ABSTRACT
We used the chamber method to measure growing season ecosystem carbon exchange and ecosystem respiration in Finnish alpine tundra. The average ecosystem respiration in the sites was 0.8–0.9 µmol m−2 s−1 and the daytime net ecosystem exchange (NEE) was around −0.4 to −0.5 µmol m−2 s−1. There were no detectable differences in cuvette-based net ecosystem exchange or ecosystem respiration between grazed fell areas and long term reindeer exclosure. Further analysis showed that net carbon exchange as well as ecosystem respiration were significantly correlated with the dwarf shrub cover, while the proportion of lichen cover (Cladina sp.) was not correlated with ecosystem carbon exchange. Clipping experiments showed that about half of the measured ecosystem respiration was heterotrophic. Plots that had been protected from reindeer grazing had almost two times higher above-ground plant biomass than grazed plots. The reason for this was 86% lower lichen biomass on the grazed side of the fell, while the biomass of Ericaceous dwarf shrubs did not differ even though there were changes in species composition. Surprisingly, the proportion of bare ground did not differ due to grazing pressure, but the reduction in biomass lead to a less stratified vegetation cover.
Introduction
Reindeer and caribou, which both belong to the same species (Rangifer tarandus L.), are widely distributed in the northern hemisphere. In Fennoscandia and Russia, reindeer pastures cover over 3,700,000 km2 and reindeer can also be found in Mongolia, China, Alaska, Canada, and Greenland. The total number of reindeer in the world is about 1.8 million (CitationTuri, 2002). While reindeer densities seem to be low compared to animal densities in more intensively managed pastoral systems, the productivity of taiga and tundra ecosystems is very low. Consequently changes in vegetation are easily observable and many regions, e.g. in northern Fennoscandia, are considered to be overgrazed.
The traditional grazing system in northern Fennoscandia is a non-rotational free grazing system with fairly low animal densities (∼1.5 animals km−2 in Finland [CitationTuri, 2002]). During winters reindeer graze mostly on felltops since the snow depth is lower there than in the forest. These felltops have a sparse and specific vegetation structure that differs from the vegetation in lower forests. In spite of the low animal densities, these felltop ecosystems have been overgrazed for a long time (CitationWielgolaski, 2002). The number of reindeer in Finland started to rise from 100,000 in the 1940s (CitationBernes, 1996), and it was around 210,000 in 1993 and 187,000 in 2001 (CitationJernsletten and Klokov, 2002). The preferred winter feed of reindeer is lichens, which during winters make up over 40% of the food, and the success of over-wintering depends on the availability of these lichens (CitationWarenberg et al., 1997). Grazing may also have a profound effect on the soil temperature due to changes in soil albedo if the white lichen cover is removed.
Grazing can have a substantial effect on the primary production of ecosystems even at moderate levels (CitationSchlesinger et al., 1990; CitationPastor et al., 1993). It can change the composition of species, as grazers usually favor certain species with low C/N ratios, high productivity and digestibility (CitationZimov et al., 1995), and low concentration of defense chemicals (CitationHobbie, 1992). It is usually thought that the highest productivity in most ecosystems is sustained by moderate grazing pressures (CitationRicklefs, 1990), even though some studies do not support this idea (CitationOlofsson et al., 2001). In the nutrient-poor continental tundra heath ecosystems, fast growing grass species do not form an essential part of the species composition even under moderate grazing pressure, and CitationOlofsson et al. (2001) concluded that in these areas graminoids are replaced by dwarf shrubs in the diet of many grazers. Grazing could have a substantial effect on the carbon balance of the felltop ecosystems due to the grazing-induced changes on the species composition and living biomass.
