Abstract
Pseudobulbar affect (PBA) consists of uncontrollable outbursts of laughter or crying inappropriate to the patient’s external circumstances and incongruent with the patient’s internal emotional state. Recent data suggest disruption of cortico–pontine–cerebellar circuits, reducing the threshold for motor expression of emotion. Disruption of the microcircuitry of the cerebellum itself may likewise impair its ability to act as a gate-control for emotional expression. Current evidence also suggests that serotonergic and glutamatergic neurotransmission play key roles. Although antidepressants have shown benefit, the supportive clinical data have often derived from small numbers of patients and unvalidated measures of PBA severity. Dextromethorphan/quinidine, the first FDA-approved PBA medication, is a novel therapy with antiglutamatergic actions. As life expectancy lengthens and the neurologic settings of PBA become more common, the need for treatment can be expected to increase.
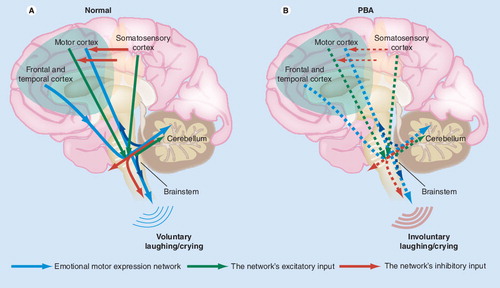
Normally (A), an emotional motor expression network including cortico–ponto–cerebellar afferentation (upper blue arrows) enables the cerebellum to act as a ‘gate-control’ for the motor expression of emotion (lower blue arrows). Inputs to this network (green and red arrows) include an inhibitory influence from sensory cortices. In PBA (B), reduced inhibitory influence at the cortical level (broken red cortical arrows) results in increased aberrant activation within the network (broken blue arrows), giving rise to the motor manifestations of pathological laughing/crying.
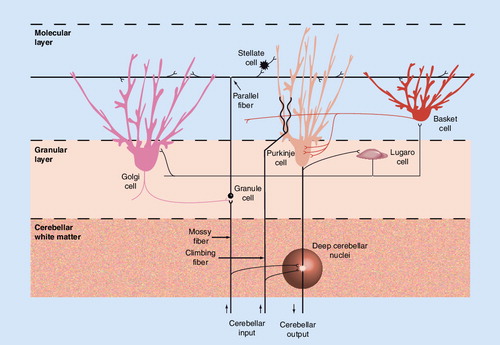
In brief, mossy fibers (from, for instance, the pontine nuclei) synapse with granule cells, which in turn distribute signals, via parallel fibers, to several cells types. Of these, only Purkinje cells generate cerebellar cortical efflux (to the deep cerebellar nuclei). Among cerebellar interneurons, stellate cells, basket cells and Lugaro cells are all inhibitory, while climbing fibers (from the inferior olivary nucleus) are strongly disinhibitory. For their part, Golgi cells are remarkable for inhibiting granule cells.
Pseudobulbar affect (PBA) is a disorder of emotional expression characterized by uncontrollable outbursts of laughter or crying that lack an appropriate environmental trigger and may be exaggerated or incongruent with the underlying emotional state. It is a distinct neurologic condition associated with various neurologic diseases or brain injuries Citation[1,2]. Although reported prevalence rates vary greatly, it may be most common in patients with amyotrophic lateral sclerosis (ALS) Citation[3] and stroke Citation[4], where rates as high as 50% or more have been estimated. PBA is also reported in patients with multiple sclerosis (MS; 10–29%) Citation[5,6], Parkinson’s disease (5–17%) Citation[7–9], Alzheimer’s disease (39%) Citation[10] and traumatic brain injury (5–11%) Citation[11,12].
Pseudobulbar affect is an added burden to patients who may already be disabled or experiencing a reduced quality of life due to their underlying neurologic disorder. Because of the embarrassment associated with an inappropriate outburst of emotion, patients’ social interaction may be impaired Citation[12]. The risk of depression and anxiety symptoms may be increased, and quality of life is often decreased Citation[9,12]. PBA can also interfere with rehabilitation Citation[13]. Although PBA continues to be underdiagnosed and undertreated Citation[14], understanding of the condition has been advancing in recent years. This review will summarize the established knowledge in PBA, as well as recent findings related to its presentation, etiology, underlying mechanisms and emerging treatments.
Taxonomy of emotional expression disorders
The terminology associated with the naming and description of disorders of emotion has been unclear and confusing, and may contribute to the under- and mis-diagnosis of these disorders. The problem is twofold, in that the same or similar disorders have been given multiple names, and the language used to describe the disorders has been inconsistent or misleading. In addition to PBA, symptomatology has been described as affective instability, compulsive laughing or weeping, emotional or affective lability, emotional incontinence, emotionalism, excessive emotionality, inappropriate hilarity, involuntary emotional expression disorder, pathological laughter and crying, pathologic emotionality and under other names Citation[1,2]. Some of these terms have been used interchangeably, while others have been used to make distinctions between similar disorders.
When describing disorders of emotion or emotional expression, it is important to differentiate between affect and mood. According to Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition – Text Revision (DSM-IV-TR), affect reflects moment-to-moment changes in emotional state that are superimposed on mood, which is a sustained emotional state. Understanding this difference helps in diagnosis. PBA is a disorder of affect. Depressive and bipolar disorders are mood disorders because of the persistent nature of the emotional state. Mood disorders may sometimes coexist with disorders of affect, but can and should be distinguished from them.
Disorders of affect may be differentiated among themselves by considering whether they involve objective (expression) versus subjective (experience) or voluntary versus involuntary displays of emotion. Affective lability and pathological laughing and crying are the two major disorders of affect Citation[15]. For patients with affective lability, the emotion expressed is exaggerated relative to the stimulus, but is congruent with the subjective (internally experienced) emotional state Citation[14]. In pathological laughter and crying, the emotion expressed is exaggerated relative to both the stimulus and the experienced emotional state. It may also be incongruent with the experienced emotional state as far as valence. The expression of emotion in pathological laughter and crying is involuntary and uncontrollable. In affective lability, the expression may be partially controllable Citation[14].
Pseudobulbar affect is a broader term defined to encompass pathological laughter and crying and affective lability Citation[14]. As such, PBA is a disorder of involuntary emotional expression (typically laughing and crying) that is uncontrollable, inappropriate to the circumstance, and may be exaggerated or incongruent with the underlying subjective emotional state Citation[14]. The disorder is called ‘pseudobulbar’ because it can be a symptom of pseudobulbar syndrome, a disorder associated with damage to corticobulbar tracts that also has features such as dysphagia, impaired facial and tongue movements, dysphonia, and slow, slurred speech. Use of the term ‘pseudobulbar’ should not be interpreted to suggest that PBA is an unreal (e.g., a factitious) disorder.
