control in 63% of patients Citation[13], which, although lower than the 77% of patients receiving the combination therapy of bevacizumab plus IFN-α who achieved at least tumor control, remains substantial. This is a further indicator that cytokine therapy still remains an option for mRCC.
Efficacy of targeted therapies
The development of targeted cancer therapies – aiming mainly at the inhibition of angiogenesis – is widely recognized as one of the major biomedical breakthroughs of the last decade and a paradigm of successful bench-to-bedside research Citation[14]. Since December 2005, six and five targeted therapies for mRCC have been approved in the USA and Europe, respectively Citation[15].
Sorafenib
Sorafenib is an oral multikinase inhibitor of several receptor tyrosine kinases, including the VEGF receptors (VEGFR)1–3, the PDGF receptor-β, Flt-3 and c-KIT. Downstream of these receptor-mediated signaling cascades, sorafenib also blocks serine–threonine kinases of the Raf family. Sorafenib is the only available inhibitor of Raf, a key protein of the MAPK pathway. MAPK signals are instrumental in cell proliferation and are overactivated in tumors. Sorafenib, therefore, targets both tumor angiogenesis and tumor proliferation Citation[16].
A Phase III trial of sorafenib in mRCC randomized 903 patients who had either received or were ineligible for cytokine therapy to receive sorafenib or placebo Citation[17]. The primary end points were OS and PFS. A planned interim analysis revealed a significant PFS advantage in the sorafenib arm of the trial (5.5 vs 2.8 months; hazard ratio [HR]: 0.44; 95% CI: 0.35–0.55; p < 0.01). Consequently, placebo patients were allowed to crossover at that time. A significant OS benefit of sorafenib was seen in a per-protocol analysis adjusting for crossover (17.8 vs 14.3 months; HR: 0.78; CI: 0.62–0.97; p = 0.0287 Citation[18]). The objective response rate was 10%. First-line PFS data from separate Phase II and IIIb studies range from 5.7 (CI: 5.0–7.4 months) to 9.3 months (CI: 5.8–not reached) Citation[19,20]. Clinical benefits of sorafenib for mRCC patients were generally confirmed in two open-label expanded access programs in North America and Europe with 2502 and 1155 patients, respectively Citation[21,22].
Sunitinib
Sunitinib is a tyrosine kinase inhibitor (TKI) that targets several VEGF and PDGF receptors Citation[23]. A Phase III trial of sunitinib in mRCC Citation[24–26] randomized 750 first-line patients to repeated 6-week cycles of sunitinib or IFN-α. The primary end point was PFS, which was significantly longer in the sunitinib group than in the IFN-α group (11 vs 5 months; HR: 0.42; CI: 0.32–0.54; p < 0.001) Citation[24]. Sunitinib demonstrated a high objective response, with a remission rate of 31% (CI: 28–38) compared with 6% (CI: 4–9; p < 0.001) in the IFN-α arm Citation[24]. However, this high remission rate could not be confirmed in the expanded access program, in which a response occurred in 9.3% of the patients. OS in the Phase III trial was 26.4 months for sunitinib versus 21.8 months for IFN-α (p = 0.051). Owing to overlapping confidence intervals, no statistical survival advantage could be demonstrated in the final OS analysis, even if adjusting for crossover Citation[26].
Pazopanib
Pazopanib is another TKI that targets several VEGF and PDGF receptors with a slightly different kinase profile than sorafenib and sunitinib. Data from a Phase III trial of pazopanib versus placebo in 435 patients who were either treatment naive or had received cytokines have recently been published Citation[27]. The primary end point of this trial was PFS, which was significantly longer in the pazopanib group than in the placebo group in the interim analysis (9.2 vs 4.2 months; HR: 0.46; CI: 0.34–0.62; p < 0.001). Pazopanib demonstrated an objective response rate of 30%, compared with 3% in the placebo arm. Critics have raised the argument that this pivotal trial was conducted with a placebo group instead of an active comparator among the therapeutic options already approved for this indication. However, pazopanib is currently tested in a Phase III head-to-head comparison against sunitinib (COMPARZ, NCT00720941).
Bevacizumab plus IFN-α
The monoclonal antibody bevacizumab binds to soluble VEGF with great affinity, thus preventing it from reaching its receptor. Bevacizumab was the first agent to prove this principle of angiogenesis inhibition in mRCC, albeit with no significant efficacy as a single agent Citation[2,28].
A Phase III trial randomized 649 first-line patients to receive IFN-α and bevacizumab or IFN-α and placebo Citation[13]. The primary end point was OS. PFS was significantly extended to 10.2 months for the combination versus 5.4 months for IFN-α (HR: 0.63; CI: 0.52–0.75; p = 0.0001). The remission rate for the combination therapy was 31% compared with 13% in the IFN-α group (p = 0.0001). Median OS with IFN-α was 21.3 versus 23.3 months for the combination (p = 0.1291) Citation[29]. In a similar trial by the Cancer and Leukemia Group B, which randomized 732 patients either to this combination or IFN-α monotherapy without placebo control, median OS was 18.3 versus 17.4 months (p = 0.069) Citation[30].
Temsirolimus
Temsirolimus is an intravenously administered inhibitor of mTOR kinase, an enzyme that is a central component of intracellular signal pathways involved in cell growth and proliferation, response to hypoxic stress, and apoptosis. Thus, temsirolimus suppresses both cell cycle and angiogenesis signals.
A Phase III trial randomized 626 mRCC patients with poor prognosis (according to modified Memorial Sloan Kettering Cancer Center [MSKCC] criteria Citation[31–33]) to receive temsirolismus, IFN-α or a combination of both Citation[34]. The primary end point was OS, which was significantly longer in the temsirolimus group (10.9 months; CI: 8.6–12.7) than in the groups treated with the combination (8.4 months; CI: 6.6–10.3) or with IFN-α alone (7.3 months; CI: 6.1–8.8). Notably, patients with intermediate risk or who were aged more than 65 years experienced a greater benefit with IFN-α than with temsirolimus.