Carbon balance of an ecosystem is measured as the small difference of two large fluxes: the carbon assimilation through photosynthesis and carbon losses through respiration. At late successional stages, carbon fixation and ecosystem respiration do usually balance (e.g. CitationLindroth et al., 1998). Besides using carbon for growth of the above-ground biomass, plants allocate significant amounts of carbon to the root systems for root growth and maintenance (CitationHögberg et al., 2001). A large proportion of this carbon is released to the atmosphere from soil through respiration by roots, root mycorrhizal fungi, and other soil microorganisms (CitationGaudinski et al., 2000; CitationChapin and Ruess, 2001).
In this study we determine the impact of reindeer grazing on the vegetation structure and carbon exchange of felltop ecosystems in eastern Finnish Lapland. We measure the differences in species composition and above-ground biomass of vegetation, growth, ecosystem respiration, and net ecosystem exchange on ungrazed and grazed sites of fell fields. We hypothesize that grazing changes the vegetation composition of the fell fields. We also hypothesize that ecosystem respiration on fell fields is higher on ungrazed sites than on grazed sites due to higher biomass and productivity. Finally, we will investigate how soil respiration is divided between roots and microbial respiration and what is the temperature dependency of respiration.
Materials and Methods
Site Description
The measurements were conducted in Värriö Nature Park, which is located close to the Russian border in Finnish Lapland. Lowlands in the park are covered by taiga (main species Pinus sylvestris L. and Picea abies Karst.). Upper slopes of the hills are covered by mountain birch forest (Betula pubescens ssp. czerepanovii L.) with some scattered P. sylvestris trees forming the uppermost tree limit; felltops are treeless. Treeline is around 470 m a.s.l. The area has been considered to be overgrazed for a long time even though the grazing pressure has slightly decreased during the last 15 years. The Nature Park is a part of Northern-Salla reindeer owners' association, where the density of reindeer is 2.3 km−2 (data for 1998; CitationMaa-ja Metsätalousministeriö, 1999).
Annual mean precipitation in the area is about 600 mm and average annual mean temperature −1 °C (at Värriö research station, altitude 380 m). The climate in the Värriö area is subcontinental and the soil has no underlying permafrost. The growing season in the area (mean monthly temperature >5 °C) lasts for 4 months. The average temperature during the growth season (June to August) in the area was 12.6 °C in 2002 and 11.9 °C in 2004. The annual precipitation during 2002 was 580 mm and during 2004 was 530 mm. Our study was carried out on two fells, Nuortti and Värriö, which are located inside the Nature Park. The felltop of Nuortti (67°47′N, 29°42′E, altitude 481 m) overtops the treeline, and it represents a kind of a “political” grazing exclusion experiment. This fell is divided by a fence to prevent reindeer from crossing the border to Russia. The Russian side of the fence has been essentially ungrazed (the nearest Russian reindeer herding areas in the Kola Peninsula are almost 100 km east [CitationNieminen, 1983], and wild reindeer densities are very low) since the fence was built in the 1940s (CitationVäre et al., 1996; CitationStark et al., 2002), where as the Finnish side has been grazed at high reindeer densities since the 1960s and at lower densities before then (CitationBernes, 1996). Due to this we have an opportunity to study the long-term effects of grazing on the vegetation structure and the carbon balance of the fell ecosystems.
The Värriö–fell chain is situated about 11 km south of Nuortti. Our measurements were done on peak two of the Värriö fells (67°43′N, 29°36′E, altitude 475 m). The fell just overtops the treeline. The area where soil respiration was measured was treeless, while there exist scattered trees elsewhere on the felltop (at slightly lower altitudes).
Experiment 1: Effects of Grazing on Vegetation Structure and Carbon Exchange
Vegetation analyses (biomass and coverage of each species) were carried out during summer 2002. Species frequencies were analyzed as percentage of cover from 50 cm × 50 cm plots in mid-July on both sites (20 plots on Nuortti on each side of the fence, and 80 plots on Värriö-fell). Biomasses were measured from 30 cm × 30 cm plots and the vegetation was divided into mosses, lichens, annuals, grasses, and to new (ongoing year) and old growth of dwarf shrubs. The dry weights of each group were measured after drying them for 20 hours at 60 °C. Biomass was harvested three times from Värriö-fell: on 5–9 June, 1–7 July, and 7–11 August 2002 (10 plots during each month); and once, on 12–16 July 2002, on Nuortti (10 plots from each side of the fence). In calculations the area of block-scree (including the area, which was covered by thin lichen coverage [usually from rock covering species]) was accounted for in the biomass calculations. We assumed that scree was bare of vegetation, since we observed only thin and discontinuous lichen cover on the blocks.