The spectrum of PBA & its diagnosis
The confusion attached to the terminology for disorders of emotion affects the question of whether some terms are ‘umbrella terms’ spanning multiple conditions. Conceivably, PBA may be a spectrum ranging from affective lability at one end to severe pathological laughing and crying (with uncontrollable, mood-incongruent displays of emotion, including outbreaks of uncontrollable anger) at the other end. Nevertheless, the boundaries of such a spectrum have not been clearly defined, nor have the diagnostic criteria for PBA been well established. Moreover, PBA and other disorders of affect are not included in DSM-based diagnostic criteria or International Classification of Diseases coding systems Citation[14]. The most recently proposed diagnostic criteria for PBA (termed involuntary emotional expression disorder) are listed in Box 1Citation[16]. The episodes of laughing or crying in PBA are stereotyped in that the frequency, severity, duration and type of episode are similar in a given patient, but may differ among patients. A patient may, however, have both laughing and crying episodes Citation[16].
Pseudobulbar affect must be distinguished from other disorders of affect and from mood and personality disorders. Depression is probably the most common misdiagnosis for PBA. However, many clinical features distinguish PBA episodes from depression symptoms. The most prominent difference is duration. Depression symptoms, including depressed mood, typically last weeks to months, while an episode of PBA lasts seconds to minutes Citation[16]. In addition, crying, as a symptom of PBA, may be unrelated or exaggerated relative to subjective mood, while crying is congruent with subjective mood in depression. Other symptoms of depression, such as fatigue, anorexia, insomnia, anhedonia and feelings of hopelessness and guilt, are not associated with PBA Citation[16]. PBA can also be differentiated from bipolar disorders with rapid cycling or mixed mood episodes because of the relatively brief duration of laughing or crying episodes (with no mood disturbance between episodes), compared with the sustained changes in mood, cognition and behavior recognized in bipolar disorders Citation[14].
Other disorders in the differential diagnosis are relatively rare. Essential crying is a lifelong propensity for crying not accompanied by an underlying psychiatric or neurologic disorder. It does not interfere with function and may be a variant of normal emotion in which the threshold for sadness and crying are lowered Citation[15]. In Witzelsucht, patients frequently and inappropriately experience situations as being funny, and laugh in response. Facetiousness, sarcasm and irritability can also be facets of this condition. Witzelsucht has been associated with brain insult affecting the function of frontal cortex Citation[15]. Crying and laughing that occur in epilepsy, as dacrystic and gelastic episodes, respectively, may be confused with PBA, but these disorders may be accompanied by other epilepsy symptoms, such as impaired consciousness Citation[16]. Gelastic epilepsy has been associated most frequently with hypothalamic tumors (hamartoma).
Pathophysiology of PBA
The pathophysiology of PBA is not fully understood. Along with neuroanatomical forms of evidence (as described below), now augmented by direct neurophysiological studies (also described below), the available evidence derives from studies of PBA treatment, for example, with antidepressants. On the latter basis, the pathophysiology of PBA appears to be at least partly distinct from that of depression, despite similarities in symptomatology. One study in patients with stroke and PBA found no difference in response to nortriptyline among patients with and without coexisting depression; this study also found no correlation between scores on an assessment of PBA symptoms (Pathological Laughter and Crying Scale) and on the Hamilton Rating Scale for Depression (HRSD) Citation[17]. Similarly, in a study of patients with MS and PBA, most patients’ PBA showed a response to amitriptyline, but there was no significant change from baseline in mean HRSD or Beck Depression Inventory (BDI) scores, which were low at baseline Citation[18]. Several studies have found little or no association between depression symptoms or diagnoses and PBA symptoms or diagnoses Citation[6,7,19]. Still other studies have found some association Citation[12,20]. However, the response of PBA to an antidepressant has often been observed within a few days after initiation of treatment Citation[21–23], much sooner than would be expected for an antidepressant effect Citation[24]. Moreover, response to an antidepressant has been reported for laughing as well as crying episodes Citation[25].
Whether or not PBA symptoms correlate with depression symptoms may relate to the differences in location of the specific neuropathology. In some patients, neuropathology may be limited to substrates involved primarily in the motor expression of emotion, whereas in other patients, damage may extend to areas and neuronal systems involved in sustained emotional state (mood). The neural pathways believed to be involved in depression are widespread, complex and involve multiple neurotransmitter systems Citation[26], while those proposed to be involved in emotional expression are more limited Citation[27]. Understanding such differences may be important because of the implications for PBA-specific treatment.
Neuroanatomical clues to pathophysiologic mechanisms
Because PBA occurs in a wide variety of neurologic disorders, commonalities in the location of brain lesions appear to be more important to the pathophysiology of PBA than the specific pathological mechanism(s) of the underlying disorder. Studies of brain lesion location have suggested specific neural substrates and circuits that are disrupted in PBA. In one such study, comparing 14 MS patients with PBA and 14 MS patients without PBA, MRI findings for parcellated brain regions identified significantly greater lesion volume in the PBA group for six regions: brainstem hypointense lesions, bilateral inferior parietal and medial inferior frontal hyperintense lesions, and right medial superior frontal hyperintense lesions Citation[28]. The data were interpreted as implicating “a widely-dispersed neural network involving frontal, parietal and brainstem regions in the pathophysiology of PBA.”
The accepted theory of PBA pathophysiology was based on post-mortem studies. Wilson hypothesized that lesions to the motor cortex resulted in a loss of voluntary inhibition of brainstem nuclei that control emotional expression Citation[29]. In this way, involuntary laughing and crying were believed to be disinhibited. This theory has been revised and extended, based on more recent data from neuroimaging studies, which allow for more precise localization of brain lesions than post-mortem analysis permits. In particular, Parvizi et al. proposed that dysfunction in a cortico–pontine–cerebellar circuit is responsible for PBA symptoms Citation[27,30]. This circuit includes motor, limbic and association cortices with descending pathways to the brainstem, basis pontis and cerebellum. Within this proposed circuit, the cerebellum automatically (unconsciously) modulates emotional expression, scaling it appropriately and producing an emotionally congruent response, according to the contextual information received from the cortex. In PBA, disruption of the cortico–pontine–cerebellar connection would therefore produce a lowered threshold for emotional response or a response incongruent to the patient’s circumstances.
