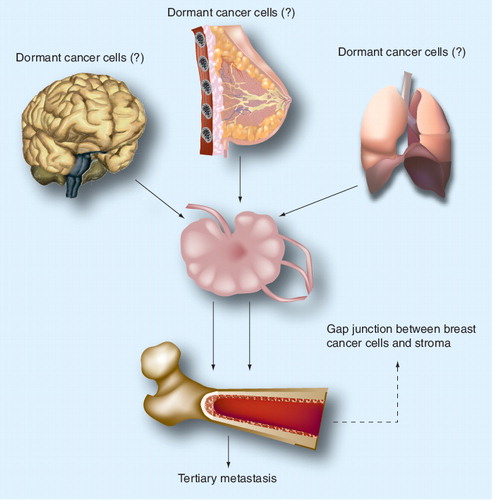
Dormant breast cancer cells can be attained in the primary mammary gland and then enter the bone marrow and other organs through the lymphatic system. Alternatively, dormancy could be acquired once the breast cancer cells enter the marrow and interact with the microenvironment. Dormant breast cancer cells can be found close to the endosteum through gap junctional intercellular interactions with the hematopoietic-supporting stroma. It is likely that dormancy is partly achieved by mechanisms in which small molecules can be exchanged between stroma and breast cancer cells through the gap junctions.
Specific clinical dilemma
A major obstacle for the effective treatment of breast cancer relates to the adaptation of the cancer cells to a dormant phenotype in bone marrow. The continued clinical dilemma for breast cancer eradication occurs even with aggressive intervention and compliance for routine mammograms. An examination of the literature indicates that cancer could resurge from bone marrow after more than 10 years of remission, which is consistent with breast cancer cells showing preference for bone marrow Citation[1].
Dormant breast cancer cells are expected to be at the G1 phase of cycling checkpoint. This cycling quiescence phase of the cancer cells would provide them with a survival advantage, owing to reduced sensitivity to common anticancer agents. In most cases, efficient treatments with anticancer agents require the cancer cells to be proliferating Citation[2–4]. If breast cancer can resurge from cancer cells in the bone marrow, it is highly likely that a subset of cancer cells in the marrow could resist cancer treatments and the surviving cells remain dormant within the microenvironment. In another view, perhaps the treatment induces resistance, which provides the cancer cells with the ability to integrate more efficiently within the microenvironment of the bone marrow.
Possible mechanisms in breast cancer dormancy
The question is whether the cancer cells attain dormancy through mechanisms involving factors within the microenvironment versus intrinsic properties of dormancy in the originating cancer cells. If there are intrinsic properties of dormancy, then these cells will resist treatment regardless of the organ or tissue. These cells could represent the long-term existing cells within the bone marrow niche. If breast cancer dormancy, is caused by intrinsic properties, this brings up a significant question regarding similarities with other dormant breast cancer cells that might be generated through changes by factors and/or cells within the bone marrow microenvironment. Regardless of the mechanism, the dormant subsets in bone marrow, and perhaps other organs, are likely to be the sources of tertiary metastasis. Eradication of these dormant cells will depend on their differences and/or similarities, as well as overlaps with endogenous stem cells.
There are several questions regarding breast cancer dormancy within the bone marrow microenvironment. First, is there a specific location for dormant cancer cells in the bone marrow? Second, does dormancy occur via a special subset of cancer cells? Third, what could explain the failure of the bone marrow, an immune organ, to clear residual cancer cells? Fourth, what are the mechanisms by which the cancer cells take advantage of the bone marrow microenvironment to evade treatment and detection? Lastly, and perhaps most importantly, what are the most efficient methods to target the cancer cells while they are within the bone marrow microenvironment?
An understanding of the mechanisms by which breast cancer cells acquire dormancy in bone marrow first requires the identification of this(these) cell subset(s). While there are widespread discussions regarding cancer stem cells, with reports on their phenotype, the literature remains open for further investigation. If there is heterogeneity of breast cancer cells within a patient, the cancer stem cells in the lung or brain might be different from those within the bone marrow. If this is the case, does this indicate that there would be different cancer stem cells within the bone marrow, or do the variations transition to a common type when the cancer cells contact the bone marrow microenvironment? These arguments/questions underscore the challenges that face the future treatment of dormant breast cancer cells within the bone marrow microenvironment.
Bone marrow microenvironment in breast cancer dormancy
The literature provides experimental evidence that underlies the assumptions on the facilitating roles of distinct regions of the bone marrow for survival of dormant cancer cells. It is likely that dormant cancer cells are present close to the endosteal region where they form gap junctional intercellular communications (GJICs) with hematopoietic-supporting cells, stroma and perhaps osteoblasts Citation[5–13]. Consistent with this assumption is a report demonstrating noncycling breast cancer cells close to the endosteum of a xenogenic mouse model, whereas proliferating cancer cells are observed within the cellular compartment of the marrow Citation[4]. The microenvironment of the bone marrow makes this organ ‘inviting’ for breast cancer cells where they take advantage of the microenvironment for their survival. Upon crossing the blood vessels from the periphery to bone marrow, the breast cancer cells encounter mesenchymal stem cells on the abluminal side of blood vessels where they interact via CXCL12–CXCR4 Citation[2]. This initial interaction with mesenchymal stem cells is interesting since these stem cells are immune suppressors and could elicit immune protection on the cancer cells Citation[14]. The role of mesenchymal stem cells in breast and other cancers is a rapidly growing field and their role would lead to further investigation of treatments. At this time, it is difficult to predict how information on mesenchymal stem cells would lead to new treatments. This is mainly due to the ubiquitous presence of these stem cells. More importantly, it is believed that pericytes, which surround blood vessels, are mesenchymal stem cells, or at least share functions and phenotypic markers.