We measured ecosystem respiration (CO2 efflux from soil surface and the plants) and net ecosystem exchange on Nuortti and Värriö fells during summer 2004. On Nuortti the first measurement campaign was carried out on 8 May and the second one on 4 June. After this the measurements were repeated at ten day intervals. On Värriö the first campaign was done on 14–15 May and the second on 10 June, after which the measurements were repeated at ten day intervals. The last measurements were done on 31 August on Värriö and on 6 September on Nuortti.
During the respiration measurements a closed dynamic chamber (volume 0.0076 m3, covered with aluminum foil) was placed on PP polypropylene collars (diameter 20.3 cm), which were permanently installed on the soil in the spring 2002 to 4 cm depth. Twelve collars were situated on each side of the fence on Nuortti-fell and 12 collars on Värriö-fell. CO2 concentration in the chamber was measured with an EGM-4 infrared gas analyzer (PPSystems, Hertfordshire, U.K.) connected to the chamber (diameter 195 mm, height 255 mm). The EGM-4 was calibrated before the measurements using a 400 ppm standard calibration gas with 1% accuracy (AGA, Lidingö, Sweden) and during the measurements the EGM-4 was zeroed after every sixth measurement to ensure the stability of the CO2 reading. We monitored the increase in carbon dioxide concentration during a period of five minutes and during that time the analyzer recorded the carbon dioxide concentration of air five times. These five readings we used to calculate the slope of CO2 concentration with time using linear regression. The pressure pulse generated in the soil upon the chamber placement was attenuated through a hole, 30 mm in diameter, on top of the chamber. The hole was closed with a rubber septum immediately after the chamber was placed on the collar.
Net ecosystem exchange (NEE) was measured in varying light conditions during daytime with the same method as the ecosystem respiration, but by using a transparent chamber having the same dimensions as the respiration chamber. Each collar was measured once, with both dark and transparent chamber, during each measurement day. As the solar elevation in these latitudes is high during summer and the upper edge of the collars was only 2 cm above-ground, the vegetation was not significantly shaded during the NEE measurements. Possible pressure increase within the chamber headspace resulting from temperature increase was balanced with a small three-way valve (BD ConnectaTM Stopcock, Beckton Dickinson, New Jersey, U.S.A.) connected on the side of the NEE chamber with a 200 cm PVC tube with 5 mm inner diameter. The valve was kept open while the chamber was on the collar to enable pressure equilibrium between the outside and inside of the chamber.
We also measured the soil temperature at 2 cm depth with a portable digital thermometer (manufacturer Suomen lämpömittari Oy, Finland) during each measurement. We determined the coverage of each species in the middle of the growing season from each collar. Soil moisture was measured during each measuring campaign by collecting five 5-cm-thick soil samples (the humus layer was removed) weighing 100–300 grams each. The samples were sealed in plastic bags until weighed and dried at the station during the same day, to prevent any loss of moisture. The samples were dried at 105 °C for 24 hours. The soil humidity was calculated as a percentage value from the fresh and dry weights of the samples.