Everolimus
Everolimus is an oral inhibitor of mTOR kinase. A Phase III trial randomized 410 patients with mRCC in a two-to-one ratio to receive either everolimus or placebo Citation[35,36]. Approximately 75% of the 410 patients were at least third-line patients who had progressed on sunitinib, sorafenib or both. Prior therapy with cytokines or bevacizumab was also allowed. The primary end point was PFS. The trial was stopped prematurely after the second interim analysis had shown a significant difference between the two groups (HR: 0.30; CI: 0.22–0.40; p < 0.0001). Median PFS in the everolimus group was 4.0 months (CI: 3.7–5.5) versus 1.9 months (CI: 1.8–1.9) in the placebo group. A more detailed analysis demonstrated different PFS rates according to individual prior therapy Citation[37], which will be discussed in more detail in the ‘Discussion’ section.
Exploratory substances
Axitinib (AG-013736; Pfizer) and regorafenib (BAY 73-4506, Bayer) are prominent examples of over 20 different VEGFR-based agents in currently ongoing clinical trials Citation[14,38]. Both have shown activity in mRCC.
Discussion
In general, targeted therapies are better tolerated by cancer patients than chemotherapies. Nevertheless, they still act as cytotoxic substances in a broader sense and can, therefore, cause side effects that must not be neglected. Fatigue, hypertension and diarrhea are common side effects of all targeted therapies. Characteristic potential adverse events also include rash, hand/foot/skin reactions and mucositis, as well as neutropenia and anemia for the kinase inhibitors; and gastrointestinal perforation, bleeding, thromboembolic events, anorexia and proteinuria for the antibody–cytokine combination Citation[4,13,17,24,39].
However, there are important differences in the tolerability of targeted therapies. In the Phase III trial of bevacizumab plus IFN-α, 60% of patients in the verum arm of the trial suffered from grade 3/4 side effects. A total of 28% of the patients in this group dropped out during the trial, compared with 12% in the placebo plus IFN-α group Citation[13].
In the Phase III trial of sunitinib, 21% of sunitinib-treated patients developed congestive heart failure symptoms with their left ventricular ejection fraction declining below normal. This specific sunitinib cardiotoxicity was confirmed in a single-center study with 48 patients at Stanford (CA, USA) Citation[40].
More than the other kinase inhibitors, sunitinib induces thyroid dysfunction. Approximately 30% of patients included in a prospective observational study at the Leuven Cancer Institute developed clinical hypothyroidism that required hormone-replacement therapy Citation[41–43]. Renal toxicities are not negligible under sunitinib treatment Citation[44] – no trivial side effect, given that most of these patients live with one kidney.
The adverse event profile of mTOR-inhibitors is different from VEGFR-based therapies. Hyperglycemia, hypercholesterolemia and hyperlipidemia are typical side effects of temsirolimus Citation[45]. Grade 3/4 adverse events still represented 54% of all-grade events with temsirolimus Citation[46,47], including asthenia, anemia and myelotoxicity. Pneumonitis, infections and stomatitis are distinctive, and represent severe potential side effects of everolimus Citation[36].
While showing several class effects, such as diarrhea and hypertension, the toxicity profile of pazopanib seems to be different from sunitinib, for example, in terms of fatigue Citation[24,27]. A black box warning regarding liver toxicity was included into the pazopanib label by the FDA. However, further results of the ongoing comparative Phase III trial (COMPARZ) are needed to settle open questions referring to tolerability among others.
The authors of a PubMed-based systematic review of adverse events associated with sorafenib, sunitinib and temsirolimus concluded that, overall, sunitinib causes the most, and sorafenib the fewest, grade 3/4 side effects, with the temsirolimus adverse event profile between those agents Citation[48]. However, in patients pretreated with sunitinib or sorafenib, temsirolimus showed considerably increased adverse events Citation[49].
Criteria for differential treatment decisions
Based on the efficacy and safety data from Phase III trials and the approval status of targeted therapies for mRCC, sunitinib is widely regarded as the preferred first-line and sorafenib as the preferred second-line treatment. Everolimus has demonstrated activity after VEGFR-based therapy in a Phase III study after various prior therapies Citation[35]. PFS was 3.9, 4.0 or 5.9 months after sunitinib, sorafenib or both, respectively, with the majority of patients receiving everolimus as a third-line therapy. Even though the PFS was different across those groups, the hazard ratios obtained were comparable, 0.25, 0.34 and 0.32 for the order above and statistically significant against placebo Citation[37]. These data support the assumption that a more differentiated approach can be applied.
As mentioned earlier, immunomodulatory therapies are well tolerated and effective only in younger patients. Targeted therapies can be beneficial to all age groups, although bevacizumab plus IFN-α leads to increased fatigue and asthenia in patients over 65 years Citation[50]. Sorafenib appears to be suitable for patients of any age, with respect to efficacy and safety. In the expanded-access programs, cardiac events did not accumulate in patients over 65 or 70 years of age Citation[51,52].
In the pivotal Phase III trials, patients benefiting from therapy with sorafenib, sunitinib or bevacizumub plus IFN-α presented with a comparable performance status (Eastern Cooperative Oncology Group score: 0–1; Karnofsky score: > 80%) and risk profile (MSKCC: low and intermediate). By contrast, immunomodulatory therapy is suitable for patients with a low MSKCC score only, and temsirolimus is intended for the treatment of patients with poor performance and risk (Karnofsky score: 60–70%; MSKCC: high).
