Abstract
As the Global Polio Eradication Initiative progresses toward the eradication of wild polioviruses, national and global health leaders must still actively consider options for managing poliovirus risks, including risks associated with using oral poliovirus vaccine. Oral poliovirus vaccine continues to represent a highly effective tool, but its use causes noticeable, rare cases of vaccine-associated paralytic polio and with low coverage it can evolve to become circulating vaccine-derived polioviruse that causes outbreaks. National leaders face a wide range of options, but their choices depend in part on global policies. This article explores the current set of global options for poliovirus eradication or control, discusses constraints and prerequisites for their implementation and offers some insights based on dynamic modeling to inform discussions and frame future economic analyses.
bOPV: Bivalent OPV; IPV: Inactivated poliovirus vaccine; mOPV: Monovalent OPV; OPV: Oral poliovirus; tOPV: Trivalent OPV; WPV: Wild poliovirus.
Progress toward the eradication of wild polioviruses (WPVs) continues with global efforts relying primarily on routine immunization and supplemental immunization activities (SIAs) that use oral poliovirus vaccine (OPV) to prevent and respond to WPV outbreaks Citation[1]. Achieving the 1988 World Health Assembly goal of eradicating poliomyelitis requires completely stopping the transmission of the three WPV serotypes (Types 1, 2 and 3) Citation[2]. The Global Polio Eradication Initiative (GPEI) successfully eradicated WPV Type 2 (WPV2) in 1999 Citation[3], but WPV1 and WPV3 continue indigenous circulation in endemic areas of steadily decreasing size Citation[1]. Although OPV remains an effective tool for the GPEI, its use leads to cases of vaccine-associated paralytic polio (VAPP) in a very small fraction of vaccine recipients or their close contacts Citation[4–6]. In addition, in populations that continue to use OPV with insufficient levels of coverage, OPV viruses can evolve to become circulating vaccine-derived polioviruses (cVDPVs) that lead to paralytic polio cases and outbreaks Citation[6,7]. Due to the concerns about VAPP and VDPVs after WPV eradication, the World Health Assembly recommended cessation of all routine use of OPV after global eradication of WPVs Citation[8]. The GPEI originally anticipated complete eradication of all three WPV serotypes around the same time and stopping the use of trivalent OPV (tOPV), and as of the end 2011, the global policy goal remained the eradication of all three WPVs followed by coordinated tOPV cessation. Shortly after WPV2 eradication, discussions identified the theoretically possible global vaccination options to achieve eradication, which included continuing tOPV or switching to inactivated poliovirus vaccine (IPV), bivalent OPV for Types 1 and 3 (bOPV), monovalent OPV (mOPV) for Types 1 and 3 (mOPV1 and mOPV3) or a new vaccine Citation[9]. At that time, no licensed bOPV or mOPV products existed and new vaccines remained a topic for research. Now, given >10 years since the eradication of WPV2, delays in achieving WPV1 and WPV3 eradication and the availability of an increasing variety of poliovirus vaccines, this represents a logical time to re-examine the global options.
Although no analyses exist that comprehensively explore the health and economic consequences of the currently existing global poliovirus management options, several policy analyses provide important context. Economic analyses of regional or global eradication efforts suggested net benefits Citation[10–17]. One study estimated hundreds of billions of dollars in net benefits for the USA associated with its efforts to control and eliminate polio Citation[15] and tens of billions of dollars in net benefits associated with the GPEI, which primarily impacted 104 low and lower middle-income countries Citation[16]. Another study demonstrated that the eradication of WPVs represents a preferred economic and humanitarian strategy in low-income countries compared with control as long as eradication is technically and operationally feasible within a limited time frame Citation[17]. Analysis of post-eradication policy options found that following the eradication of WPVs, coordinated OPV cessation represents a better option than the continued use of OPV, particularly continued OPV use with insufficient coverage to prevent the occurrence of cVDPV outbreaks Citation[18]. Although this analysis focused on post-eradication options following the complete eradication of all three WPV types, the insights and results extend more generally to each WPV serotype Citation[18]. For example, given successful WPV2 eradication and after stopping current cVDPV2 outbreaks, continued use of tOPV may represent a less than optimal policy (i.e., due to the risk of Type 2 cVDPVs and VAPP cases associated with continued use of OPV2 in tOPV). As long as tOPV use continues anywhere, countries should seek to achieve and maintain very high levels of coverage for all three serotypes Citation[19]. Quantitative analyses will need to assess the health and economic impacts of the current vaccine options used for routine immunization (and SIAs as needed). Performing a systematic identification of the viable policy options, including any constraints that exist with respect to implementation, represents a precursor to such analyses.
Scope
This article develops the set of current global policy vaccine options for managing the risks of polioviruses, discusses constraints and prerequisites for their implementation and offers some key dynamic insights based on modeling. Similar to prior efforts that focused on national decisions until and after global eradication [Thompson KM et al. Pre-eradication national vaccine policy options for poliovirus infection and disease control, Submitted] Citation[20], this article reviews the literature to develop a decision tree that identifies the current set of global options. All countries currently use either tOPV or IPV for routine vaccination to protect their citizens from all three poliovirus serotypes, and some countries currently perform SIAs to increase their population immunity using tOPV, bOPV, mOPV1 and/or mOPV3 [Thompson KM et al. Pre-eradication national vaccine policy options for poliovirus infection and disease control, Submitted]. The dynamic complexity and variability, the roles of different and diverse stakeholders, and the option to change policies with time complicate global policies. Using a process of systematically identifying the options helps to ensure consistency and reveals constraints in the system. Some options may only exist after time delays (e.g., some vaccine options that require significantly more capacity become available only after the construction and licensing of new facilities), and some theoretical options (e.g., increasing routine vaccine coverage to 100% Citation[21]) face practical constraints in the context of becoming viable options Citation[17]. Thus, in contrast to simply suggesting all possibilities, a thorough analysis requires specification of the options with sufficient detail to support the characterization of the associated costs and risks. The decision tree should provide useful information for ongoing discussions and future economic analyses about global poliovirus management policies and disease eradication efforts. Different national and regional policy makers may bring large differences in perspective to global discussions due to variability in their available resources and current practices.