Experiment 2: Soil Autotrophic and Heterotrophic Respiration
The contribution of soil respiration to total ecosystem respiration was studied with a trench-plot method during the summer of 2004. In the beginning of June 2004, we established four measurement plots on Nuortti on both Russian and Finnish sides of the fence. Root respiration was measured using a trenching method. Above-ground vegetation was removed from the collars by clipping. The roots growing into the collars were cut by digging a 15- to 20-cm-deep trench around the vegetation-free area. In addition, we established four similar control plots without plant and root exclusion. Respiration was measured from the collars during four measurement campaigns: before trenching, immediately after trenching, and one and two months following the trenching. From now on, the respiration measured from collars where vegetation was left intact, is called “ecosystem respiration,” whereas respiration measured without vegetation is called “soil respiration.”
The relative change in respiration rate (PR) after trenching and removal of vegetation was calculated as
where C1 = respiration in control group before the trenching, C2 = respiration in control plots at a given measurement time, T1 = respiration of the clipped group before the trenching, and T2 = respiration of the clipped group at a given measurement time.
Statistical Analyses
Statistical analyses for carbon exchange and biomass data were done with Systat and R statistical software packages. Differences were tested by analysis of variance (ANOVA). Correlations between carbon exchange and vegetation cover were calculated using a t-test for statistical comparisons between treatments. Canoco for Windows 4.5 was used in the non-linear canonical correlation analysis (CCA) for the analysis of vegetation data. Q10 values were calculated by the following equation:
where a is constant (µmol m−2 s−1), R = respiration (µmol m−2 s−1), and T = soil temperature (°C) at 2 cm at a given moment.
To calculate the effect of reindeer exclusion and bare ground on soil temperature we used the Linear Mixed Effect model using measurement date as a random variable. The calculations were done with the R 2.4.1 Statistical Software Package (CitationR Development Core team, 2007) and the NLME library (CitationPinheiro et al., 2007). The soil temperature measurements were done simultaneously with the ecosystem respiration measurements, and there were 12 points in each measurement group.
Results
1. Effects of Grazing on Vegetation Structure and Carbon Exchange
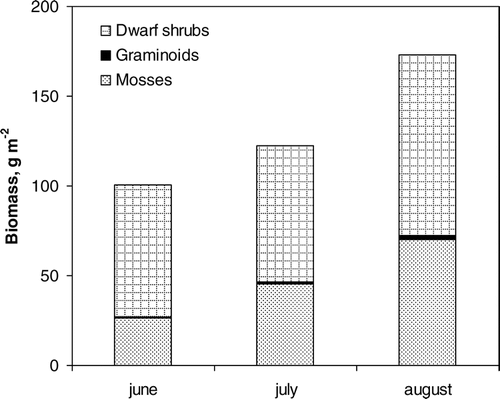
Reindeer grazing changed the above-ground biomass and composition of the vegetation. The total biomass was almost double on the ungrazed side of the fence (). The main impact of the grazing on the structure of the vegetation was a reduction in the lichen biomass (Cladina sp. and Cladonia sp.) which was 355 g m−2 (70% of all the biomass) in the ungrazed area and 50 g m−2 in the grazed area (). The difference was statistically significant at p < 0.001 using a t-test. Grazing had, also, a small effect on the composition of dwarf shrub species () and on the amount of mosses (). The area covered by Vaccinium vitis-idea (L.) was significantly higher on the grazed side of the fence on Nuortti (t-test, p < 0.001), while Arctostaphylos uva-ursi (L.) was more common on the ungrazed side of the fence (p < 0.05; , ). Other differences in the plant cover data of different species were not significant. An analysis of similarity (ANOSIM in the Vegan package of R using the Bray distance) (CitationOksanen, 2005) showed that the grazed sites (Nuortti grazed and Värriö) differed significantly from each other (p < 0.001, r = 0.20). A Canonical Correspondence Analysis showed that these differences were statistically significant (p < 0.01). However, the eigenvalues of the first two eigenvectors explained only 12.6% of the species variance (). Most of the species favored by grazing were deciduous while evergreen shrubs were more dominant on the ungrazed side. The mass of dwarf shrubs was almost equal on both sides of the fence on Nuortti; on the grazed area 204 g m−2 and on the ungrazed 228 g m−2 (the difference was not statistically significant according to Tukey's test). The lichen and moss biomasses were almost two times larger on the grazed Värriö-fell than on the grazed side of the Nuortti-fell, but as the amount of dwarf shrubs was clearly lower on the Värriö-fell, the difference in the total biomass between these two grazed sites was less than 2/3. The growth of above-ground biomass during the growing season on Värriö was about 70 g m−2 (). The area covered by bare ground did not increase due to grazing (), but the total vegetation cover was higher (calculated as the sum of area covered by each species in each measured plot) on the ungrazed site (average 1.27 m2 m−2) than on the grazed site (average 1.01 m2 m−2).