Except for temsirolimus, most clinical trials of targeted therapies enrolled patients with clear-cell RCC exclusively. With a prevalence of approximately 80%, this is the most common histological subtype of RCC Citation[2,3]. Little or minimal efficacy was ascribed to the TKIs in patients with papillary RCC (PRCC) or chromophobe RCC (CRCC), the next most frequent histological subtypes Citation[53]. According to data from expanded-access trials, both sorafenib and sunitinib may also have antitumor activity in PRCC and CRCC Citation[33,54,55]. A small retrospective multicenter study recently suggested that PFS of selected PRCC and CRCC patients may be prolonged by sunitinib and sorafenib, with a small advantage of sunitinib in PRCC and vice versa in CRCC Citation[56]. However, this is inconsistent with preliminary results of an ongoing Phase II trial that demonstrated disappointing data for sunitinib efficacy in non-clear-cell RCC Citation[57]. Taken together, the available data indicate a clinical benefit of TKIs for non-clear-cell RCC Citation[58].
Brain metastases occur in approximately 10% of mRCC patients and worsen their prognosis Citation[59]. The risk of cerebral bleedings, antiedema activity and other side effects originally led to great caution in the application of TKIs to these patients. However, results from the international expanded-access programs demonstrated that both sorafenib and sunitinib are relatively safe and effective treatments for mRCC patients with brain metastases Citation[60,61]. However, these agents differ in their ability to prevent the occurrence of brain metastases: in a subgroup analysis of the pivotal Phase III trial with sorafenib, Massard et al. demonstrated that sorafenib can significantly reduce the incidence of brain metastases in mRCC patients Citation[62]. According to a study by Helgason et al., sunitinib does not exhibit this prophylactic property Citation[63]. The authors suggest that sunitinib may even temporarily mask the existence of brain metastases and that its pharmacokinetics limit its distribution within the brain Citation[63].
Bevacizumab plus IFN-α achieves relatively high objective response rates and leads sporadically to complete remissions. However, these responses were also observed in the IFN-α-monotherapy arm of the clinical trial (1 vs 2%) Citation[13]. Notably, patients without lung metastases did not significantly benefit from the antibody combination therapy. summarizes all criteria that, at least to our knowledge, need to be taken into consideration for differential treatment decisions.
In our experience, one of the most important, yet not rigidly defined, criteria for individualized treatment decisions is the tumor burden of a patient. This criterion comprises both organ-specific and systemic parameters, such as tumor size, growth kinetics, number and sites of metastases, deviances in certain lab values and time from diagnosis to treatment; that is, variables that represent the sum of all symptoms of a patient. It is the tumor burden on which the oncologist’s most important therapeutic decision depends: remission or control? First and foremost, the answer to this question should direct the timing and type of therapy in order to reach an optimal balance between longevity and quality of life for mRCC patients, as Rini convincingly emphasized in his presentation at ASCO 2008 Citation[64]. Therefore, we envision the therapy decision making process as a triangle in which a balance is found between the most crucial, yet contrary points – tumor burden and quality of life – in order to obtain the most positive outcome for the patient.
Determination of therapeutic focus
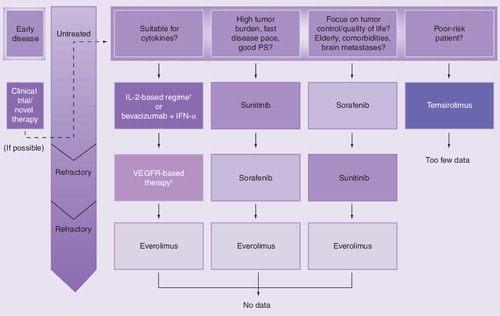
Kidney cancer is one of the biologically most diverse solid tumors; indolent and almost stable in some patients, aggressively growing and symptomatic in others. While the former subset of patients may not require immediate systemic therapy and will best be served by careful surveillance, the latter group needs to be treated by agents with a potentially high remission rate Citation[65]. Because targeted therapies rarely achieve durable and complete remissions, mRCC patients have to undergo chronic treatment. Therefore, it is important to balance treatment benefits against adverse events, duration and costs. For example, tumor control is often preferable to a high remission rate for patients who present with a low tumor burden because it results in a better quality of life. By contrast, patients with a high tumor burden will more willingly accept the side effects of a treatment that prolongs their life. Therefore, Rini suggests considering a three-stage treatment scheme along the spectrum of RCC, where tumor burden is defined along a linear scale Citation[4,64–66]. Patients with a low tumor burden and slow tumor progression should be closely monitored and potentially be offered exploratory substances. Targeted agents with proven efficacy for tumor control should – with an emphasis on their tolerability – be administered as a first-line treatment choice to patients whose tumor burden is up to approximately 2.5-times higher than that of patients in the first segment. Finally, for patients with a high tumor burden, sunitinib should be the initial treatment of choice .
Patient-based approaches were also suggested independently by Bellmunt et al.Citation[67] and by Gore, during his presentation at European Society for Medical Oncology 2008 on how to turn cancer into a chronic disease Citation[68]. The patient-based treatment algorithm for mRCC that we propose in this section fits well into the second segment of Rini’s treatment scheme. In this stage, patients present with a tumor burden that requires – given the multitude of options we have today – the start of a sequential therapy. For the majority of patients, three therapeutic options (sorafenib, sunitinib or bevacizumab plus IFN-α) are advisable as first-line treatments, according to our algorithm .
As of today, the available clinical evidence supports the benefit of sequential therapies with sorafenib and sunitinib. Most notably, the results of several studies suggest that the sequential use of both of these TKIs is not hampered by cross-resistance, despite partly blocking the same signaling pathways Citation[69–74]. With regard to the most advisable succession in this sequence, these studies evince a trend towards more clinical benefits when sorafenib is administered in the first line, followed by sunitinib as a second-line treatment.