Recent data from an electrophysiologic study of event-related potentials (ERPs) support the concept of cortico–pontine–cerebellar circuit disruption in PBA Citation[31]. ERPs are transient voltage waveforms in brain tissue, as recorded, in this case by scalp electrodes, after experimental stimuli, in this case a spoken list of bisyllabic first names chosen for being subjectively significant or neutral to a given subject. In 11 patients with PBA in the setting of MS, as compared with 11 controls without MS or PBA, ERP current densities were greater in the MS plus PBA group, suggesting that stimulus processing differs between these groups. In response to subjectively significant stimuli, the differences were seen at later stages of processing (in pre-motor and supplementary motor cortical areas), consistent with a difference in the processing of meaning and context. In response to both neutral and subjectively significant stimuli, the differences were seen at early processing stages (in sensory areas), during stimulus discrimination. The sensory processing differences between the groups preceded the motor processing differences, and for both subjectively significant and neutral stimuli, activation of motor areas was significantly greater in MS patients with PBA than in controls.
These data are the first to show evidence of sensory in addition to motor involvement in PBA. Based on these findings, the authors proposed a ‘gate-control’ theory of emotional expression Citation[31]. According to this theory, in patients with PBA, inhibitory transmission from sensory cortices to motor and limbic cortex is reduced. This results in ‘disinhibition’ of a gate-control mechanism in the cerebellum, and therefore a lowered emotional expression threshold .
Neurochemistry
The neurotransmitters and neuromodulators involved in PBA pathophysiology could potentially include any that play a role in emotion and its expression, including serotonin, norepinephrine, glutamate, dopamine, acetylcholine, GABA, adenosine, corticotropin-releasing hormone and corticosteroids Citation[2]. However, serotonin and glutamate appear to be particularly relevant, based not only on their participation in circuits thought to underlie PBA pathophysiology, but also on the types of therapies (serotonergic or antiglutamatergic) used to treat PBA.
Serotonergic projections and serotonin receptors are widespread in the CNS, with potential to modulate most neural functions. In particular, serotonergic neuronal cell bodies are situated predominantly in the brainstem raphé nuclei, with axon terminals diffusely distributed throughout the brain. The terminals are most densely localized in cortico-limbic areas, including cerebral cortex, hippocampus and amygdala; density is relatively low in cerebellum and ventral pons Citation[32]. The role of serotonin in memory, learning, sleep, sex and appetite is well known, and disturbance of these functions is characteristic of mood and anxiety disorders Citation[32]. Serotonin has been viewed as a nonspecific neuropsychiatric stabilizer because of its potential to modulate numerous psychobiological functions and the pervasive usefulness of serotonergic drugs across a variety of psychiatric disorders Citation[33]. Serotonin may be involved in the pathophysiology of PBA through the diffuse cortico-limbic networks involved in emotion or via serotonergic neurotransmission in the cerebellum. Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants increase the synaptic availability of serotonin and are believed to improve PBA symptoms through their serotonergic actions.
Glutamate is the major excitatory neurotransmitter in the CNS. Unlike serotonin and other monoamines, glutamate cell bodies are not limited to brainstem areas but are disseminated throughout the brain, particularly in the cortex. They are also present in the thalamus, hippocampus and cerebellum Citation[34]. Glutamate terminals are also widespread and have been identified in cortex, hippocampus, striatum, amygdala, substantia nigra, pons, cerebellum, and other areas Citation[34]. Drugs modulating glutamatergic transmission could potentially affect diverse neural circuits, depending on the types and location of the glutamate receptors modulated. The efficacy of dextromethorphan/quinidine (DMQ), recently US FDA-approved as therapy for PBA, is believed to be related to antiglutamatergic effects at NMDA and σ-1 receptors Citation[35]. An ERP study in patients with MS and PBA, as compared with healthy controls, found that waveforms elicited by subjectively significant verbal stimuli tended to normalize after treatment with DMQ Citation[36]. The authors speculate that the normalization may have resulted from agonism of presynaptic σ-1 receptors or uncompetitive antagonism of postsynaptic NMDA receptors, leading to reduced glutamatergic activity in the cortex. Such reduction may contribute to improvements in PBA symptoms by reducing transmission to the brainstem, thus compensating for the proposed gate-control disinhibition deemed responsible for PBA Citation[31].
Cerebellar mechanisms
As the CNS structure proposed to monitor context and modulate emotional expression accordingly, the cerebellum may be a key substrate in PBA pathophysiology Citation[27]. Lesions confined to the cerebellum Citation[37–39], to the basis pontis, a relay center to the cerebellum Citation[40–42], or to the thalamus, an output node from the cerebellum Citation[43], have been sufficient to produce PBA symptoms without any other known brain pathology. Indeed, in a recent detailed review of the available neuroanatomical evidence for localization of PBA Citation[30], the basis pontis, a convergence point for descending pathways carrying cerebellar input, “stands out as the only identified site where a discrete lesion can cause [PBA].” Understanding the functional anatomy and neurochemistry of the cerebellum might therefore be particularly relevant for PBA diagnosis and treatment.
The functions attributed to the cerebellum now extend beyond sensorimotor control to include cognitive function and affective processing Citation[44,45]. Based on data from anatomical, functional-imaging and clinical studies in patients with cerebellar damage, distinct cortico–pontine–cerebellar loops have been proposed to underlie motor, cognitive and affective function Citation[45]. Motor function is believed to be subserved by a circuit from sensorimotor cortex to pontine nuclei to anterior parts of the cerebellum, while cognitive and affective function may involve association cortex and limbic areas with connections through pontine nuclei to posterior regions of the cerebellum. Each loop is completed through output connections to thalamus that then return to the originating cortical or limbic area Citation[45]. It has been suggested that affective processing may be further subdivided, in that connections between association cortex and cerebellar hemispheric lobules VI and VII may relate especially to cognitive aspects of emotional processing, such as empathy, while connections between limbic structures and the posterior cerebellar vermis may be most relevant to processes such as emotion-related autonomic function Citation[45]. Potential vermis involvement in autonomic responses was demonstrated in a patient with a tumor in the vermis who had pathological laughter followed by syncope Citation[38].
In consonance with the hypothesized cerebellar involvement in PBA, the disproportionate laughter and crying characteristic of PBA have been viewed as an ‘affective dysmetria’ related to posterior cerebellar lesions, much as overreaching a target is defined as a limb dysmetria related to anterior cerebellar lesions Citation[46]. The cytoarchitectonic structure of the cerebellar cortex supports this concept, in that its uniformity across cerebellar regions Citation[44] would allow for uniform processing of information, with the connections to specific areas of the cerebrum determining whether limb dysmetria, PBA (affective dysmetria), or dysregulation of some other function results from cerebellar damage. PBA may be more common in patients with cerebellar damage than has been recognized. Affective dysregulation, emotional lability and behavioral disinhibition are common features of cerebellar cognitive affective syndrome, a disorder characterized by abnormalities in executive, visuospatial, linguistic and emotional function in patients with infection, degeneration or lesions confined to the cerebellum Citation[47]. In a case-series study of patients with multiple system atrophy–cerebellar type (MSA-C), a chart review (triggered by the discovery of PBA in a single patient with MSA-C) determined that 36% met PBA criteria Citation[48]. This is the highest prevalence rate reported for PBA outside the ALS population. All patients in this series had cerebellar and brainstem atrophy and, in addition to PBA, they demonstrated symptoms of autonomic dysfunction (orthostatic hypotension), dysarthria and other dysmetrias.