TABLE 1 Biomass distribution on Värriö-fell and on Nuortti-fell.
TABLE 2 Species composition on Värriö-fell and on ungrazed and grazed sites of Nuortti, presented as a percentage of covered ground area. If area is less than 0.5%, the number is replaced by +.
FIGURE 1 CCA analysis for study sites. Triangles refer to places as follows: V2 is the Värriö fell, N. graze is the grazed part of Nuortti, and N. ungra is ungrazed part is of Nuortti. Circles in the figure describe species as follows: Vmyrt = Vaccinium myrtillus, Dicran = Dicranum sp¸Vvitisid = Vaccinium vitis-idea, Vulig = Vaccinium uliginosum, Lboreali = Linnaea borealis, Auvaursi = Arctostaphylos uva-ursi, Aalpinus = Arctostaphylos alpinus, Enigrum = Empetrum nigrum, Lground = lichens on ground. The eigen value of first eigenvector explains 8.4% of the species variance and the second eigenvector explains 12.6% of the species variance.
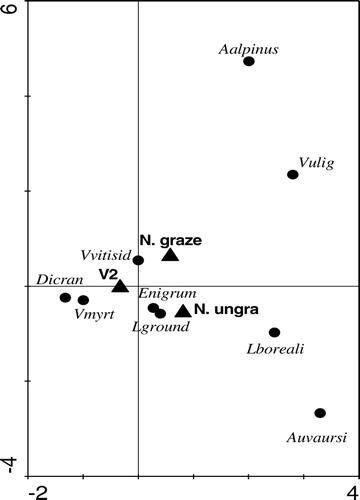
FIGURE 2 Biomass growth on Värriö during growing season 2002 (June describes the situation before growth has begun). Biomass of annuals was less than <0.02 g m−2.
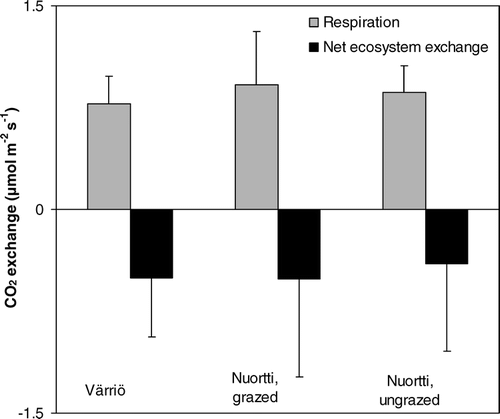
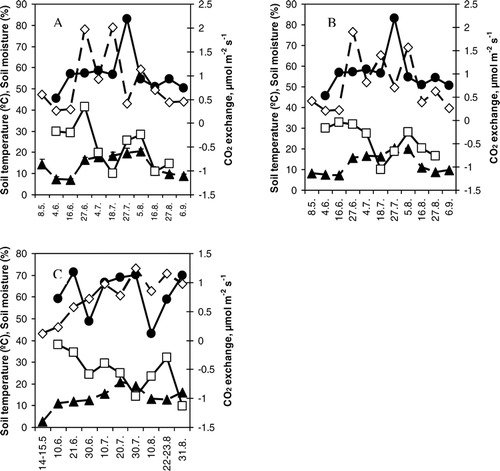
The average ecosystem respiration during the measurements on both Värriö and Nuortti was about 0.8–0.9 µmol CO2 m−2 s−1. There were no statistically significant differences between the sites (). Reindeer grazing did not have a significant effect, neither on the daytime NEE nor on the ecosystem respiration rate (). A Tukey test showed that there were no differences in the ecosystem respiration between the grazed and ungrazed collars on the Nuortti-fell. There was a correlation between the percentage of dwarf shrub cover and ecosystem respiration (R2 = 0.22, p < 0.01, when the results from both fells were used in the calculations), even though the correlations calculated separately for each site were smaller (, ). Also NEE was correlated (in each fell site p < 0.02, correlation of all sites was R2 = 0.69) with dwarf shrub cover (, ). (Note that we define NEE using the atmospheric definition, where negative NEE values mean a carbon sink and positive a carbon source.) There was a positive correlation between NEE and lichen cover (, ) and a weak negative correlation between ecosystem respiration and lichen cover (, ). These relations may be a result of the negative correlation between dwarf shrub cover and lichen cover (R2 = 0.27, p < 0.01, data not presented). Generally, the daytime NEE measured by cuvettes on both fells was negative (), showing that daytime photosynthesis was higher than daytime respiration.