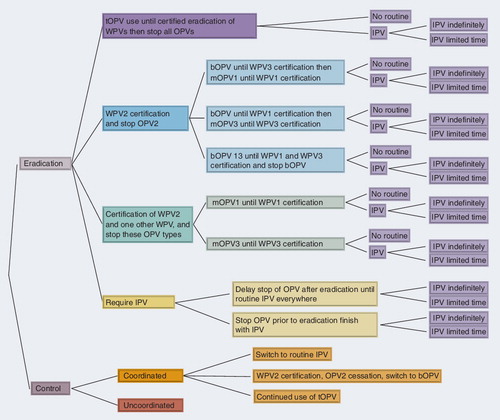
Current options
presents the decision tree of the complete set of minimum global policy options. The first branch represents the decision to continue to pursue WPV eradication or switch to control, and subsequent branches focus on the selection of the ‘minimum’ vaccine for global use in routine immunization. The minimum global options imply that all countries agree to do at least the minimum, but any individual country could always choose to do more. This approach explicitly recognizes the variability that currently exists in the world with respect to the use of IPV, the extent of existing circulation of live polioviruses (LPVs; i.e., WPV1, WPV3, cVDPVs, OPV, Sabin-like polioviruses) and levels of population immunity. For example, for the eradication options, any country could always opt to use IPV (in addition to or instead of OPV for vaccination for those options that include OPV), as long as global IPV production capacity meets demand. In addition, countries could opt to use OPV formulations containing fewer serotypes than the vaccine used for routine immunization in their SIAs (e.g., SIAs with bOPV or a mOPV could supplement the use of tOPV for routine immunization). For any option that involves OPV cessation of one or more serotypes, countries can opt to do more than the minimum global policy by using IPV or by conducting SIAs with OPV serotypes that remain, but they cannot choose to use any OPV serotypes that have previously been stopped, except perhaps in the limited context of outbreak response shortly after cessation. Those countries that elect to use IPV will need to achieve and maintain a high enough level of coverage to prevent or stop sustained transmission of LPVs within their borders. Most developed countries currently use and will probably continue to use IPV with high coverage, and this goes beyond the minimum of all identified global options. For the control options, any country can opt to use IPV in addition to or instead of OPV. Under either an eradication or control strategy, any countries using IPV may prefer to use it in a combined form to share administration costs and minimize the number of injections [Thompson KM et al. Pre-eradication national vaccine policy options for poliovirus infection and disease control, Submitted] Citation[18], but this choice may constrain schedule choices and the availability of vaccine for outbreak response Citation[22].
Any branch in that involves a choice between ‘No routine’ and IPV (light purple) ends with the choice of requiring IPV indefinitely or for a limited time, which would presumably be specified as part of the minimum option. Thus, any options that require IPV as the minimum might do so only for a limited period of time (e.g., only during the first 3, 5 or 10 years, or some other number of years following tOPV cessation, or cessation of OPV2 with or without cessation of OPV1 or OPV3). By contrast, any eradication branch that ends with a minimum option of no routine vaccination implies that countries stop all use of OPV without a global effort to implement IPV everywhere at that time, but this still allows individual countries to decide to use IPV if they wish to do so and if sufficient production capacity exists to support their use.
Focusing on the specific eradication options in , the top three branches of the tree show the multiple different pathways that exist to get from the current routine use of tOPV to no OPV. The top branch (dark purple; i.e., tOPV use until certification of eradication of all WPVs and then cessation of all OPVs) represents the current status quo option and baseline for comparison with any alternative options. The second eradication branch (dark blue) represents a global decision to declare victory with respect to the eradication of WPV2 (using the term certification although the level of formality of the process for this branch remains somewhat ambiguous) and implement OPV2 cessation. Selection of this option creates subsequent choices (light blue) related to the sequential eradication of WPV1 then WPV3 or WPV3 then WPV1, or parallel eradication of WPV1 and WPV3, with appropriate associated cessation of OPVs. The third branch (dark green) represents a global decision to declare victory with respect to the eradication of WPV2 and the next eradicated serotype of either WPV1 or WPV3 with cessation of two of the three types and a switch to the mOPV for the remaining type. With any of these top three branches, the implementation of OPV cessation will most likely depend on the availability of a low-cost IPV option for any countries that wish to use it, but the world will face a choice related to whether to require IPV use or allow no routine polio vaccination (i.e., with optional IPV as a minimum global policy). Finally, the last eradication branch (dark yellow) requires some IPV before eradication occurs, which covers a wide spectrum of possible options represented by two branches. The top light yellow branch represents the option of requiring complete phase-in of one or more doses of IPV prior to allowing any OPV cessation, which implies a potential delay of the cessation of one or more serotypes of OPV until after universal adoption of one or more doses of IPV. This option assumes that eradication efforts will continue to use OPV as the primary tool, but that adoption of at least one dose of IPV should occur (presumably with some minimal level of routine immunization coverage) before global cessation of one or more OPV serotypes to minimize the creation of immunity gaps at the time of coordinated global cessation of the serotype(s) stopped. After the universal adoption of at least one dose of IPV, the process of phasing out the three OPV serotypes would proceed along paths similar to the first (light purple), second (light blue) and third (light green) eradication branches (with IPV use continued). The costs of this option will depend on IPV costs (i.e., the vaccine and its delivery), coverage, the speed of IPV phase-in and OPV phase-out, and length of time for which the global minimum strategy requires IPV. Although it might appear and some analysts might incorrectly assume Citation[21,23] that switching to IPV could provide a ‘no risk’ option, some gaps in coverage will always exist and susceptible individuals will exist with this option Citation[18,22]. For example, even the populations in high-income countries currently using IPV with very high coverage at a high cost still contain unvaccinated individuals Citation[22].
Proper economic analyses require the use of dynamic disease models to appropriately characterize the impacts of the changing risks of polio and polio outbreaks, for example in the context of exploring the role of one or more doses of IPV in global policy Citation[18,24]. Changes in vaccination impact the probability of outbreaks and thus the number of expected cases Citation[25], and outbreak response efforts significantly influence costs and cases Citation[18,26]. A simple, static economic analysis Citation[23] that ignored important dynamic aspects of poliovirus transmission Citation[27], complexities associated with characterizing the risks Citation[25] and costs Citation[28], the impact of outbreaks and outbreak response Citation[26], and the synthesis of all these Citation[18,29], and that did not follow existing guidelines for conducting economic analyses Citation[24] provided potentially misleading results by making IPV appear as a no-risk, low-cost option. However, despite its deficiencies, that study Citation[23] confirms our point Citation[18] that the estimated cost–effectiveness ratios for the switch from OPV to IPV will depend on the assumptions about whether the use of routine OPV alone or routine OPV with SIAs represents the appropriate comparator. IPV offers some advantages due to its better immunogenicity compared with OPV, particularly in developing countries Citation[30–32], but informed policy discussions require integrated, detailed analyses that account for all costs, risks and benefits of the various vaccination options.