Dormancy of breast cancer cells during remission or before detection would not also accompany bone marrow failure. Experimental studies show GJIC between stroma and the cancer cells without affecting hematopoietic activity Citation[3]. It is paramount for research to continue along this area to address the mechanisms by which the microenvironment of bone marrow can protect the cancer cells without affecting hematopoietic activity. Information on this topic could lead to targeting of the cancer cells without disruption of the hematopoietic system.
The integration of breast cancer cells among bone marrow cells is expected to involve cytokines, which are major growth factors for hematopoietic support. However, as new categories of molecules are identified, for example miRNAs and RNA-binding proteins, the complexity of networks among cancer cells and endogenous bone marrow cells becomes evident. These networks might overlap those of the hematopoietic system, which would make it more challenging to target the cancer cells without toxicity to the hematopoietic system.
The generation of GJIC between breast cancer cells and bone marrow stroma suggests that small molecules could be exchanged through the GJIC. Studies in this area are required as this could be paramount to the quiescent phase of the cancer cells. While stromal cells have been studied, the osteoblasts could be also relevant in the generation of GJIC as indicated by the growing literature to suggest that osteoblasts are significant to hematopoiesis. If miRNAs are relevant to breast cancer cell dormancy, their roles will be complex and not limited to the cell cycle checkpoint, but might involve mechanisms of protection during chemotherapy treatment and also against other toxic agents.
The location of dormant breast cancer cells close to the endosteal region adds to the complexity in developing strategies for treating cancer cells in regions close to the bone. This area is also the ‘home’ of hematopoietic stem cells that are sensitive to the toxic effects of drugs, including cancer drugs. The maximum dose of a drug that can be given for cancer treatment is limited to the concentration that causes no toxic effect on hematopoietic stem cells. Thus, by protecting the hematopoietic stem cells, the treatment could be, unknowingly, protecting the regional breast cancer cells that are likely in dormancy.
The impact of studies to understand how breast cancer cells adapt within the bone marrow microenvironment would be appreciated if one revisits the failed autologous transplantation of the hematopoietic system for breast cancer patients. If the quiescent breast cancer cells are located within the endosteal region, forming GJIC with stroma, then high-dose chemotherapy will be inadequate to eliminate the cancer cells within the region. In this case, the autologous transplantation with hematopoietic stem cells was performed in a setting in which dormant cancer cells existed.
Failed clinical trials can also provide insights on the targeted disease. Autologous transplantation with hematopoietic stem cells for breast cancer revealed substantial information on the biology of patients’ bone marrow. The cohort of patients with inflammatory breast cancer responded positively to autologous hematopoietic stem cell transplantation. This raises the question of differences in the cancer cells, based on their functions and hormone expressions. The questions posed in this editorial, if answered, could lead to the identification of new targets to translate the science to patients, ultimately to apply innovative methods to target the dormant breast cancer cells without harm to the endogenous hematopoietic stem cells in the same region. On the other hand, if mesenchymal stem cells are critical to the dormancy, an understanding of the mechanism by which these stem cells facilitate dormancy could be relevant to future treatments, either as absolute treatments or for prevention. The discussion is relevant to all phases of breast cancer since dormant cancer cells could be present during periods of overt metastasis, during remission and also before detection since the entry of cancer cells into bone marrow might occur long before detection.
Conclusion
The discussion on challenges for treating breast cancer cells while they are in the marrow microenvironment is summarized in . If dormancy is derived from the primary site in the mammary gland, the challenge could be limited to the intrinsic properties of the cancer cells. On the other hand, if dormancy is dictated by the bone marrow microenvironment, the biology of these cancer cells might be different, depending on the region of the marrow where they are located. As the science proceeds, consideration will need to be placed on the first contacting cells, mesenchymal stem cells and the gradient changes in growth factors, such as CXCL12 towards the marrow cavity. Another major issue is the reduced oxygen close to the bone, which might be important to understand the protective mechanism on the cancer cells in this region of low oxygen.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Mansi JL, Berger U, McDonnell T et al. The fate of bone marrow micrometastases in patients with primary breast cancer. J. Clin. Oncol.7(4), 445–449 (1989).
- Corcoran KE, Fernandes H, Bryan M, Taborga M, Srinivas V, Rameshwar P. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS One3, e2563 (2008).
- Moharita AL, Taborga M, Corcoran KE, Bryan M, Patel PS, Rameshwar P. SDF-1a regulation in breast cancer cells contacting bone marrow stroma is critical for normal hematopoiesis. Blood108(10), 3245–3252 (2006).
- Rao G, Patel PS, Idler SP et al. Facilitating role of preprotachykinin-I gene in the integration of breast cancer cells within the stromal compartment of the bone marrow: a model of early cancer progression. Cancer Res.64(8), 2874–2881 (2004).
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat. Rev. Immunol.8(4), 290–301 (2008).
- Muller-Sieburg CE, Deryugina E. The stromal cells’ guide to the stem cell universe. Stem Cells13(5), 477–486 (1995).
- Calvi LM, Adams GB, Weibrecht KW et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature425(6960), 841–846 (2003).
- Taichman RS, Emerson SG. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells16(1), 7–15 (1998).
- Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J. Exp. Med.179(5), 1677–1682 (1994).
- Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. I. Krogh’s model. Biophys. J.81(2), 675–684 (2001).
- Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys. J.81(2), 685–696 (2001).
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu. Rev. Immunol.8(1), 111–137 (1990).
- Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood110(8), 3056–3063 (2007).
- Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: Functional discrimination between cellular responses to alloantigens and recall antigens. J. Immunol.171(7), 3426–3434 (2003).