FIGURE 4 (a) Correlation between average net ecosystem exchange (NEE) and dwarf shrub coverage of each cuvette collar. (b) Correlation between average ecosystem respiration and dwarf shrub (%) cover of each cuvette collar. (c) Correlation between average net ecosystem exchange and lichen (%) cover of each cuvette collar. (d) Correlation between average respiration and lichen (%) cover of each cuvette collar. • = Nuortti, grazed (broad dashed line), o = Nuortti, ungrazed (narrow dashed line), and ▴ = Värriö (solid line). The statistical calculations for the images are presented in . The correlations were statistically significant (t-test, p < 0.05) between dwarf shrub coverage and NEE on all sites, between dwarf shrub coverage and respiration on Värriö, and between lichen coverage and respiration on Värriö.
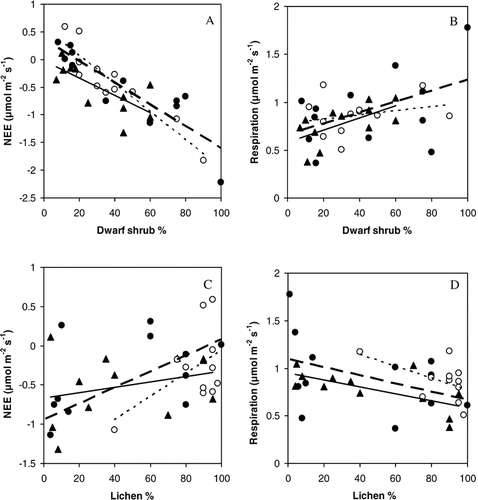
TABLE 3 Relationships between dwarf shrub and lichen cover (X, %) and ecosystem respiration and net ecosystem exchange (NEE) (Y, µmol m−2 s−1) on Nuortti and Värriö using correlation analysis.
Soil moisture did not have a significant effect on average daily carbon exchange in any of the sites (). Soil moisture values were also always comparatively high. Also soil temperature measured at 2 cm depth did not affect on average daily NEE, but it did have a positive effect on ecosystem respiration (p < 0.05 on ungrazed side of Nuortti and on Värriö; ).
FIGURE 5 Average soil temperature, soil moisture, respiration and net ecosystem exchange in (A) Nuortti grazed area, (B) Nuortti ungrazed area, and (C) Värriö fell during each measuring day. Symbols: ▴ (dashed line) = soil temperature (°C), • (solid line) = soil moisture (%), □ (solid line) = net ecosystem exchange (µmol m−2 s−1), and ◊ (dashed line) = respiration (µmol m−2 s−1). There were no statistically significant correlations between carbon exchange and soil moisture or between NEE and soil temperature, but soil temperature had a positive effect on ecosystem respiration (on Värriö R2 = 0.54 and p < 0.02; on Nuortti, ungrazed R2 = 0.51 and p < 0.02; and on Nuortti grazed R2 = 0.36 and p < 0.1). P-values were calculated using the t-test.