The bottom light yellow branch represents the option of switching from OPV to IPV as the primary tool for eradication. This option represents the extreme view that all countries should switch to the exclusive use of IPV as quickly as possible, and it implicitly assumes the possibility of achieving the eradication of WPV1, WPV3 and cVDPVs using IPV alone. This option suggests that IPV should represent the global vaccine of choice immediately such that using IPV essentially represents the primary objective, and eradication represents a secondary goal (but still a goal, because otherwise this would not be included as an eradication option). For this branch, many choices exist and they depend on specification of the time course for phasing in the IPV requirement as sufficient global vaccine supplies become available, the costs of IPV, the costs associated with achieving the levels of coverage required to prevent and/or stop outbreaks, and the health and financial costs associated with outbreaks. Selection of this option may possibly delay WPV eradication if insufficient financial and/or vaccine resources exist, and consequently characterization of this option in the eradication branch will require making IPV use assumptions consistent with achieving eradication and costing those assumptions. Any sets of assumptions that fail to explicitly target eradication belong in the control options (in the top light orange branch in ).
The bottom part of shows the complete set of minimum control options (dark gray), which explicitly includes the choice of coordinated or uncoordinated efforts. Coordination (dark orange) implies a comprehensive global management policy with ongoing global programmatic activities and costs, whereas the uncoordinated branch (dark red) entirely defers to countries to implement their preferred policy independent of the actions taken by other countries. The coordinated control branch assumes that countries will agree to maintain some level of global surveillance and outbreak response capability and agree on a minimum recommended poliovirus vaccination strategy, presumably coordinated by a group that evolves out of the GPEI Citation[33]. shows several possible minimum vaccination strategies for coordinated control (light orange). These include requiring IPV, but doing so without requiring coverage levels sufficient to achieve eradication, making a coordinated switch from tOPV to bOPV for control, or continued use of tOPV. Uncoordinated control, which represents the concept that global negotiations and planning stop and the GPEI disbands without replacement, assumes no minimum, so some countries might choose to stop all use of poliovirus vaccines, even with WPVs still circulating and OPV still in use.
Constraints
All the eradication options identified on the top branch of the tree must satisfy the constraint of achieving the global eradication goal, which requires achieving population immunity levels high enough everywhere to stop the transmission of WPVs Citation[34](which occurred in 1999 for WPV2 Citation[3]). Thus, all the options coming off the eradication branch in require active management of population immunity to achieve eradication of WPVs. Most poliovirus infections occur asymptomatically and vaccinated individuals can potentially get re-infected and participate in poliovirus transmission Citation[25,27,35], although these asymptomatic individuals remain outside of the acute flaccid paralysis surveillance system, which detects paralytic cases. Achieving WPV eradication requires the complete prevention of cases (i.e., taking action to stop the cases before they occur), which depends on stopping poliovirus transmission (i.e., the spread of all symptomatic and asymptomatic infections) Citation[34,35].
Population immunity represents a dynamic stock, like the level of water in a bath tub or a bank balance, and the level depends on inflows and outflows. Population immunity increases when individuals get infected with LPVs (either from OPV vaccination or infection with a WPV, VDPV or Sabin-like virus) or receive IPV. Population immunity decreases due to waning of immunity, deaths of individuals with immunity and births of unvaccinated individuals into a population (although maternal antibodies may provide some protection during the first few months of life, this wanes fairly quickly) Citation[30,34]. However, unlike a bank account, all population immunity cannot be instantly withdrawn, because it takes time for new susceptibles to accumulate and for immunity to wane. Population immunity requires ongoing and active management, and countries that achieve a level of population immunity high enough to stop poliovirus transmission only temporarily (i.e., countries that do not maintain population immunity above the threshold required to stop poliovirus transmission after local WPV elimination) remain vulnerable to subsequent outbreaks from WPVs that may get imported, most likely by asymptomatic individuals, and from cVDPVs Citation[34]. For example, some of the largest recent outbreaks occurred in previously WPV-free areas, and these outbreaks revealed gaps in immunity Citation[2,36].
All of the eradication options assume coordination and continued maintenance of the functionality of the GPEI through the complete achievement of WPV eradication and for as long as required to manage post-eradication risks Citation[18]. All of the eradication options that involve the cessation of any OPV further assume that the coordination of OPV cessation will represent the optimal strategy, because the lack of coordination would generally increase the opportunities for cVDPVs to develop by creating immunity gaps across borders Citation[37,38]. However, countries can always opt to switch to IPV and go beyond the minimum global policy as discussed earlier, but they will need relatively higher overall coverage to maintain the same level of population immunity as with OPV Citation[34]. Any country that switches from OPV to IPV should recognize the need to attain and maintain relatively higher overall coverage (i.e., routine immunization plus SIAs) with IPV than with OPV to maintain the same level of population immunity to compensate for the loss of secondary spread of OPV and intestinal immunity, with appropriate adjustments for different take rates Citation[34], as discussed later. Coordination will require planning to ensure that global manufacturing capacity can meet the demand for the switch (e.g., for OPV2 cessation to produce enough bOPV or IPV for global use) and coordination will come with financial costs Citation[18,37]. Cessation of any OPV serotype will also require incurring costs associated with containment, outbreak response preparedness and the creation of an appropriate vaccine stockpile for outbreak response Citation[18,37]. Recent experience with uncoordinated use of mOPVs and bOPV clearly demonstrates the potential impact of not coordinating the cessation or reduction of the use of one or more vaccine serotypes. Specifically, the GPEI introduced and prioritized the use of mOPV1 beginning in 2005 in the majority of SIAs in some endemic areas, because the relatively higher paralytic rate and apparent transmissibility associated with Type 1 (i.e., propensity to spread more rapidly geographically than Type 3) and the better seroconversion for Type 1 with mOPV1 compared with tOPV is recognized Citation[5,39]. Not surprisingly, this created immunity gaps to Types 2 and 3 in areas with demonstrated low-routine tOPV coverage, which left these areas vulnerable to WPV3 importations and the creation of cVDPV2s Citation[39,40]. The use of mOPV1 and mOPV3 led to the potential for alternating outbreaks of WPV3 and WPV1, and in 2010 the GPEI began to use bOPV for SIAs to simultaneously fight WPV1 and WPV3 Citation[39]. The GPEI continues to respond to cVPDV outbreaks in nonendemic countries as emergencies (i.e., it implements or encourages outbreak response efforts mainly with tOPV or the mOPV that corresponds to the outbreak serotype). However, the existence of the largest cVPDV2 outbreak to date in Nigeria, which reflects the emergence of multiple separate lineages Citation[41], did not immediately lead to a shift in vaccination strategy to reprioritize Type 2-containing tOPV, most likely due to greater concern about exportation of its endemic WPV1 and WPV3 and much more limited exportation of cVDPV2 Citation[39,40].