Previous data had raised doubts regarding sorafenib’s first-line efficacy. A prospective study that had randomized 189 treatment-naive mRCC patients to receive either sorafenib or IFN-α, showed a median PFS of 5.7 months in the sorafenib arm versus 5.6 months in the IFN-α arm (HR: 0.883; p = 0.504) Citation[19]. But then the median PFS of 224 first-line patients in the expanded-access program extension protocol was 8.1 months Citation[21]. In a retrospective analysis of 90 patients, sorafenib followed by sunitinib showed a median disease stabilization of 61 versus 49 weeks for the sequential therapy started with sunitinib. Median OS was also longer in the group that started with sorafenib (135 vs 82 weeks; p = 0.04) Citation[69]. Another retrospective study with 37 patients revealed a significant advantage in the median time to tumor progression for patients treated with sorafenib first (69.4 vs 36.1 weeks; p = 0.016) Citation[70]. In a study conducted at the University of Hamburg (Germany), 30 patients who were first treated with sorafenib and switched to sunitinib after progression under sorafenib, achieved a median PFS of 8.7 and 10.3 months under sorafenib and sunitinib, respectively. In the subgroup of second-line sunitinib responders, the overall median PFS was 26.6 months, a result that underscores the potential value of a sorafenib–sunitinib sequence in mRCC treatment Citation[71]. Recently, a retrospective analysis of 144 patients, which investigated every possible sequence of sorafenib, sunitinib and bevacizumab, again concluded that sorafenib followed by sunitinib is preferable Citation[73]. Regarding the fact that today a patient should be able to benefit from more than one line of therapy, another important finding was that more than half of the patients were able to receive a second-line treatment after sorafenib or bevacizumab, but less than every fifth patient after sunitinib therapy upfront Citation[73]. At ASCO 2009, Vickers et al. presented a first retrospective comparison of VEGFR-based therapy with mTOR-inhibitors after failure of initial VEGFR-based therapy. Time to treatment failure was 4.9 versus 2.5 months (p = 0.014) in favor of VEGFR-based agents. However, different patient numbers and patient selection may have contributed to the findings of this ongoing study Citation[75]. Of note, only 33% of patients had received second-line therapy. shows the efficacy of sequence therapy in retrospective studies currently available in which PFS, time to progression or duration of therapy is known for both lines.
While most of these data are retrospective with limited patient numbers and have to be interpreted with caution, a prospective study with 42 patients refractory to either first-line bevacizumab (n = 18) or sunitinib (n = 24) was able to demonstrate a median PFS of 3.7 months with sorafenib as a second-line treatment after failure of VEGFR-based therapy Citation[76].
Clear clinical benefits with sunitinib or sorafenib as second-line treatment after failure of prior antiangiogenic therapy were demonstrated in a detailed analysis by Tamaskar et al.Citation[72]. Both sunitinib and sorafenib led to significant reductions in tumor size (median 35%, p < 0.001; 14%, p = 0.07, respectively). Greater tumor burden reduction was observed under sunitinib, a result that supports the value of sunitinib when the therapeutic focus is tumor remission.
When tumor control is the main focus, keeping available sequence data in mind, sorafenib should be considered as the first-line therapy of choice also because of its relatively good tolerability Citation[48]. Especially in elderly patients and in patients with comorbidities including cardiovascular disease, sorafenib is – subject to further prospective investigation – arguably regarded as the preferred initial therapy for mRCC Citation[21]. By contrast, for sunitinib a recent analysis of expanded access results in 82 unselected mRCC patients revealed that 57% of them required a dose reduction due to adverse events and that grade 3 or higher adverse events from sunitinib were significantly related to higher age, female gender and a low body surface area Citation[77].
To date, there are still too few clinical studies with bevacizumab plus IFN-α or with mTOR inhibitors to assess their utility as sequential therapies for mRCC. In general, first-line application of the bevacizumab combination is limited by its relatively high rate of adverse events, even though a reduction of the IFN-α dose probably does not have a negative impact on efficacy Citation[13,78]. Whereas the mTOR inhibitor everolimus has proven efficacy after VEGFR-based therapy, the mTOR inhibitor temsirolimus has been shown to be ineffective or to have an increased rate of adverse events in the very same setting Citation[79,80]. Data regarding the sequential use of pazopanib with other TKIs or mTOR inhibitors are not available yet.
Combination therapies
Combination therapies promise to be more effective than single targeted therapies. It is hoped that they may induce better responses because they interfere with sequential steps in a single pathway or attack a tumor from two sides. However, they tend to be associated with more adverse events than single therapies. In a palliative situation, their application can only be justified if they far exceed the efficacy of monotherapy – or if their synergistic effects allow for a dose reduction in each single therapy that decreases overall adverse events and treatments costs while maintaining antitumor activity Citation[79].
Since most combination data to date are preliminary, no combination can be said to fulfill this requirement. A Phase I study with 25 patients showed an objective response rate of 52% at a maximum tolerated dose of sunitinib 50 mg plus bevacizumab 10 mg/kg. However, adverse events required dose reductions in 40% of the patients and for the same reason 44% had to be withdrawn from the study Citation[81]. Several ongoing studies were stopped because about a third of the RCC patients developed a rare type of hemolytic anemia.
The dose-limiting adverse events resulting from the use of the combination of sunitinib plus temsirolimus, at a concomitant use of intravenous temsirolimus 15 mg weekly and sunitinib 25 mg daily, affected as many as two out of three patients observed in a Phase I study Citation[82] and led to a warning in temsirolimus’ US label.
By contrast, the combination of everolimus plus bevacizumab appears to be an active and tolerable treatment, as the results of a Phase II study with 59 patients suggest Citation[83]. Combining everolimus with sorafenib led to encouraging results in two recent Phase I studies Citation[84,85].