The cell types and their functional interactions within the microcircuitry of the cerebellum suggest that it could perform a gate-control function. The structure of the cerebellar cortex consists of three organized cell layers (granular, Purkinje cell and molecular), with Purkinje cells serving as the main processor of information. Granule cells receive information, via mossy fibers, from the pontine nuclei. From granule cells, as many as 200,000 parallel fibers then transmit information to each Purkinje cell Citation[44]. Axons from Purkinje cells project to the deep cerebellar nuclei, from which projections return through the thalamus to the cerebral cortex Citation[44]. Within the cerebellar microcircuitry, it has been proposed that Golgi cells may perform a gating function on cerebellar output. In a study in rats, Holtzman et al. noted that activation of Golgi cells inhibits the firing of weakly activated granule cells and leads to decreased firing in Purkinje cells Citation[49]; however, when afferents from many parts of the periphery are activated (by tactile stimulation in their study), most Golgi-cell firing is depressed, allowing for an increase in granule-cell firing and consequent Purkinje-cell firing . The researchers proposed that the convergence of signals from spatially distinct areas and possibly even from different sensory modalities that occurs in Golgi cells and Purkinje cells sets up this circuit as a context-specific gate for cerebellar output. Disruptions of inhibitory pathways and related regulatory circuits would interfere with this gate-control, lower the threshold for emotional expression, and lead to uncontrolled cerebellar output and associated pathological laughter and crying in patients with PBA.
Holtzman et al. also hypothesized that the mechanism underlying depression of Golgi-cell firing in their study may have involved an increase in serotonergic activation of inhibitory cerebellar Lugaro cells synapsing with Golgi cells, or possibly glutamatergic activation of inhibitory metabotropic glutamate receptors on Golgi cells Citation[49]. Although these speculations are intriguing because of the use of serotonergic and antiglutamatergic drugs in PBA, the function of various cells and neurotransmitters within the cerebellar microcircuitry, or their specific role in PBA, has not been clearly defined, and researchers continue their efforts to elucidate potential mechanisms within this circuitry and their potential role in cerebro–cerebellar loops Citation[50–53].
Treatment of PBA
As mentioned above, treatment of PBA has primarily involved use of medications that modulate serotonergic or glutamatergic neurotransmission. Serotonergic therapies such as amitriptyline and fluoxetine may exert effects by increasing serotonin in the synapse, and dextromethorphan may act via antiglutamatergic effects at NMDA receptors and σ-1 receptors. However, it is important to note that SSRIs and tricyclic antidepressants have affinities to a wide variety of receptor types, including σ-1 receptors, although binding affinities at the σ-1 receptor are lower for fluoxetine and amitriptyline than for dextromethorphan Citation[54]. Similarly, the binding profile of dextromethorphan is not limited to σ-1 or NMDA receptors, but also includes affinities for the 5-HT transporter and α-2 noradrenergic receptors (although lower than those reported for fluoxetine or amitriptyline) Citation[54].
Serotonergic agents
Selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants are used off-label to treat PBA, with evidence of efficacy based on findings from case studies Citation[4,27,39,55,56], open-label trials Citation[21,57] and placebo-controlled trials Citation[17,18,22,23,58,59], most of which have been small. The data from the placebo-controlled trials may be most helpful for assessing efficacy, although these studies have been criticized for not clearly defining PBA symptoms or requiring standard diagnostic criteria and for using ill-defined or inconsistent efficacy measures Citation[1,30,60,61]. We identified six placebo-controlled trials that evaluated PBA symptoms treated with fluoxetine, sertraline, citalopram, nortriptyline or amitriptyline . Five of the six studies enrolled post-stroke patients only Citation[17,22,23,58,59], and one enrolled patients with MS Citation[18].
The largest of the trials had a double-blind, parallel-group design Citation[59]; it assessed fluoxetine 20 mg/day versus placebo in 91 patients with post-stroke ‘emotional incontinence’, defined as excessive or inappropriate laughing or crying or both (compared with pre-stroke status) that had occurred on at least two occasions, as judged by the patients and their relatives without use of assessment tools. Some of these patients also had depression or ‘anger proneness’. The intensity of emotional incontinence was measured on a visual analogue scale (VAS) at baseline and after 1, 3 and 6 months of treatment. Compared with the placebo group, the fluoxetine-treated patients had significant mean percent decreases from baseline at all follow-up visits for VAS crying scores, but there were no significant differences in VAS laughing scores at any follow-up. The percent of fluoxetine-treated patients with self-reported improvement in emotional incontinence was significantly greater than in the placebo group at all follow-up visits in both the group with crying and the group with laughing as a symptom. Adverse events were reported only for the study group as a whole, which included patients with other disorders of emotion.
Another, smaller parallel-group study of fluoxetine 20 mg/day recruited 20 patients with a 4-week history of ‘emotionalism’ (undefined) Citation[23]. After 3 and 10 days of treatment with fluoxetine, scores on the modified Lawson and MacLeod scale (a measure of severity of laughing or crying episodes) were significantly reduced and the proportion of patients with >50% reduction in emotional outbursts were significantly increased compared with placebo. Adverse event rates were not reported. One sertraline-treated patient withdrew because of skin rash.
Two small studies evaluated other SSRIs for PBA symptoms in post-stroke patients. In a double-blind, parallel-group trial, 28 nondepressed patients with ‘lability of mood’ (undefined) were treated with sertraline 50 mg or placebo daily for 8 weeks Citation[58]. A significantly higher proportion of the sertraline group had improvement on a lability scale that measured frequency and other characteristics of tearfulness, and also on a clinician’s impression of global change. Laughing episodes were not reported. It was also not specified whether patients’ tearfulness was exaggerated or incongruent with experienced emotion. Skin rash, hip fracture and death (due to stroke) were reported as causes of study withdrawal in three patients from the sertraline group.
The other study of an SSRI in post-stroke patients was a double-blind crossover trial comparing citalopram 10–20 mg/day with placebo, each administered for 3 weeks to patients with ‘involuntary outbursts of crying’ Citation[22]. Six of 16 enrolled patients reported that crying was evoked by nonemotional stimuli, and three of 16 reported laughing in addition to crying. Among 13 patients whose crying frequency could be assessed, the proportion of patients with ≥50% decrease in crying episodes was significantly greater during citalopram treatment compared with placebo. HRSD scores were also significantly decreased (vs baseline). Changes in laughing episodes were not described. Although adverse events were not comprehensively described, a trend for increased incidence of orthostatic dizziness and insomnia and increased spasticity was reported during citalopram treatment.