Manufacturers are key stakeholders, and vaccine production constraints place real limits on options. Although only relatively expensive licensed formulations of IPV currently exist, which require injection of a minimum of two scheduled doses, innovations continue with respect to the development of IPV products (e.g., fractional dose, adjuvant formulations, combination vaccines), delivery mechanisms (e.g., intradermal) and schedules (e.g., one-dose, two-dose, sequential IPV/OPV) Citation[42,43]. The development of these options will change the attractiveness of IPV as an option. Any discussions of IPV options must recognize the supply constraints that exist and consider realistic timelines with respect to current and projected production capacity (e.g., a minimum IPV strategy may emerge in the form of a single fractional IPV dose only at the point in time when such an option becomes practically available). Although a prior analysis identified IPV as a more effective, but not a more cost-effective option than no routine vaccination after eradication of WPVs Citation[18], ongoing efforts to reduce IPV costs may significantly improve its cost–effectiveness Citation[42,43]. However, adoption of these efforts will take time and depend on the availability of appropriate vaccine supplies, and manufacturers may need to receive non-market incentives to produce low-cost IPV. Evolution of the IPV options will necessitate iteration of economic analyses, which can help to prioritize areas for research that may then further improve the cost–effectiveness, availability and acceptability of available options Citation[18].
In addition to vaccine supply constraints, resource, logistical and other constraints may also represent important considerations, because countries will need to budget for the purchase and delivery of the vaccines that they will use. It is important to link the costs of the options in (or the set of options that each branch represents) with the associated risks and risk-management constraints. For example, establishing a minimum that requires high IPV coverage to lower risks will imply relatively higher costs (i.e., for IPV and its administration), and by contrast, minimum requirements for using IPV with low coverage will imply relatively higher risks (i.e., larger numbers of unvaccinated people). IPV production involves the use of LPVs and this implies a non-zero risk associated with the release of virus from vaccine-manufacturing facilities Citation[25]. Ongoing efforts seek to develop IPV production methods that replace the currently used WPV seed strains with safer OPV seed strains to reduce, but not eliminate, the risk of an outbreak due to an inadvertent release of virus Citation[8,44,45,101].
Evaluation and comparison of different options require making consistent assumptions about numerous issues that impact the costs and benefits of the different policy options. These assumptions include: the timing of major policy changes, such as cessation of one or more OPVs and/or implementation of routine and/or supplemental IPV use; the strategy used to increase IPV supply and the achieved levels over time, which constrain the timing of potential IPV use; the strategy to respond to post-cessation outbreaks over time given that OPV may generate new cVDPVs when population immunity becomes low; the level of surveillance required after WPV eradication to rapidly respond to outbreaks and the level of surveillance assumed for the control options; confidence about actual eradication of WPV1 and WPV3 as a function of time since the last observed case; and the specific details (e.g., products, schedule, use of SIAs, vaccine price projections) of vaccination policies adopted by countries in each branch of the tree . Due to the dynamic nature of population immunity, short-term decisions can affect long-term consequences. Careful analysis and explicit discussion of the assumptions associated with each option will help policy makers determine the best options and manage the expectations of countries, donors and the global health community.
Prerequisites to OPV cessation
Several prerequisites to OPV cessation exist. OPV cessation promises to end cases of VAPP and stop the introduction of new Sabin-like viruses that can potentially begin to circulate and evolve to cVDPVs. However, stopping new introductions of OPV does not eliminate any existing cVPDVs or any Sabin-like viruses already circulating silently (i.e., without causing detected cases) or that may begin to circulate Citation[25]. This implies that OPV cessation should only occur once global resources exist for outbreak response, including an appropriate vaccine stockpile and emergency response plans Citation[37], and following the implementation of containment efforts. Switching to IPV at the time of OPV cessation may help countries by moderating the decrease in population immunity that comes from OPV cessation, but introducing IPV will not completely eliminate the risks of potential cVDPVs Citation[18,25]. Notably, changes in the inflows and outflows that impact population immunity do not instantly lead to a change in the overall level of the stock (e.g., shutting off the water to a bathtub does not instantaneously empty it), and for those countries with population immunity levels above the threshold required to stop transmission, it will take some time for population immunity to drop below the threshold after stopping the inflow of OPV-induced immunity. Increasing the use of IPV may also imply the need to look longer for potential cVDPVs, because by removing some (but not all) susceptibles and not necessarily stopping transmission, it may take longer for a case to occur that would reveal ongoing circulation of a cVDPV Citation[46]. This leads to identification of the OPV cessation prerequisite that all countries should maintain population immunity levels above the thresholds required to prevent continued transmission of all LPVs before OPV cessation. This implies stopping all of the existing cVDPVs, which essentially behave like WPVs. Outbreaks of cVDPVs provide evidence of existing immunity gaps after it is too late to prevent them, so stopping cVDPVs should already represent a high priority. Other prerequisites for OPV cessation include developing a plan for managing the risks associated with the very small number of immunodeficient VDPV excretors, maintaining high-quality surveillance and ensuring sufficient quantities of the remaining vaccine options (e.g., IPV following tOPV cessation or bOPV and IPV following OPV2 cessation).