Bevacizumab plus sorafenib combination therapies are still debated regarding dose but have shown encouraging efficacy. A Phase I study with 39 patients suffering from various solid tumors revealed promising clinical activity. Partial response or disease stabilization for 4 months or longer occurred in 22 patients (median: 6 months; range: 4–22+ months). Dose limiting was necessitated by adverse events in response to the bevacizumab plus sorafenib combination at a dosage of sorafenib 200 mg twice daily and bevacizumab 5 mg/kg every 2 weeks Citation[86], indicating this dosage is not tolerable in the long term and that alternate dosing schedules should be explored. In a Phase I study with the same combination in 48 mRCC patients, dose modifications became necessary in 11 patients, but only four had to stop therapy owing to adverse events. Partial response was observed in 46% of patients while the PFS was 14 months Citation[87]. Combined treatment using bevacizumab and temsirolimus demonstrated improved antitumor efficacy compared with results of monotherapies Citation[88]. A prospective Phase III trial comparing bevacizumab plus IFN-α versus the combination of bevacizumab plus temsirolimus is ongoing (NCT00631371). summarizes current data on the feasibility of combination therapies.
Conclusion
The new targeted therapies offer hitherto unexpected opportunities for the treatment of mRCC in a palliative setting. An integration of all options into one standard therapy is not yet in sight. Head-to-head comparisons between the currently available agents are desirable to learn more about their respective benefits. Prospective studies are also needed to assess optimal sequences and combinations.
In this situation, a number of patient-specific criteria, most importantly tumor burden, will best define the therapeutic goal and help to develop patient-specific treatment plans. An optimal balance between quality of life and prolongation of survival will only be achieved by individualized considerations of both benefits and risks of the new targeted therapies. In this respect, the treatment scheme we present in this review is intended to be a decision guide for optimizing patient care in mRCC in clinical routine.
Owing to the development of resistance against antiangiogenetic treatment strategies, all of the new therapies have only transitory effects. While the problem of resistance has neither been solved nor fully understood and more tumor biology studies are warranted, targeted therapies certainly have radically improved the prospects for patients suffering from mRCC.
Table 1. Important criteria that should be considered in differential treatment decisions.
Table 2. Currently available feasibility data for possible combination strategies.
Key issues
• Targeted therapies have radically improved the prospects for patients suffering from metastatic renal cell carcinoma (mRCC).
• In 2010, six novel targeted therapeutics are available for the treatment of mRCC.
• Because targeted therapies rarely achieve durable and complete remissions, mRCC patients have to undergo chronic treatment.
• Most patients today will receive several targeted therapies in a treatment sequence.
• Retrospective data on sequential treatment in mRCC show there is no cross resistance between available VEGF receptor–tyrosine kinase inhibitor (TKI) targeted agents, such as sorafenib and sunitinib.
• Everolimus showed activity in mRCC in later lines of therapy, including the use after several consecutive VEGF receptor–TKI-targeted agents.
• Patients at high risk, according to modified Motzer criteria, benefit from temsirolimus.
• Patient-specific criteria, most importantly tumor burden, define the therapeutic goal and help to develop individual treatment plans.
• Before starting first-line therapy, the whole potential therapeutic sequence should be considered for the individual patient.
• An optimal balance between quality of life and prolongation of survival will only be achieved by putting both, benefits and risks of the new targeted therapies into context.
Financial & competing interests disclosure
Hartmut Kirchner has received speaking fees and/or honoraria for advisory board meetings from Pfizer, Roche, Wyeth, GlaxoSmithKline and Bayer Healthcare. Amit Bahl received speaking fees and/or honoraria from Pfizer, Roche and Bayer Healthcare. Friedrich Overkamp and Dirk Strumberg have no real or apparent conflicts of interest to report. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors received editorial support in the preparation of this article, supported by Bayer Healthcare. The authors, however, were fully responsible for content and editorial decisions and received no compensation for this manuscript.
References
- Jemal A, Siegel R, Ward E et al. Cancer statistics 2009. CA Cancer J. Clin.59, 225–249 (2009).
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N. Engl. J. Med.353, 2477–2490 (2005).
- Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J. Urol.163, 408–417 (2000).
- Rini BI, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma: a home run or a work in progress? Oncology22, 388–396 (2008).
- Djulbegovic B, Loughran TP, Hornung C et al. The quality of medical evidence in hematology–oncology. Am. J. Med.106, 198–205 (1999).
- Kirchner H, Heinzer H, Roigas J, Overkamp F. Differenzialtherapie beim metastasierenden Nierenzellkarzinom. Der Onkologe14, 191–197 (2008).
- Coppin C, Porszolt F, Autenrieth M et al. Immunotherapy for advanced renal cell cancer (Review). Cochrane Database Syst. Rev.CD001425 (2005).
- Atzpodien J, Kirchner H, Jonas U et al. Interleukin-2- and interferon α-2a-based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). J. Clin. Oncol.22, 1188–1194 (2004).
- Atzpodien J, Reitz M. Metastatic renal carcinoma long-term survivors treated with s.c. interferon-α and s.c. interleukin-2. Cancer Biother. Radiopharm.20, 410–416 (2005).
- Bordin V, Giani L, Meregalli S et al. Five-year survival results of subcutaneous low-dose immunotherapy with interleukin-2 alone in metastatic renal cell cancer patients. Urol. Int.64, 3–8 (2000).
- Escudier B, Chevreau C, Lasset C et al. Cytokines in metastatic renal cell carcinoma: is it useful to switch to interleukin-2 or interferon after failure of a first treatment? J. Clin. Oncol.17, 2039–2043 (1999).
- Gore ME. interferon-α (IFN), interleukin-2 (IL2) and 5-fluorouracil (5FU) vs IFN alone in patients with metastatic renal cell carcinoma (mRCC): results of the randomised MRC/EORTC RE04 trial. J. Clin. Oncol.26, (2008) (Abstract 5039a).
- Escudier B, Pluzanska A, Koralewski P et al. Bevacuzimab plus interferon a-2a for treatment of metastastic renal cell carcinoma: survival and biomarker analysis. Lancet370, 2103–2111 (2007).
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat. Rev. Cancer8, 579–591 (2008).
- Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nat. Rev. Cancer2, 672–682 (2002).
- Wilhelm SM, Carter C, Tang L et al. Bay 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res.64, 7099–7109 (2004).