A single study evaluated a tricyclic antidepressant (nortriptyline) in post-stroke patients with “pathological emotions or depression with excessive crying”, as defined by the patient or a referring physician Citation[17]. During 6 weeks of double-blind treatment, patients received nortriptyline (titrated up to 100 mg/day) or placebo. Two patients (one in each group of 14) had pathological laughter; the remainder had pathological crying. Mean scores on the Pathological Laughter and Crying Scale (PLACS) decreased significantly in the nortriptyline group after 4 and 6 weeks of treatment compared with the placebo group. A subset analysis of patients with comorbid major depression (n = 19) compared with nondepressed patients (n = 9) found that response on PLACS did not differ based on depression status. One patient discontinued because of sedation on nortriptyline.
Only one double-blind study evaluated serotonergic medications in a population other than post-stroke patients. This crossover trial evaluated 12 patients with MS and ‘involuntary laughing or weeping’ who completed a month of amitriptyline treatment (75 mg/day maximum dose) and a month of placebo separated by a 1-week wash-out Citation[18]. At baseline, two of these patients had laughing and weeping episodes, two had laughing only, and eight had weeping only. Reduction in weekly episode rate was significantly greater after amitriptyline treatment compared with placebo, as was the number of patients with a clinical judgment of improvement in pathologic emotionality. All patients with laughing episodes were considered responders to amitriptyline. Overall, BDI scores and HRSD scores did not change significantly; analysis by treatment group or in the group of responders was not reported. Four patients required amitriptyline dose reduction because of side effects including dry mouth and drowsiness.
A recent Cochrane review analyzed studies of antidepressants (SSRIs and tricyclic antidepressants) used for ‘emotionalism’ after stroke Citation[61]. Four of the studies reviewed above met the criteria for inclusion Citation[17,23,58,59]; in addition, the review included a study that enrolled patients with depression without a requirement of emotionalism Citation[62]. The analysis found that the frequency and intensity of emotionalism were reduced by use of antidepressants; however, the 95% confidence intervals were wide, indicating that benefit may be small, or possibly in the wrong direction. The authors concluded that recommendations on treatment of post-stroke emotionalism could not be made until better-designed trials were performed. Recommended methodological improvements included standardization of methods for diagnosing emotionalism and measuring changes, use of a standard assessment of depression (to be treated as a confounder), increase in the numbers of patients enrolled, and systematic collection and reporting of adverse event data.
Dextromethorphan/quinidine
Dextromethorphan/quinidine has antiglutamatergic properties and is an emerging therapy in PBA Citation[35,63]. Of its constituent agents, dextromethorphan has shown neuroprotective effects in animal models and, for this reason, it was studied as a potential treatment for ALS. Although neuroprotective effects were not seen, it was serendipitously noted that PBA symptoms decreased Citation[63]. Because of the rapid metabolism of dextromethorphan via CYP2D6 enzymes in the liver, it has been combined with a low dose of quinidine (a CYP2D6 inhibitor) in order to increase dextromethorphan plasma concentrations Citation[64].
Although dextromethorphan may affect serotonergic neurotransmission through binding at 5-HT transporters and receptors involved in serotonergic function Citation[54,65], its effects on glutamatergic transmission via σ-1 agonism could be particularly important for PBA treatment. Dextromethorphan binding is most prominent in the brainstem and cerebellum Citation[66], brain areas known to be rich in σ-1 receptors Citation[67] and key sites implicated in the pathophysiology of PBA Citation[37–42]. Evidence from an ERP study also suggests glutamatergic modulation at the cortical level Citation[36]. Although the precise mechanisms of DMQ in ameliorating PBA are not known, modulation of excessive glutamatergic transmission within cortico–pontine–cerebellar circuits may contribute to its benefits.
Three large, parallel-group, double-blind, multicenter, industry-sponsored trials have demonstrated efficacy of DMQ in patients with PBA Citation[35,63,68]. The first of these trials evaluated twice-daily dosing with a combination of dextromethorphan 30 mg plus quinidine 30 mg (DMQ 30/30, n = 65) compared with each drug taken individually at the same dose (dextromethorphan [DM] 30, n = 30, or quinidine [Q] 30, n = 34) for 28 days Citation[35]. Enrolled patients had ALS and PBA, as indicated by history and a score ≥13 on the Center for Neurologic Study Lability Scale (CNS-LS) Citation[69,70]. Improvement in CNS-LS score (average of day 15 and 29) was significantly greater in the DMQ 30/30 group than in either the DM 30 or Q 30 groups. Weekly laughing and crying episode rates, as recorded on patient diaries, were reduced significantly, by approximately twice as much in the DMQ 30/30 group as in the DM 30 or Q 30 groups. Scores on HRSD were low at baseline (<6 in each treatment group) and did not correlate with CNS-LS scores, indicating that patients were not depressed and suggesting that PBA improvements were not mediated by improvements in depression. Nausea, diarrhea, dizziness and headache were the most frequently reported adverse events for DMQ. Withdrawals due to adverse events were reported in 24% of the DMQ group versus 6% of the DM group and 5% of the Q group.
A second double-blind trial of DMQ 30/30 enrolled patients with MS and clinically diagnosed PBA with baseline CNS-LS scores ≥13 Citation[63]. Screening for depression symptoms was not reported; however, antidepressant use was not permitted. Patients received DMQ 30/30 (n = 76) or placebo (n = 74) twice daily for 12 weeks. Adjusted mean scores on CNS-LS (averaged across weeks 2, 4, 8 and 12) were significantly reduced in the DMQ 30/30 group versus placebo. The mean number of episodes of laughing and crying per week (from diaries) was also significantly lower for DMQ 30/30 than for placebo. Dizziness, nausea and headache were the most common adverse events in the DMQ group, although headache was reported by a greater percentage of placebo-treated patients than DMQ-treated patients. Adverse events led to treatment discontinuation in 14.5% of the DMQ group and 10.8% of the placebo group.