2. Soil Autotrophic and Heterotrophic Respiration
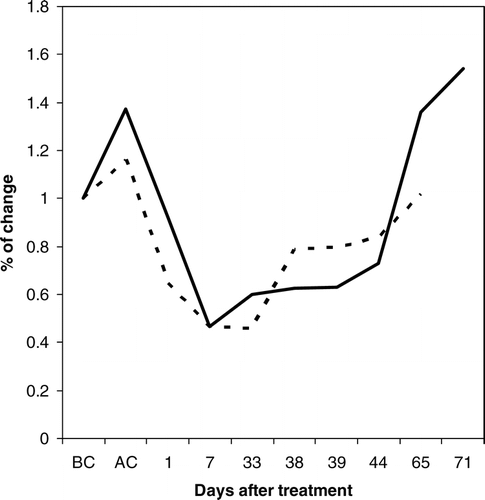
The relative respiration rate in clipped collars declined by about 50% during the first week after cutting the vegetation on Nuortti (). One month after clipping, soil respiration started to increase on Nuortti, even though there was variation in respiration between the measurement dates.
FIGURE 6 Relative change in respiration ratio (Rx) rate during the clipping experiment in grazed (dashed line) and ungrazed (solid line) sites of Nuortti. X-axis stands for days after clipping. BC = before clipping and AC = immediately after clipping. The values are normalized with respect to the values of unclipped plots as described in the materials and methods section.
Ecosystem respiration correlated with soil temperature at 2 cm depth (). The Q10 values for the sites were 1.70 for plots on Värriö fell and 2.00 for grazed and 2.33 for ungrazed side on Nuortti (). Mean soil temperature during the ecosystem respiration measurements measured at 2 cm depth was higher in measurement points situated on bare ground than in plots, where vegetation was intact on Värriö (14.8 °C and 13.5 °C, respectively; ). It was also higher on the grazed side of the fence of Nuortti (13.7 °C [grazed] and 13.1 °C [ungrazed]). According to the Linear Mixed-Effects Model the differences in temperature were statistically significant between the grazed and ungrazed sites of Nuortti (p < 0.001). The results between bare ground plots and the plots where vegetation was left intact were also statistically significant on Värriö (p < 0.001). Also the variance of temperature at 2 cm depth was higher in the bare ground plots than in the plots where vegetation was intact on Värriö (0.8 and 0.17) and on the grazed than on the ungrazed side of Nuortti (0.68 and 0.51; ). The highest correlation between soil temperature and ecosystem respiration was observed on the ungrazed side of Nuortti (R2 = 0.40; ).
FIGURE 7 Correlation between ecosystem respiration and soil temperature at 2 cm depth on Värriö and Nuortti during growing season of 2004 (June–August). Symbols: ▴ (dashed, wider line) = Värriö (R2 = 0.39), o (dashed line) = Nuortti, ungrazed (R2 = 0.40), and • (solid line) = Nuortti, grazed (R2 = 0.22).
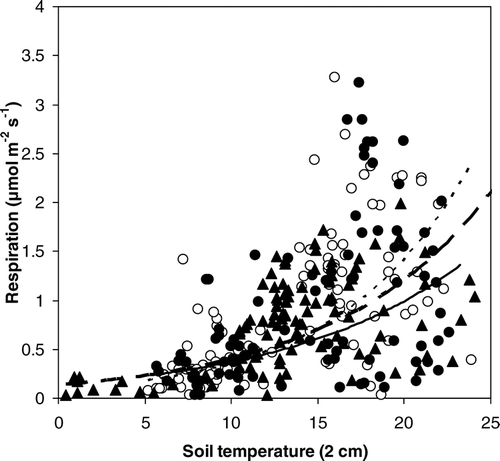
TABLE 4 Parameter values of the parameter a in Q10 formula, Q10 values, average soil temperatures (average of average value in each measurement point) measured from 2 cm depth and their variances. The average temperatures of grazed and ungrazed sites of Nuortti and bare ground and vegetated places of Värriö, respectively, differ statistically from each other using Linear Mixed-Effects Model (at p < 0.001).