- Escudier B, Eisen T, Stadler WM et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med.356, 125–134 (2007).
- Bukowski RM, Eisen T, Szczyklik C et al. Final results of the randomized Phase III trial of sorafenib in advanced renal cell carcinoma: survival and biomarker analysis. J. Clin. Oncol.25 (2007) (Abstract 5023a).
- Szczylik C, Demkow T, Staehler M et al. Randomized Phase II trial of first-line treatment with sorafenib versus interferon in patients with advanced renal cell carcinoma: final results. J. Clin. Oncol.25 (2007) (Abstract 5025a).
- Jonasch E, Corn P, Ashe RG et al. Randomized Phase II study of sorafenib with or without low-dose IFN in patients with metastatic renal cell carcinoma. J. Clin. Oncol.25 (2007) (Abstract 5104a).
- Knox JJ, Figlin RA, Stadler WM et al. The advanced renal cell carcinoma sorafenib (ARCSS) expanded access trial in North America: safety and efficacy. J. Clin. Oncol.25 (2007) (Abstract 5011a).
- Beck J, Bajetta E, Escudier B et al. A large open-label, non-comparative, Phase III study of the multi-targeted kinase inhibitor sorafenib in European patients with advanced renal cell carcinoma. Eur. J. Cancer Suppl.5, 4506 (2007).
- Motzer RJ, Michaelson MD, Redman BG et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol.24, 16–24 (2006).
- Motzer RJ, Hutson TE, Tomczak P et al. Sunitinib versus interferon a in metastatic renal-cell carcinoma. N. Engl. J. Med.356, 115–124 (2007).
- Motzer RJ, Figlin RA, Hutson TE et al. Sunitinib versus interferon-α (IFN-α) as first-line treatment of metastatic renal cell carcinoma (mRCC): updated results and analysis of prognostic factors. J. Clin. Oncol.25 (2007) (Abstract 5024).
- Figlin RA, Hutson TE, Tomczak P et al. Overall survival with sunitinib versus interferon (IFN)-a as first-line treatment of metastatic renal cell carcinoma (mRCC). J. Clin. Oncol.26 (2008) (Abstract 5024).
- Sternberg CN, Davis ID, Mardiak J et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized Phase III trial. J. Clin. Oncol. DOI: 10.1200/JCO.2009.23.9764 (2010) (Epub ahead of print).
- Yang JC, Haworth L, Sherry RM et al. A randomized trial of bevacizumab, an antivascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med.349, 427–434 (2003).
- Escudier B, Bellmunt J, Negrier S et al. Final results of the Phase III, randomized, double-blind AVOREN trial of first-line bevacizumab (BEV) + interferon-α2a (IFN) in metastatic renal cell carcinoma (mRCC). 2009 ASCO Annual Meeting; May 29-June 2; Orlando, FL. J. Clin. Oncol.27(Suppl.) 15s (2009) (Abstract 5020a).
- Rini BI, Halabi S, Rosenberg JE et al. CALGB 90206: a Phase III trial of bevacizumab plus interferon-α versus interferon-α monotherapy in metastatic renal cell carcinoma. 2009 ASCO Annual Meeting; May 29-June 2; Orlando, FL. J. Clin. Oncol.27(Suppl.) 15s (2009) (Abstract 5019a).
- Motzer RJ, Mazumdar M, Bacik J et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol.17, 2530–2540 (1999).
- Motzer RJ, Bacik J, Murphy BA et al. interferon-α as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J. Clin. Oncol.20, 289–296 (2002).
- Mekhail TM, Abou-Jawde RM, BouMerhi G et al. Validation and extension of the Memorial Sloan-Kettering Prognostic Factors Model for Survival in patients with previously untreated metastatic renal cell carcinoma. J. Clin. Oncol.23, 832–841 (2005).
- Hudes G, Carducci M, Tomczak P et al. Temsirolimus, interferon a or both for advanced renal-cell carcinoma. N. Engl. J. Med.356, 2271–2281 (2007).
- Motzer R, Escudier B, Oudard S et al. RAD001 vs placebo in patients with metastatic renal cell carcinoma (RCC) after progression on VEGFr-TKI therapy: results from a randomized, double-blind, multicenter Phase-III study. J. Clin. Oncol.26, (2008) (Abstract LBA5026).
- Motzer R, Escudier B, Oudard S et al. Efficacy of everolimus in adavanced renal cell carcinoma: a double-blind, randomised, placebo-controlled Phase III trial. Lancet372, 449–456 (2008).
- Escudier B, Ravaud A, Oudard S et al. Phase-3 randomized trial of everolimus (RAD001) vs placebo in metastatic renal cell carcinoma. Ann. Oncol.19, 72O (2008).
- Sternberg CN, Szczylik C, Lee E et al. A randomized, double-blind Phase III study of pazopanib in treatment-naive and cytokine-pretreated patients with advanced renal cell carcinoma (RCC). J. Clin. Oncol.27(Suppl.), 15s (2009) (Abstract 5021a).
- Dutcher JP, Wilding G, Hudes GR et al. Sequential axitinib (AG-013736) therapy of patients (pts) with metastatic clear cell renal cell cancer (RCC) refractory to sunitinib and sorafenib, cytokines and sorafenib, or sorafenib alone. J. Clin. Oncol.26, 5127 (2008) (Abstract 5127).
- Hutson TE, Davis ID, Machiels JH et al. Biomarker analysis and final efficacy and safety results of a Phase II renal cell carcinoma trial with pazopanib (GW786034), a multi-kinase angiogenesis inhibitor. J. Clin. Oncol.26, (2008) (Abstract 5046).
- Witteles RM, Telli ML, Fisher GA et al. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. J. Clin. Oncol.26, (2008) (Abstract 9597).
- Clement P, Wolter P, Stefan C et al. Thyroid dysfunction in patients (pts) with metastatic renal cell cancer (RCC) treated with sorafenib. J. Clin. Oncol.26, (2008) (Abstract 16145).