A recent placebo-controlled trial for new-drug submission to the FDA evaluated lower quinidine doses in DMQ for PBA Citation[68]. Nondepressed patients with MS or ALS and clinically significant PBA, as defined by a CNS-LS score ≥13, received DM 30 mg plus Q 10 mg (DMQ 30/10; n = 110), DM 20 mg plus Q 10 mg (DMQ 20/10; n = 107) or placebo (n = 109) twice daily for 12 weeks. Episodes of laughing and crying were reported on patient diaries throughout the study, and CNS-LS scores were obtained on weeks 2, 4, 8 and 12. The reduction in daily episode rate was significantly greater in both DMQ groups than for placebo, with reduction exceeding that for placebo by 46.9% in the DMQ 30/10 group and by 49.0% in the DMQ 20/10 group. Moreover, in both DMQ groups the proportion of patients reporting remission (no episodes throughout the study’s final 14 days) was significantly greater than for placebo. Mean reduction in CNS-LS score was also significantly greater than for placebo. Mental Summary mean score on the Medical Outcomes Study 36-Item Short-Form Health Survey (and its subdomains for social functioning and mental health) were significantly improved in the DMQ 30/10 group versus placebo. A significant improvement in the mean BDI II score was also demonstrated in the DMQ 30/10 group as compared with the placebo group, although patients were not clinically depressed at baseline. The authors suggested that these improvements may have resulted from quality of life improvements related to a reduction in PBA episodes, but analgesic and antidepressant effects of DMQ, as part of its more general psychotropic actions, are also possible Citation[71,72].
The overall adverse-event rate was similar regardless of treatment group. Falls, dizziness, headache, diarrhea and nausea were the most commonly reported event types; falls and headache were slightly more frequent for placebo than in either DMQ group, dizziness and diarrhea were more frequent in both DMQ groups than for placebo, and nausea rates were highest in the DMQ 30/10 group and lowest in the DMQ 20/10 group. Adverse-event-related withdrawals were most common in the DMQ 20/10 group (9.3%), followed by the DMQ 30/10 group (5.5%) and the placebo group (1.8%). Seven deaths were reported (three for DMQ 30/10, three for DMQ 20/10, and one for placebo), all of which were considered related to respiratory causes likely resulting from progression of ALS.
Although long-term DMQ usage in PBA has not been extensively studied, a 12-week open-label extension (OLE) of the above trial reported continuing efficacy of DMQ 30/10 (the only dose used). CNS-LS scores decreased further from OLE baseline, with statistical significance regardless of treatment received during the double-blind phase; however, this reduction was numerically greatest in the group that had previously received placebo Citation[72].
Expert commentary
Pseudobulbar affect is a common and burdensome disorder in patients with neurologic insult. From a patient- and family-centered perspective, the impact on daily life is not ‘pseudo’ but very real and very debilitating. Accordingly, it is important to recognize PBA and distinguish this condition from depression and other mood disorders. Although the mechanisms that underlie PBA are not fully known, recent data suggest involvement of cortico–pontine–cerebellar circuits, including potential inputs from sensory cortical areas. Disruption of frontal and cortical motor inputs into these circuits, or of the cerebellum itself, may impair its ability to act as a gate-control for emotional expression. Therapeutically, the circuits involved in PBA may be affected by drugs that modulate any number of neurotransmitters; however, current evidence suggests that serotonergic and glutamatergic transmission play key roles. As a therapeutic target, the former has been addressed by antidepressants, including SSRIs and tricyclic agents. The latter is now addressed by DMQ, a novel PBA therapy with distinctive antiglutamatergic mechanisms of action. In large, well-controlled trials, DMQ has demonstrated efficacy versus either of its components (DM or Q) and versus placebo. The recent approval of DMQ for PBA makes it the first medication with this indication and offers a needed treatment option.
Five-year view
The terminology used to describe PBA has been confusing and misleading, and despite the condition’s impact on patient quality of life, the diagnosis of PBA is often missed. Recently proposed diagnostic criteria and the availability of an FDA-approved pharmacotherapy, DMQ, may help to increase the recognition and treatment of PBA. Although SSRIs and tricyclic antidepressants have shown benefit, larger trials with better control are needed to evaluate those off-label therapies more fully. Meanwhile, elucidation of the mechanisms underlying PBA can be expected to advance. Neuroimaging studies have already refined our understanding of the condition’s neuroanatomical substrates. Electrophysiological studies may now assume a prominent role, aiding efforts not only to understand PBA, but also to differentiate it from mood disorders not simply phenomenologically but also neurophysiologically and neurochemically. As a facet of the overall research efforts, intensified study of the hypothesis that cerebellar microcircuitry performs a context-specific ‘gate-control’ function may support the conceptualization of PBA as essentially a dysmetria. As another facet of the overall research, preclinical studies of glutamatergic neurotransmission and clinical studies of its therapeutic modulation may identify potential benefits such as analgesia.
Table 1. Placebo-controlled trials of pharmacotherapeutic options in pseudobulbar affect.
Box 1. Proposed diagnostic criteria for pseudobulbar affect.
Essential criteria
• Patient experiences episodes of involuntary or exaggerated emotional expression that result from a brain disorder, including episodes of laughing, crying or related emotional displays
– Episodes represent a change in the patient’s usual emotional reactivity, are exaggerated or incongruent with the patient’s subjective emotional state, and are independent or in excess of the eliciting stimulus.
– Episodes cause clinically significant distress or impairment in social or occupational functioning.
– The symptoms cannot be attributed to another neurologic or psychiatric disorder or to the effects of a substance.
Supportive criteria
• Patient may experience accompanying autonomic changes (e.g., flushing of face) and pseudobulbar signs (e.g., increased jaw jerk, exaggerated gag reflex, tongue weakness, dysarthria and dysphagia).
• Patients may exhibit a proneness to anger.
Key issues
• Pseudobulbar affect (PBA) is a common comorbidity and source of psychosocial disability in patients with neurologic insult. However, the terminology utilized to describe it has been unclear and confusing, perhaps contributing to both under- and mis-diagnosis.
• Although the pathophysiologic mechanisms responsible for PBA are not yet well understood, recent data suggest dysfunction of cortico–pontine–cerebellar circuits, potentially affecting cerebellar input from sensory as well as frontal and motor cortical areas.
• Disruption of the microcircuitry of the cerebellum itself may likewise impair its ability to act as a gate-control for motor expression of emotion.
• Disruption of cerebellar capacity to modulate its output based on context may constitute a disinhibition reducing the threshold for emotional expression, conceivably producing an ‘affective dysmetria’ analogous to limb dysmetria.
• Neurochemically, the circuits involved in PBA may be affected by drugs that modulate any of a variety of neurotransmitters. However, current evidence suggests that serotonergic and glutamatergic transmission play key roles.
• Although antidepressants have shown benefit in PBA, interpretation of the clinical data is limited by the small numbers of patients studied and by the previous lack of standardization of PBA diagnostic criteria and measures of PBA severity.
• Dextromethorphan/quinidine is a novel PBA therapy with antiglutamatergic actions. Its recent FDA approval for treating PBA makes it the first medication with this indication.
• As life expectancy lengthens, the broad variety of neurologic disorders underlying PBA – for example, stroke, Alzheimer’s disease and Parkinson’s disease – will only become more common, creating a heightened need for PBA recognition and treatment.