Since WPV2 circulation ended over a decade ago, most of the existing population immunity for Type 2 comes from tOPV or receipt of IPV for those people living in IPV-using countries. The current relatively higher proportion of existing cVDPV events caused by Type 2 reflects both the relatively lower levels of immunity from prior exposure to LPVs, and recent policies that prioritized the use of mOPV1, mOPV3 and bOPV. Thus, although OPV2 cessation promises to end VAPP2 cases and stop the introduction of new OPV2-derived viruses that might evolve to become cVDPV2s, clearly the existing gaps in Type 2 immunity must be addressed before OPV2 cessation. This implies that some additional vaccination efforts may need to occur to increase population immunity to Type 2, and such efforts could potentially compete for resources with ongoing national WPV1 and WPV3 eradication efforts in some countries. This could lead to potentially nonoptimal priority shifting if resource constraints drive decisions Citation[47]. Thus, ensuring sufficient resources to pursue optimal strategies also represents an important prerequisite to OPV2 cessation, and this might imply the need to incur higher short-term costs to achieve greater long-term net benefits. Coordination will remain essential, and existing experience with the exportation of LPVs indicates that they can and do move between countries. Thus, the situation of creating immunity gaps that could potentially begin to support the transmission of OPV2-derived viruses at the same time that other countries continue to use OPV2 represents a high-risk strategy with effects that will only become observable after a time delay. Notably, the overall reduction in cVDPV risks after OPV2 cessation will depend on the choices made now and leading up to OPV2 cessation, and developing plans to address these risks also represents a prerequisite to OPV2 cessation.
Insights from dynamic modeling
All of the current options imply that some expected future health and financial costs and the trade-offs between options involve significant dynamic complexity. As noted above, some immunity gaps will always exist, because even populations with very high vaccine coverage include some unvaccinated individuals Citation[22]. Achieving WPV eradication requires maintaining population immunity at levels above the thresholds required to prevent sustained WPV transmission, which implies high coverage, although not 100% coverage Citation[34].
One of the most significant impacts of a shift toward greater use of vaccines to control disease and from OPV to IPV relates to their impact on population immunity. Infection from endemic circulation of WPVs provides significant population immunity, albeit at a very high human cost due to relatively high rates of paralysis (e.g., on the order of one case of paralysis per 200 infections in fully susceptibles) Citation[5]. The introduction of vaccination into a population using OPV targets susceptible individuals by focusing on young children, which gives it a competitive advantage compared with WPVs given high enough levels of coverage. In addition, secondary spread of OPV further increases immunity for individuals beyond the vaccine recipient. The extent of secondary OPV immunity depends on the transmissibility of OPV Citation[48] and probably increases as immunity from WPVs decreases and as vaccination intensity decreases (i.e., more infections due to WPVs and OPV vaccinations means relatively fewer secondary OPV infections) Citation[49]. Multiple studies provide evidence that secondary OPV infections regularly occur both in developed Citation[50,51] and in developing countries Citation[42,52–55] based on the instantaneous OPV excretion rates among subjects not challenged with OPV and serology data demonstrating the cumulative effect of OPV exposure among unvaccinated children Citation[55]. The effect of secondary OPV immunity on boosting of already immune individuals remains more uncertain, with a few longitudinal studies suggesting a limited effect Citation[56,57] and excretion studies suggesting lower secondary OPV infection rates among previously infected individuals compared with susceptible individuals Citation[50,58]. As long as endemic WPV circulation continues, population immunity remains high due to infections with WPVs. However, once OPV vaccination successfully out-competes any other circulating LPVs, the potential exists for population immunity to subsequently decrease if the vaccination intensity does not remain high enough to provide a force of infection equivalent to the force of infection associated with WPV circulation Citation[34]. For this reason, cVDPV outbreaks to date occurred only in places that previously eliminated indigenous WPV transmission of the same serotype Citation[6] and reintroduction of WPV in previously polio-free countries can lead to explosive outbreaks Citation[36,59]. Moreover, as vaccination impacts the relative role of immunity from circulating WPVs on population immunity, suboptimal coverage may actually make it more difficult to achieve high enough population immunity to interrupt transmission Citation[34]. In the context of modeling the probability of undetected WPV circulation after apparent eradication, the initial conditions with respect to population immunity can significantly impact the probability of eradication occurring (within 10 years) with all else being equal Citation[46]. This reinforces prior results demonstrating that eradication occurs more quickly as the intensity of vaccination increases above the required threshold, and that vaccination at exactly the level of the threshold will achieve eradication only after a very long time Citation[17]. The recent success in India that followed significant intensification of its vaccination efforts provides some field validation of this concept. The best biological strategy to achieve eradication in a population would be to start vaccination everywhere at the highest possible level that exceeds the coverage required to eradicate the disease, because this takes maximum advantage of the momentum of the existing force of infection from WPV. Experience in the Americas and many other countries clearly took advantage of these dynamics when they rapidly stopped WPV transmission after initiating large-scale vaccination campaigns Citation[60,61].
Timing may play a critical role with respect to decisions about cessation of OPV2 alone or in combination with another OPV serotype. Depending on the strategies used going forward, the world might achieve apparent WPV3 or WPV1 eradication before meeting the prerequisites of OPV2 cessation, at which point a switch to the mOPV of the remaining type might become a realistic option (light green branches in ). However, assurance of the true eradication of the next WPV type also takes time, and thus, decisions regarding a switch to bOPV versus the last mOPV serotype will depend on when we control all cVDPV2 outbreaks, when we achieve apparent eradication of the next serotype and how much confidence we require in the true eradication of the next serotype Citation[46]. Immunization efforts in all endemic countries should focus on intensifying as much and as quickly as possible to reach all susceptible individuals and recognize that investments made in increasing population immunity during pre-eradication will help to reduce the post-eradication risks of cVDPVs Citation[34]. For example, applying the lessons from India to Nigeria suggests the urgent need for multiple intensive OPV national campaigns that achieve high coverage while targeting all serotypes and children born since at least 2001, and it may make sense to include an even larger age range in the campaigns. Similarly, intensive immunization efforts should occur throughout Pakistan and Afghanistan, with all districts viewed as high-risk areas (i.e., instead of focusing on trying to identify current high-risk areas based on retrospective acute flaccid paralysis data, the strategy should focus on an all-out assault on all three types of polioviruses and vaccinating all potentially susceptible individuals).