- Wolter P, Stefan C, Decallonne B et al. The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br. J. Cancer99, 448–454 (2008).
- Billemont B, Rixe O, Meric JB et al. Renal safety of sunitinib treatment: a prospective study for patients (pts) with metastatic renal cell carcinoma (mRCC). Ann. Oncol.19, E01a (2008).
- De Souza PL, Radulovic S, Beck J et al. Characterization of hyperglycemia, hypercholesterolemia, and hyperlipidemia in patients with advanced renal cell carcinoma treated with temsirolimus or interferon-α. J. Clin. Oncol.26, (2008) (Abstract 5116).
- Negrier S. Temsirolimus in metastatic renal cell carcinoma. Ann. Oncol.19, 1369–1370 (2008).
- Bellmunt J, Szczylik C, Feingold J et al. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Ann. Oncol.19, 1387–1392 (2008).
- Bhojani N, Jeldres C, Patard JJ et al. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur. Urol.53, 917–930 (2008).
- Wood L, Bukowski RM, Dreicer R et al. Temsirolimus (TEM) in metastatic renal cell carcinoma (mRCC): safety and efficacy in patients (pts) previously treated with VEGF-targeted therapy. J. Clin. Oncol.26, (2008) (Abstract 16067a).
- Bajetta E, Ravaud A, Bracarda S et al. Efficacy and safety of first-line bevacizumab (BEV) plus interferon-α2a (IFN) in patients (pts) ≥ 65 years with metastatic renal cell carcinoma (mRCC). J. Clin. Oncol.26, (2008) (Abstract 5095).
- Porta C, Bracarda S, Beck J et al. Efficacy and safety of sorafenib in elderly pts: results from a large open-label, non-comparative Phase III study of sorafenib in European pts with advanced RCC (EU-ARCCS). Ann. Oncol.19 (2008) (Abstract 596P).
- Bukowski RM, Stadler WM, Figlin RA et al. Safety and efficacy of sorafenib in elderly patients (pts) ≥ 65 years: a subset analysis from the advanced renal cell carcinoma sorafenib (ARCSS) expanded access program in North America. J. Clin. Oncol.26 (2008) (Abstract 5045).
- Plantade A, Choueiri T, Escudier B et al. Treatment outcome for metastatic papillary and chromophobe renal cell carcinoma (RCC) patients treated with tyrosine-kinsase inhibitors. J. Clin. Oncol.25 (2007) (Abstract 5037).
- Gore Me, Porta C, Oudard S et al. Sunitinib in metastatic renal cell carcinoma (mRCC): preliminary assessment of toxicity in an expanded access trial with subpopulation analysis. J. Clin. Oncol.25 (2007) (Abstract 5010).
- Stadler WM, Figlin RA, Ernstoff MS et al. The advanced renal cell carcinoma sorafenib (ARCSS) expanded access trial: safety and efficacy in patients (pts) with non-clear cell (NCC) renal cell carcinoma (RCC). J. Clin. Oncol.25 (2007) (Abstract 5036).
- Choueiri TK, Plantade A, Elson P et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J. Clin. Oncol.26, 127–131 (2008).
- Plimack ER, Jonasch E, Bekele BN et al. Sunitinib in non-clear cell renal carcinoma (ncc-RCC): a Phase II study. J. Clin. Oncol.26 (2008) (Abstract 5112).
- Strumberg D. Efficacy of sunitinib and sorafenib in non-clear cell renal cell carcinoma: results from expanded access studies. J. Clin. Oncol.26, 3469–3471 (2008).
- Schouten LJ, Rutten J, Huveneers HA et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer94, 2698–2705 (2002).
- Henderson CA, Bukowski RM, Stadler WM et al. The Advanced Renal Cell Carcinoma Sorafenib (ARCCS) expanded access trial: subset analysis of patients (pts) with brain metastases (BM). J. Clin. Oncol.25(18), (2007) (Abstract 15506).
- Hariharan S, Szczyklik C, Porta C et al. Sunitinib in metastatic renal cell carcinoma (mRCC) patients (pts) with brain metastases (METS): data from an expanded access trial. J. Clin. Oncol.26 (2008) (Abstract 5094).
- Massard C, Zonierek J, Laplanche A et al. Incidence of brain metastasis in advanced renal cell carcinoma among patients randomized in a Phase III trial of sorafenib, an oral multi-kinase inhibitor. Ann. Oncol.17 (2006) (Abstract 454P).
- Helgason HH, Mallo HA, Droogendijk H et al. Brain metastases in patients with renal cell cancer receiving new targeted treatment. J. Clin. Oncol.26, 152–154 (2008).
- Rini BI. Clinical prognostic and predictive markers for metastatic RCC therapeutic choices. Presented at: ASCO Annual Meeting. Chicago, IL, USA, 30 May–3 June 2008.
- Rathmell WK, Stadler WM, Rini BI. Rational therapeutic choices and strategies for patients with metastatic renal cancer. Am. Soc. Clin. Oncol. Ed. Book192–198 (2008).
- Kirkali Z. Will we be able to ‘cure’ metastatic renal cell carcinoma like we cure testicular tumours? Eur. Urol.54, 712–714 (2008).
- Bellmunt J, Mulders P, Szczylik C et al. Defining a new patient-focused treatment approach to renal cell carcinoma (RCC). Ann. Oncol.19 (2008) (Abstract 612P).
- Gore M. What are the problems that are to be solved in the future? Presented at: ESMO Annual Meeting. Stockholm, Sweden, 12–16 September (2008).
- Sablin MP, Negrier S, Ravaud A et al. Sequential sorafenib and sunitinib for renal carcinoma. J. Urol.182, 29–34 (2009).
- Dudek AZ, Zolnierek J, Dham A, Lindgren BR, Szczylik C. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer115(1), 61–67 (2009).