Financial & competing interests disclosure
Ariel Miller has indicated that he receives travel expenses for speaking engagements from Avanir Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial assistance in the preparation of this manuscript was provided by the Curry Rockefeller Group, LLC. Support for this assistance was funded by Avanir Pharmaceuticals.
Notes
Adapted from Citation[16].
References
- Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J. Neuropsychiatry Clin. Neurosci.17(4), 447–454 (2005).
- Wortzel HS, Oster TJ, Anderson CA, Arciniegas DB. Pathological laughing and crying: epidemiology, pathophysiology and treatment. CNS Drugs22(7), 531–545 (2008).
- Gallagher JP. Pathologic laughter and crying in ALS: a search for their origin. Acta Neurol. Scand.80(2), 114–117 (1989).
- Kim SW, Shin IS, Kim JM, Lim SY, Yang SJ, Yoon JS. Mirtazapine treatment for pathological laughing and crying after stroke. Clin. Neuropharmacol.28(5), 249–251 (2005).
- Pratt RT. An investigation of the psychiatric aspects of disseminated sclerosis. J. Neurol. Neurosurg. Psychiatry14(4), 326–335 (1951).
- Feinstein A, Feinstein K, Gray T, O’Connor P. Prevalence of neurobehavioral correlates of pathological laughing and crying in multiple sclerosis. Arch. Neurol.54(9), 1116–1121 (1997).
- Petracca GM, Jorge RE, Ación L, Weintraub D, Robinson RG. Frequency and correlates of involuntary emotional expression disorder in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci.21(4), 406–412 (2009).
- Siddiqui MS, Fernandez HH, Garvan CW et al. Inappropriate crying and laughing in Parkinson disease and movement disorders. World J. Biol. Psychiatry10(3), 234–240 (2009).
- Strowd RE, Cartwright MS, Okun MS, Haq I, Siddiqui MS. Pseudobulbar affect: prevalence and quality of life impact in movement disorders. J. Neurol.257(8), 1382–1387 (2010).
- Starkstein SE, Migliorelli R, Teson A et al. Prevalence and clinical correlates of pathological affective display in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry59(1), 55–60 (1995).
- Zeilig G, Drubach DA, Katz-Zeilig M, Karatinos J. Pathological laughter and crying in patients with closed traumatic brain injury. Brain Inj.10(8), 591–597 (1996).
- Tateno A, Jorge RE, Robinson RG. Pathological laughing and crying following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci.16(4), 426–434 (2004).
- Sacco S, Sarà M, Pistoia F, Conson M, Albertini G, Carolei A. Management of pathologic laughter and crying in patients with locked-in syndrome: a report of 4 cases. Arch. Phys. Med. Rehabil.89(4), 775–778 (2008).
- Arciniegas DB, Lauterbach EC, Anderson KE et al. The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr.10(5), 1–14 (2005).
- Arciniegas DB, Topkoff J. The neuropsychiatry of pathologic affect: an approach to evaluation and treatment. Semin. Clin. Neuropsychiatry5(4), 290–306 (2000).
- Cummings JL, Arciniegas DB, Brooks BR et al. Defining and diagnosing involuntary emotional expression disorder. CNS Spectr.11(Suppl. 6), 1–7 (2006).
- Robinson RG, Parikh RM, Lipsey JR, Starkstein SE, Price TR. Pathological laughing and crying following stroke: validation of a measurement scale and a double-blind treatment study. Am. J. Psychiatry150(2), 286–293 (1993).
- Schiffer R, Herndon RM, Rudick RA. Treatment of pathological laughing and weeping with amitriptyline. N. Engl. J. Med.312(23), 1480–1482 (1985).
- Phuong L, Garg S, Duda JE, Stern MB, Weintraub D. Involuntary emotional expression disorder (IEED) in Parkinson’s disease. Parkinsonism Relat. Disord.15(7), 511–515 (2009).
- Calvert T, Knapp P, House A. Psychological associations with emotionalism after stroke. J. Neurol. Neurosurg. Psychiatry65(6), 928–929 (1998).
- Seliger GM, Hornstein A, Flax J, Herbert J, Schroeder K. Fluoxetine improves emotional incontinence. Brain Inj.6(3), 267–270 (1992).
- Andersen G, Vestergaard K, Riis JO. Citalopram for post-stroke pathological crying. Lancet342(8875), 837–839 (1993).
- Brown KW, Sloan RL, Pentland B. Fluoxetine as a treatment for post-stroke emotionalism. Acta Psychiatr. Scand.98(6), 455–458 (1998).
- Iannaccone S, Ferini-Strambi L. Pharmacologic treatment of emotional lability. Clin. Neuropharmacol.19(6), 532–551 (1996).
- Panzer MJ, Mellow AM. Antidepressant treatment of pathologic laughing or crying in elderly stroke patients. J. Geriatr. Psychiatry Neurol.5(4), 195–199 (1992).
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull.65, 193–207 (2003).
- Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR. Pathological laughter and crying: a link to the cerebellum. Brain124(Pt 9), 1708–1719 (2001).
- Ghaffar O, Chamelian L, Feinstein A. Neuroanatomy of pseudobulbar affect: a quantitative MRI study in multiple sclerosis. J. Neurol.255(3), 406–412 (2008).
- Wilson SAK. Some problems in neurology. J. Neurol. Psychopathol.4, 299–333 (1924).
- Parvizi J, Coburn KL, Shillcutt SD et al. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J. Neuropsychiatry Clin. Neurosci.21(1), 75–87 (2009).
- Haiman G, Pratt H, Miller A. Brain responses to verbal stimuli among multiple sclerosis patients with pseudobulbar affect. J. Neurol. Sci.271(1–2), 137–147 (2008).
- von Bohlen und Halbach O, Dermietzel R. Serotonin (5-hydroxytryptamine). In: Neurotransmitters and Neuromodulators: Handbook of Receptors and Biological Effects (Second Edition). von Bohlen und Halbach O, Dermietzel R (Eds). Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 132–143 (2006).
- Petty F, Davis LL, Kabel D, Kramer GL. Serotonin dysfunction disorders: a behavioral neurochemistry perspective. J. Clin. Psychiatry57(Suppl. 8), 11–16 (1996).
- von Bohlen und Halbach O, Dermietzel R. Glutamate and aspartate. In: Neurotransmitters and Neuromodulators: Handbook of Receptors and Biological Effects (Second Edition). von Bohlen und Halbach O, Dermietzel R (Eds). Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 90–107 (2006).
- Brooks BR, Thisted RA, Appel SH et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology63(8), 1364–1370 (2004).