With respect to the switch from OPV to IPV, better seroconversion rates with IPV than OPV in developing countries Citation[31,32] imply that IPV use could provide better individual protection from paralytic disease. However, IPV does not provide much (if any) increase in enteric mucosal immunity in subjects never infected with LPVs Citation[62,63] and does not spread secondarily to provide direct immunological benefits beyond the vaccine recipient (although it may provide indirect benefits from herd protection Citation[64,65]). Thus, the evidence suggests a greater ability to participate in fecal–oral transmission for IPV-only vaccinated individuals compared with individuals with a history of at least one LPV infection (OPV vaccination or infection from WPVs), although the absolute impact remains uncertain. Consequently, population immunity to poliovirus transmission changes with time and following a switch from OPV to IPV Citation[22]. The consequences of this can be viewed in terms of coverage by recognizing the likely need to achieve a relatively higher level of coverage with IPV than OPV to maintain the same level of population immunity with respect to poliovirus transmission. The required increase in coverage to stop transmissions depends on seroconversion (or ‘take’), effectiveness of the vaccines in the population and the relative importance of different modes of transmission, because IPV provides relatively better protection from oropharyngeal than from fecal poliovirus excretion Citation[66]. Actual vaccine ‘take’ depends on maintenance of the cold chain and integrity of the vaccine delivery, monitoring and reporting systems. Issues related to apparent low vaccine efficacy and failure of OPV to take in some countries warrant focused attention, particularly in the context of paralysis reported in children who reportedly received multiple doses of OPV Citation[67]. In some cases, using IPV may offer an opportunity to bypass non-poliovirus enteric infections that may impact the ‘take’ of OPV Citation[5], but countries will need to carefully weigh the trade-offs associated with administration of an injected vaccine. Failure to vaccinate represents a significant issue for both OPV and IPV, and switching the vaccine used will not in itself take care of the problem of missing susceptible individuals, although it may impact the ability to reach some susceptible individuals (directly and/or indirectly) with vaccine.
Successful vaccination efforts can decrease public perception of the benefits of vaccines. Public perception of the relative benefits and risks of vaccines compared with the disease will most likely decline with the success of vaccination efforts (i.e., as successful vaccination efforts lead to the disappearance of the disease, perceptions about the adverse events associated with the vaccines may dominate perceptions about the risks of the disease). Thus, increasing coverage may make it more difficult to further increase and/or sustain coverage, and to eradicate Citation[17,68]. Although phasing-in and ramping-up vaccination may represent better options from a vaccine supply, budgetary and/or staff management perspective, this approach paradoxically may make eradication harder, take longer and cost more in the long run. Circulation of LPVs and the associated immunity and boosting provided by ongoing exposure play an underappreciated role in increasing population immunity and getting it closer to the threshold required to stop WPV transmission, and understanding these issues requires appreciating the dynamic complexity of population immunity. Poliovirus transmission can die out due to chance alone, particularly in small populations, and the absence of observed cases after the passage of several years after the last outbreak does not necessarily mean that the population cannot sustain transmission. Modeling populations that successfully eliminated an agent represents a much more difficult task than characterizing endemic transmission due to challenges associated with predicting when importations may occur and whether the agent will generate enough infections to sustain transmission. In addition, the thresholds required to sustain transmission in a specific population may vary by season Citation[46], which may also impact the optimal dynamics for achieving and maintaining eradication.
Expert commentary
Achieving a global goal such as eradication depends on all individual countries planning to achieve the goal on time within their borders, and this implies the need to begin efforts at the time of making the commitment. Although the World Health Assembly in 1988 committed to global polio eradication by the year 2000, remarkably, some countries only seriously focused their national efforts shortly before 2000 and some regions only began coordinated vaccination campaigns after 2000 Citation[13]. Thus, it should come as no surprise that polio eradication will take longer and cost more than expected, because the global approach reflected more of a gradual ramp-up than an intensive assault, and the GPEI operates in the context of constrained resources Citation[19]. At the same time, the enormous net health and financial benefits derived from national polio control efforts and the GPEI deserve recognition. Policy makers should continue to look for strategies that create an optimal path forward given resource constraints and societal values and preferences for health.
Five-year view
The options for managing polio continue to evolve. During the next 5 years many of the current uncertainties about potential low-cost IPV options will get resolved, and this will make the choice to switch to IPV relatively easier. However, after eradication of WPVs, even with a low-cost IPV option, some countries may prefer to use their scarce resources for vaccines of noneradicated diseases, particularly diseases with high burdens. The road that the global health leaders will choose remains unpredictable, but by clearly laying out the options, this work should stimulate further analyses and discussions of the risks, costs and benefits of the different options with full consideration of the variability in the conditions that currently exist in the world. Hopefully, ongoing discussions will better consider the dynamics that should factor into decisions. Given the large space of global options, finding the optimal path will require defining the specific details of each of the options. Economic models will need to carefully ensure consistency in assumptions to evaluate the risks, costs and benefits of the current and future global options for poliovirus management.
Increasing population sizes and the numbers of different types of vaccines will continue to make the management of vaccine-preventable diseases challenging. Public perceptions of vaccines will continue to evolve, and the trend toward parental preferences not to vaccinate children based on their perception that the risks of the vaccine exceed the risks of the disease remains a concern. Communication efforts will need to provide effective messages about the benefits of vaccines, including poliovirus vaccines. Communicating benefits of continued vaccination (with IPV) after eradication and OPV cessation may become increasingly difficult as cases of poliomyelitis fade further into memory.
Key issues
• National public health leaders face a wide range of options with important trade-offs, and collectively these complicate the development of global policy.
• Oral poliovirus vaccine (OPV) continues to represent a highly effective tool, but its use causes noticeable, rare cases of vaccine-associated paralytic polio and with low coverage it can also evolve to become circulating vaccine-derived polioviruses, which can cause outbreaks.
• All populations will include some susceptible individuals, even countries with high coverage, which suggests the need for active national risk-management efforts.
• Numerous different eradication and control options exist, and implementation of most of the options requires some level of global coordination.
• After eradication of all wild polioviruses, coordinated trivalent OPV cessation represents a better policy than continued trivalent OPV use, and this may generalize to each individual serotype (e.g., Type 2).
• Cessation of OPV Type 2 now represents a potentially viable option, with the licensure of bivalent Types 1 and 3 OPV, but we must address existing circulating vaccine-derived poliovirus Type 2 outbreaks before making a switch.