- Eichelberg C, Heuer R, Chun FK et al. Sequential use of the tyrosine kinase inhibitors sorafenib and sunitinib in metastatic renal cell carcinoma: a retrospective outcome analysis. Eur. Urol.54, 1373–1378 (2008).
- Tamaskar A, Garcia JA, Elson P et al. Antitumor effects of sunitinib or sorafenib in patients with metastatic renal cell carcinoma who received prior antiangiogenic therapy. J. Urol.179, 81–86 (2008).
- Choueiri TK, Brick AJ, McDermott S et al. Treatment and dosing patterns for angiogenesis inhibitor (AIS) therapies in patients with metastatic renal cell carcinoma (MRCC). Ann. Oncol.19(Suppl. 8), (2008) (Abstract 593P).
- Porta C, Procopio G, Sabbatini R et al. Retrospective analysis of the sequential use of sorafenib and sunitinib in patients with advanced renal cell carcinoma (RCC). Eur. Urol. Suppl.8(4), 183(252) (2009).
- Vickers MM, Choueiri TK, Percy A et al. Failure of initial VEGF-targeted therapy in metastatic renal cell carcinoma (mRCC): what next? J. Clin. Oncol.27(Suppl.), 15s (2009) (Abstract 5098a).
- Shepard DR, Rini BI, Garcia JA et al. A multicenter prospective trial of sorafenib in patients with metastatic clear cell carcinoma refractory to prior sunitinib and bevacizumab. J. Clin. Oncol.26 (2008) (Abstract 5123a).
- Van der Veeldt AAM, Boven E, Helgason HH et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br. J. Cancer99, 259–265 (2008).
- Melichar B, Koralewski P, Pluzanska A et al. First-line bevacizumab improves progression-free survival with lower doses of interferon a2a in the treatment of patients with metastastic renal cell carcinoma (AVOREN). Eur. J. Cancer Suppl.5, 304–304 (2007).
- Dutcher J, Szczylik C, Tannir N et al. Correlation of survival with tumor histology, age, and prognostic-risk group for previously untreated patients with advanced renal cell carcinoma receiving temsirolimus or interferon a. J. Clin. Oncol.25 (2007) (Abstract 5033a).
- Richter S, Pfister D, Thüer D et al. Second line treatment of progressive metastatic renal cell cancer with temsirolimus following first-line therapy with sunitinib or sorafenib. Onkologie31 (2008) (Abstract V684a).
- Feldman DR, Ginsberg MS, Baum M et al. Phase I trial of bevacizumab plus sunitinib in patients with metastatic renal cell carcinoma. J. Clin. Oncol.26 (2008) (Abstract 5100a).
- Fischer P, Patel P, Carducci MA et al. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. J. Clin. Oncol.26, (2008) (Abstract 16020a).
- Whorf RC, Hainsworth JD, Spigel DR et al. Phase II study of bevacizumab and everolimus (RAD001) in the treatment of advanced renal cell carcinoma (RCC). J. Clin. Oncol.26 (2008) (Abstract 5010a).
- Rosenberg JE, Weinberg VK, Claros C et al. Phase I study of sorafenib and RAD001 for metastatic clear cell renal cell carcinoma. J. Clin. Oncol.26 (2008) (Abstract 5109a).
- Giessinger S, Amato RJ, Jac J et al. A Phase I study with a daily regimen of the mTOR inhibitor RAD001 (everolimus) plus sorafenib for patients with metastatic renal cell cancer (mRCC). J. Clin. Oncol.26 (2008) (Abstract 14603a).
- Azad NS, Posadas EM, Kwitkowski VE et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J. Clin. Oncol.26, 3709–3714 (2008).
- Sosman JA, Flaherty KT, Atkins MB et al. Final results of a Phase I trial of sorafenib and bevacizumab in patients with metastatic renal cell cancer. J. Clin. Oncol.26 (2008) (Abstract 5011a).
- Merchan JR, Liu G, Fitch T et al. Phase I/II trial of CCI-779 and bevacizumab in stage IV renal cell carcinoma: Phase I safety and activity results. J. Clin. Oncol.25 (2007) (Abstract 5034a).
- Brunello A, Sacco C, Barile C et al. Sunitinib in elderly patients (>=70 years) with metatsatic renal cell carcinoma (mRCC): multicenter analysis of tolerability and efficacy. Ann. Oncol.19 (2008) (Abstract 657P).
- Bokemeyer C, Porta C, Beck J et al. Efficacy and safety of sorafenib in pts with brain and bone metastases: Results from a large openlabel, non-comparative Phase III study of sorafenib in European pts with advanced rcc (EU-ARCCS). Ann. Oncol.19 (2008) (Abstract 595P).
- Eisen T, Beck J, Procopio G et al. Large open label, non-comparative Phase III study of sorafenib in European pts with advanced RCC (EU-ARCCS): subgroup analysis of pts with and without baseline clinical cardiovascular diseases (CCD). Ann. Oncol.19 (2008) (Abstract 602P).
- Tolcher A, Appleman L, Mita A et al. Effect of sorafenib treatment on left ventricular ejection fraction in cancer patients: an open-label, Phase I study. Ann. Oncol.19 (2008) (Abstract 552P).
- Snow H, Brueckner A, Gelder M et al. Sorafenib is not associated with a high incidence of cardiovascular events in many tumor types. Ann. Oncol.19 (2008) (Abstract 553P).
- Zimmermann K, Schmittel A, Steiner U et al. Sunitinib treatment for patients with advanced clear-cell renal-cell carcinoma after progression on sorafenib. Oncology76(5), 350–354 (2009).
- Patnaik A, Ricart A, Cooper J et al. A Phase I, pharmacokinetic and pharmacodynamic study of sorafenib (S), a multi-targeted kinase inhibitor in combination with temsirolimus (T), an mTOR inhibitor in patients with advanced solid malignancies. J. Clin. Oncol.25 (2007) (Abstract 3512a).