In the Clinical Trial Report by Claudio Borghi, Stefano Bacchelli and Daniela Degli Esposti, ‘Long-term clinical experience with zofenopril’, published in the August 2012 issue of Expert Review of Cardiovascular Therapy (Expert Rev. Cardiovasc. Ther. 10[8], 973–982 [2012]), the following sentence appeared as:
“The SMILE trial was designed to test the hypothesis that the administration of zofenopril (15–60 mg twice daily or placebo) administered within 24 h of the onset of symptoms for 6 weeks would improve the clinical outcome of 1556 high-risk patients with acute anterior MI not receiving thrombolysis [21,22].”
The sentence should have read:
“The SMILE trial was designed to test the hypothesis that the administration of zofenopril (15–60 mg/day or placebo) administered within 24 h of the onset of symptoms for 6 weeks would improve the clinical outcome of 1556 high-risk patients with acute anterior MI not receiving thrombolysis [21,22].”
In addition, the following figures appeared as:
RR: Risk reduction.
Adapted with permission from [26].
![Figure 4. One-year survival in patients with acute myocardial infarction and history of hypertension treated with zofenopril or placebo.RR: Risk reduction.Adapted with permission from [26].](/cms/asset/79aeef3f-101a-4e3c-9ffc-42c1deb416b5/ierk_a_11209842_f0002_b.jpg)
RR: Risk reduction.
Data taken from [28].
![Figure 5. Combined occurrence of death and severe congestive heart failure (primary end point) during the 6 weeks of double-blind treatment in the diabetic and nondiabetic population of the SMILE study.RR: Risk reduction.Data taken from [28].](/cms/asset/452c8597-0fbc-4d22-9b44-ebb108212242/ierk_a_11209842_f0003_b.jpg)
RR: Risk reduction.
The figures should have appeared as:
RR: Risk reduction.
Adapted with permission from [26].
![Figure 4. One-year survival in patients with acute myocardial infarction and history of hypertension treated with zofenopril or placebo.RR: Risk reduction.Adapted with permission from [26].](/cms/asset/237800d8-fa37-46da-859b-40274916a164/ierk_a_11209842_f0006_b.jpg)
RR: Risk reduction.
Data taken from [28].
![Figure 5. Combined occurrence of death and severe congestive heart failure (primary end point) during the 6 weeks of double-blind treatment in the diabetic and nondiabetic population of the SMILE study.RR: Risk reduction.Data taken from [28].](/cms/asset/83d19570-deea-4be0-b005-44c3a4ae79f6/ierk_a_11209842_f0007_b.jpg)
RR: Risk reduction.
The authors and editors of Expert Review of Cardiovascular Therapy would like to sincerely apologize for any inconvenience or confusion this may have caused our readers.
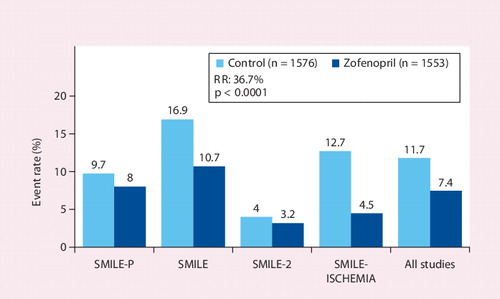
![Figure 3. One-year mortality rate in the SMILE study.Adapted with permission from [22].](/cms/asset/fafe1246-294f-417e-858c-7d740e25e493/ierk_a_11209842_f0001_b.jpg)
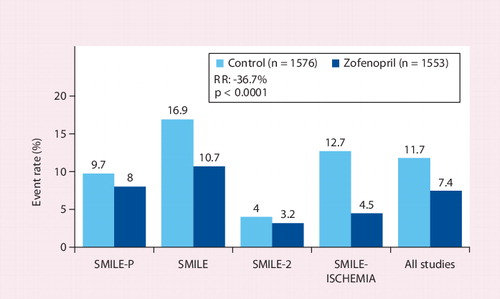
![Figure 3. One-year mortality rate in the SMILE study.Adapted with permission from [22].](/cms/asset/bd35f58b-fc4f-48e5-96a2-1d90b8159a4a/ierk_a_11209842_f0005_b.jpg)