• Ongoing efforts to develop lower-cost inactivated poliovirus vaccine (IPV) options could significantly alter the relative desirability of IPV, particularly for lower-income countries, and analyses should explicitly address the change in population immunity to poliovirus transmission associated with a switch from OPV to IPV.
Acknowledgements
The authors wish to thank L Venczel, M Pallansch and three anonymous reviewers for helpful comments.
Disclaimer
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Bill and Melinda Gates Foundation.
Financial & competing interests disclosure
The authors thank the Bill & Melinda Gates Foundation for providing a contract to Kid Risk, Inc. to support the completion of this work under Work Order 4533-18487. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- WHO. Global Polio Eradication Initiative—Strategic Plan 2010–2012. WHO, Geneva, Switzerland (2010).
- World Health Assembly. Global Eradication of Poliomyelitis by the Year 2000 (Resolution 41.28). WHO, Geneva, Switzerland (1988).
- WHO. Transmission of wild poliovirus Type 2: apparent global interruption. Wkly Epidemiol. Rec. 76, 95–97 (2001).
- Alexander LN, Seward JF, Santibanez TA et al. Vaccine policy changes and epidemiology of poliomyelitis in the United States. J. Am. Med. Assoc. 292(14), 1696–1701 (2004).
- Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine-live. In: Vaccines. Plotkin SA, Orenstein WA, Offit PA (Eds). Saunders Elsevier, PA, USA, 631–686 (2008).
- Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 59, 587–635 (2005).
- Kew O, Morris-Glasgow V, Landaverde M et al. Outbreak of poliomyelitis in Hispaniola associated with circulating Type 1 vaccine-derived poliovirus. Science 296(5566), 356–359 (2002).
- World Health Assembly. Poliomyelitis: Mechanism for Management of Potential Risks to Eradication (Resolution 61.1). WHO, Geneva, Switzerland (2008).
- Cochi SL, Sutter RW, Aylward RB. Possible global strategies for stopping polio vaccination and how they could be harmonized. Dev. Biol. (Basel) 105, 153–158; discussion 159 (2001).
- Musgrove P. Is polio eradication in the Americas economically justified? Bull. Pan Am. Health Organ. 22(1), 1–16 (1988).
- Bart K, Foulds J, Patriarca P. Global eradication of poliomyelitis: benefit–cost analysis. Bull. World Health Organ. 74, 35–45 (1996).
- Kahn MM, Ehreth J. Costs and benefits of polio eradication: a long-run global perspective. Vaccine 21, 702–705 (2003).
- Aylward RB, Acharya A, England S, Agocs M, Linkins J. Global health goals: lessons from the worldwide effort to eradicate poliomyelitis. Lancet 362(9387), 909–914 (2003).
- Barrett S, Hoel M. Optimal disease eradication. Environ. Dev. Econ. 12(5), 627–652 (2007).
- Thompson KM, Duintjer Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 26(6), 1423–1440 (2006).
- Duintjer Tebbens RJ, Pallansch MA, Cochi SL et al. Economic analysis of the global polio eradication initiative. Vaccine 29(2), 334–343 (2011).
- Thompson KM, Duintjer Tebbens RJ. Eradication versus control for poliomyelitis: an economic analysis. Lancet 369(9570), 1363–1371 (2007).
- Thompson KM, Duintjer Tebbens RJ, Pallansch MA et al. The risks, costs, and benefits of future global policies for managing polioviruses. Am. J. Public Health 98(7), 1322–1330 (2008).
- Thompson KM. The role of risk analysis in polio eradication: modeling possibilities, probabilities, and outcomes to inform choices. Expert Rev. Vaccines 11(1), 5–7 (2012).
- Sangrujee NK, Duintjer Tebbens RJ, Cáceres VM, Thompson KM. Policy decision options during the first five years following certification of polio eradication. MedGenMed 5(4), 35 (2003).
- Kimman TG, Boot H. The polio eradication effort has been a great success – let’s finish it and replace it with something even better. Lancet Infect. Dis. 6(10), 675–678 (2006).
- Thompson KM, Wallace GS, Duintjer Tebbens RJ et al. Trends in the risk of U.S. polio outbreaks and poliovirus vaccine availability for response. Public Health Rep. 127(1), 23–37 (2012).
- Khan MM. Economics of polio vaccination in the post-eradication era: should OPV-using countries adopt IPV? Vaccine 26, 2032–2040 (2008).
- WHO Guide for Standardization of Economic Evaluations of Immunization Programmes. WHO, Geneva, Switzerland (2008).
- Duintjer Tebbens RJ, Pallansch MA, Kew OM et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Anal. 26(6), 1471–1505 (2006).
- Thompson KM, Duintjer Tebbens RJ, Pallansch MA. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal. 26(6), 1541–1556 (2006).
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Sutter RW, Thompson KM. A dynamic model of poliomyelitis outbreaks: learning from the past to help inform the future. Am. J. Epidemiol. 162(4), 358–372 (2005).
- Duintjer Tebbens RJ, Sangrujee N, Thompson KM. The costs of future polio risk management policies. Risk Anal. 26(6), 1507–1531 (2006).
- Duintjer Tebbens RJ, Pallansch MA, Kew OM et al. Uncertainty and sensitivity analyses of a decision analytic model for post-eradication polio risk management. Risk Anal. 28(4), 855–876 (2008).
- Plotkin SA, Vidor E. Poliovirus vaccine-inactivated. In: Vaccines. Plotkin SA, Orenstein WA, Offit PA (Eds). Saunders Elsevier, PA, USA, 631–686 (2008).
- Sutter RW, Cáceres VM, Más Lago P. The role of routine polio immunization in the post-certification era. Bull World Health Organ. 82(1), 31–38 (2004).
- WHO. Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Wkly Epidemiol. Rec. 85, 213–228 (2010).
- Arita I, Nakane M, Fenner F. Public health. Is polio eradication realistic? Science 312(5775), 852–854 (2006).
- Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SGF, Cochi SL. Modeling population immunity to support efforts to end the transmission of live polioviruses. Risk Analysis. (2012) (In Press).
- Fine PE. Herd immunity: history, theory, practice. Epidemiol. Rev. 15(2), 265–302 (1993).
- CDC. Resurgence of wild poliovirus Type 1 transmission and consequences of importation—21 countries, 2002–2005. MMWR Morb. Mortal. Wkly Rep. 55(6), 145–150 (2006).