- Haiman G, Pratt H, Miller A. Effects of dextromethorphan/quinidine on auditory event-related potentials in multiple sclerosis patients with pseudobulbar affect. J. Clin. Psychopharmacol.29(5), 444–452 (2009).
- Andersen G, Ingeman-Nielsen M, Vestergaard K, Riis JO. Pathoanatomic correlation between poststroke pathological crying and damage to brain areas involved in serotonergic neurotransmission. Stroke25(5), 1050–1052 (1994).
- Famularo G, Corsi FM, Minisola G, De Simone C, Nicotra GC. Cerebellar tumour presenting with pathological laughter and gelastic syncope. Eur. J. Neurol.14(8), 940–943 (2007).
- Parvizi J, Schiffer R. Exaggerated crying and tremor with a cerebellar cyst. J. Neuropsychiatry Clin. Neurosci.19(2), 187–190 (2007).
- Tei H, Sakamoto Y. Pontine infarction due to basilar artery stenosis presenting as pathological laughter. Neuroradiology39(3), 190–191 (1997).
- Arif H, Mohr JP, Elkind MS. Stimulus-induced pathologic laughter due to basilar artery dissection. Neurology64(12), 2154–2155 (2005).
- Oh K, Kim HJ, Kim BJ, Park KW, Lee DH. Pathological laughter as an unusual manifestation of acute stroke. Eur. Neurol.59(1–2), 83–84 (2008).
- Lauterbach EC, Price ST, Spears TE, Jackson JG, Kirsh AD. Serotonin responsive and nonresponsive diurnal depressive mood disorders and pathological affect in thalamic infarct associated with myoclonus and blepharospasm. Biol. Psychiatry35(7), 488–490 (1994).
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nature Rev.7(7), 511–522 (2006).
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex46(7), 831–844 (2010).
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum – insights from the clinic. Cerebellum6(3), 254–267 (2007).
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain121(Pt 4), 561–579 (1998).
- Parvizi J, Joseph J, Press DZ, Schmahmann JD. Pathological laughter and crying in patients with multiple system atrophy-cerebellar type. Mov. Disord.22(6), 798–803 (2007).
- Holtzman T, Rajapaksa T, Mostofi A, Edgley SA. Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J. Physiol.574(Pt 2), 491–507 (2006).
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J. Neurosci.28(5), 1140–1152 (2008).
- Crowley JJ, Fioravante D, Regehr WG. Dynamics of fast and slow inhibition from cerebellar Golgi cells allow flexible control of synaptic integration. Neuron63(6), 843–853 (2009).
- Prsa M, Dash S, Catz N, Dicke PW, Thier P. Characteristics of responses of Golgi cells and mossy fibers to eye saccades and saccadic adaptation recorded from the posterior vermis of the cerebellum. J. Neurosci.29(1), 250–262 (2009).
- Dean P, Porrill J, Ekerot CF, Jörntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat. Rev. Neurosci.11(1), 30–43 (2010).
- Werling LL, Keller A, Frank JG, Nuwayhid SJ. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp. Neurol.207(2), 248–257 (2007).
- Kim JS. Pathologic laughter after unilateral stroke. J. Neurol. Sci.148(1), 121–125 (1997).
- Ferentinos P, Paparrigopoulos T, Rentzos M, Evdokimidis I. Duloxetine for pathological laughing and crying. Int. J. Neuropsychopharmacol.12(10), 1429–1430 (2009).
- Müller U, Murai T, Bauer-Wittmund T, von Cramon DY. Paroxetine versus citalopram treatment of pathological crying after brain injury. Brain Inj.13(10), 805–811 (1999).
- Burns A, Russell E, Stratton-Powell H, Tyrell P, O’Neill P, Baldwin R. Sertraline in stroke-associated lability of mood. Int. J. Geriatr. Psychiatry14(8), 681–685 (1999).
- Choi-Kwon S, Han SW, Kwon SU, Kang DW, Choi JM, Kim JS. Fluoxetine treatment in poststroke depression, emotional incontinence, and anger proneness: a double-blind, placebo-controlled study. Stroke37(1), 156–161 (2006).
- Miller A, Panitch H. Therapeutic use of dextromethorphan: key learnings from treatment of pseudobulbar affect. J. Neurol. Sci.259(1–2), 67–73 (2007).
- Hackett ML, Yang M, Anderson CS, Horrocks JA, House A. Pharmaceutical interventions for emotionalism after stroke. Cochrane Database Syst. Rev. (2), CD003690 (2010).
- Murray V, von Arbin M, Bartfai A et al. Double-blind comparison of sertraline and placebo in stroke patients with minor depression and less severe major depression. J. Clin. Psychiatry66(6), 708–716 (2005).
- Panitch HS, Thisted RA, Smith RA et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann. Neurol.59(5), 780–787 (2006).
- Pope LE, Khalil MH, Berg JE, Stiles M, Yakatan GJ, Sellers EM. Pharmacokinetics of dextromethorphan after single or multiple dosing in combination with quinidine in extensive and poor metabolizers. J. Clin. Pharmacol.44(10), 1132–1142 (2004).
- Bermack JE, Debonnel G. Distinct modulatory roles of σ receptor subtypes on glutamatergic responses in the dorsal hippocampus. Synapse55(1), 37–44 (2005).
- Craviso GL, Musacchio JM. High-affinity dextromethorphan binding sites in guinea pig brain. I. Initial characterization. Mol. Pharmacol.23(3), 619–628 (1983).
- Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and σ-1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res. Rev.37(1–3), 116–132 (2001).
- Pioro EP, Brooks BR, Cummings J et al. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann. Neurol.68(5), 693–702 (2010).
- Moore SR, Gresham LS, Bromberg MB, Kasarkis EJ, Smith RA. A self report measure of affective lability. J. Neurol. Neurosurg. Psychiatry63(1), 89–93 (1997).
- Smith RA, Berg JE, Pope LE, Callahan JD, Wynn D, Thisted RA. Validation of the CNS emotional lability scale for pseudobulbar affect (pathological laughing and crying) in multiple sclerosis patients. Mult. Scler.10(6), 679–685 (2004).
- Thisted RA, Klaff L, Schwartz SL et al. Dextromethorphan and quinidine in adult patients with uncontrolled painful diabetic peripheral neuropathy: a 29-day, multicenter, open-label, dose-escalation study. Clin. Ther.28(10), 1607–1618 (2006).
- Pioro EP, Brooks BR, Cummings J et al. Persistent efficacy of dextromethorphan (DM)/quinidine (Q) for pseudobulbar affect (PBA): results from a 12-Week, Open-Label Extension (OLE) Study. Presented at: 62nd American Academy of Neurology Annual Meeting. Toronto, Ontario, Canada, 10–17 April 2010.