- Thompson KM, Duintjer Tebbens RJ. The case for cooperation in managing and maintaining the end of poliomyelitis: stockpile needs and coordinated OPV cessation. Medscape J. Med. 10(8), 190 (2008).
- Barrett S. Stop! The polio vaccination cessation game. World Bank Econ. Rev. 24(3), 361–385 (2010).
- Sutter RW, John JT, Jain H et al. Immunogenicity of bivalent Types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 376, 1682–1688 (2010).
- CDC. Laboratory surveillance for wild and vaccine-derived polioviruses—Worldwide, January 2008–June 2009. MMWR Morb. Mortal. Wkly Rep. 58, 950–954 (2009).
- Wassilak S, Pate MA, Wannemuehler K et al. Outbreak of Type 2 vaccine-derived poliovirus in Nigeria: emergence and widespread circulation in an underimmunized population. J. Infect. Dis. 203(7), 898–909 (2011).
- Mohammed AJ, AlAwaidy S, Bawikar S et al. Fractional doses of inactivated poliovirus vaccine in Oman. N. Engl. J. Med. 362(25), 2351–2359 (2010).
- Resik S, Tejeda A, Lago PM et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J. Infect. Dis. 201(9), 1344–1352 (2011).
- Doi Y, Abe S, Yamamoto H, Horie H et al. Progress with inactivated poliovirus vaccines derived from the Sabin strains. Dev. Biol. 105, 163–169 (2001).
- Kreeftenberg H, van der Velden T, Kersten G, van der Heuvel N, de Bruijn M. Technology transfer of Sabin-IPV to new developing country markets. Biologicals 34(2), 155–158 (2006).
- Kalkowska D, Duintjer Tebbens RJ, Thompson KM. The probability of undetected wild poliovirus circulation after apparent global interruption of transmission. Am. J. Epidemiol. doi:10.1093/aje/kwr399 (2012) (Epub ahead of print).
- Duintjer Tebbens RJ, Thompson KM. Priority shifting and the dynamics of managing eradicable infectious diseases. Manag. Sci. 55(4), 650–663 (2009).
- Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am. J. Epidemiol. 150(10), 1001–1021 (1999).
- Chen RT, Hausinger S, Dajani AS et al. Seroprevalence of antibody against poliovirus in inner-city preschool children. J. Am. Med. Assoc. 275(21), 1639–1645 (1996).
- Benyesh-Melnick M, Melnick JL, Rawls WE et al. Studies of the immunogenicity, communicability and genetic stability of oral poliovaccine administered during the winter. Am. J. Epidemiol. 86(1), 112–136 (1967).
- Mas Lago P, Ramon Bravo J, Andrus JK et al. Lessons from Cuba: mass campaign administration of trivalent oral poliovirus vaccine and seroprevalence of poliovirus neutralizing antibodies. Bull. World Health Organ. 72(2), 221–225 (1994).
- El-Sayed N, El-Gamal Y, Abbassy AA et al. Monovalent Type 1 oral poliovirus vaccine in newborns. N. Engl. J. Med. 359(16), 1655–1665 (2008).
- Grassly NC, Jafari H, Bahl S et al. Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J. Infect. Dis. 200(5), 794–801 (2009).
- John TJ, Jain H, Ravishankar K et al. Monovalent Type 1 oral poliovirus vaccine among infants in India: report of two randomized double-blind controlled clinical trials. Vaccine 29(34), 5793–5801 (2011).
- WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in the Gambia, Oman, and Thailand. WHO collaborative study group on oral and inactivated poliovirus vaccines. Bull. World Health Organ. 74(3), 253–268 (1996).
- Nishio O, Ishihara Y, Sakae K et al. The trend of acquired immunity with live poliovirus vaccine and the effect of revaccination: follow-up of vaccinees for ten years. J. Biol. Stand. 12(1), 1–10 (1984).
- Faden H, Duffy L, Sun M, Shuff C. Long-term immunity to poliovirus in children immunized with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines. J. Infect. Dis. 168(2), 452–454 (1993).
- Gelfand HM, Potash L, Leblanc DR, Fox JP. Intrafamilial and interfamilial spread of living vaccine strains of polioviruses. J. Am. Med. Assoc. 170(17), 2039–2048 (1959).
- Outbreaks following wild poliovirus importations – Europe, Africa, and Asia, January 2009–September 2010. MMWR Morb. Mortal. Wkly Rep. 59(43), 1393–1399 (2010).
- Sabin AB. Strategy for rapid elimination and continuing control of poliomyelitis and other vaccine preventable diseases of children in developing countries. Br. Med. J. 292(6519), 513–533 (1986).
- Hull HF, Ward NA, Hull BP, Milstein JB, de Quadros C. Paralytic poliomyelitis: seasoned strategies, disappearing disease. Lancet 343(8909), 1331–1337 (1994).
- Ghendon YZ, Sanakoyeva II. Comparison of the resistance of the intestinal tract to poliomyelitis virus (Sabin’s strains) in persons after naturally and experimentally acquired immunity. Acta Virol. 5, 265–273 (1961).
- Duintjer Tebbens RJ, Pallansch MA et al. Expert review on poliovirus immunity and transmission. Risk Analysis (2012) (In Press).
- Halloran ME, Struchiner CJ, Longini IM. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am. J. Epidemiol. 146(10), 789–803 (1997).
- Paul Y. Herd immunity and herd protection. Vaccine 22, 301–302 (2004).
- Onorato IM, Modlin JF, McBean MA, Thoms ML, Losonsky GA, Bernier RH. Mucosal immunity induced by enhanced-potency inactivated and oral polio vaccines. J. Infect. Dis. 163, 1–6 (1991).
- Grassly NC, Wenger J, Durrani S et al. Protective efficacy of a monovalent oral Type 1 poliovirus vaccine: A case-control study. Lancet 369(9570), 1356–1362 (2007).
- Geoffard PY, Philipson T. Disease eradication private versus public vaccination. Am. Econ. Rev. 87, 222–230 (1997).
Website
- WHO. Global Polio Eradication Initiative: WHO calls for expressions of interest in developing Sabin-IPV: safe and affordable inactivated polio vaccine for low income countries. www.polioeradication.org/tabid/408/iid/127/Default.aspx (Accessed 7 July 2011)