Discussion
We found that grazing changed strongly vegetation structure of the fell ecosystems, but that it did not have an influence on the carbon exchange of the vegetation. We think that this paradox can be explained by the fact that some functional groups fix carbon and grow actively, while other groups have a large biomass, but low rates of photosynthesis and growth.
Grazing had a profound effect on the abundance and biomass of lichens, while changes in other biomass components were quite small. Nevertheless, the total biomass on grazed plots was only half of the biomass of ungrazed plots. CitationManseau et al. (1996) found that caribou grazing and trampling removed more than 90% of the lichen mat, which is very close to our value of 85% reduction of the lichen biomass. It is likely that the lower level of lichens in the grazed area on Nuortti compared to Värriö was due to higher grazing pressure, as grazing pressure is known to be higher next to the reindeer fence (e.g. CitationOksanen, 1978).
The results of CitationManseau et al. (1996) showed that the biomass of vascular species is clearly reduced under high grazing pressure by caribou in northern Canada. Though, according to our results and results of CitationKumpula et al. (2004), the total biomass of dwarf shrubs is not reduced due to grazing in Finland, even though there are some changes in species composition. Vaccinium uliginosum, which benefited strongly from grazing, is one of the main forage plants of reindeer during summertime (CitationKumpula et al. 2004). Also Vaccinium myrtillus is an important forage species of reindeer, but it seems to tolerate well, or according to CitationKumpula et al. (2004), even benefit from strong summertime grazing, though this was not true in our study area. Also CitationManseau et al. (1996) discovered that some food plants of reindeer can benefit from grazing pressure. They found that grazing increased the covered ground area of Vaccinium uliginosum, whereas the ground area covered by Vaccinium caespitosum (Michx.), another foraged species, decreased clearly. These small differences in the non-lichen species composition between grazed and ungrazed sites can occur either due to the direct effect of grazing and trampling (CitationOksanen and Virtanen, 1995), due to reduced amount of allelopathic extracts produced by lichens (CitationBrown and Mikola, 1974), or as a result of changed microclimatic conditions in the soil (Herder et al., 2003). According to CitationKumpula et al. (2004), grazing should also decrease the biomass of mosses, which was not the case in our study site. In fact, in our site mosses seemed to benefit substantially from grazing, most likely due to decreased competition with lichens. Also, reindeer urine and feces are known to increase the availability of nitrogen, which might have an effect on the species composition (CitationStark et al., 2002).
In spite of the large changes in the vegetation composition, carbon dioxide exchange did not differ between grazed and ungrazed areas of the fell. The dependence of NEE on dwarf shrub cover was evident on the Nuortti-fell. This suggests that dwarf shrubs dominate the carbon exchange of the system. As grazing did not have an impact on the dwarf shrub coverage of these areas, grazing could be considered “carbon neutral” in these ecosystems. Daytime NEE depended positively and respiration negatively on lichen cover on both our study sites. There was a negative correlation between NEE and dwarf shrub cover (implying higher daytime carbon sinks on dwarf shrub–dominated areas) and a positive correlation between respiration fluxes and dwarf shrub cover (implying higher respiration rates). This implies that the influence of dwarf shrubs outweighed the influence of lichens on carbon exchange, since the coverage of these two groups correlate negatively with each other. Even though lichens formed a dominant part of the living above-ground biomass on the ungrazed tundra ecosystem (with more than 60% of the total above-ground biomass), they did not seem to exchange much carbon with the environment.
Photosynthesis rates of lichens are highly variable and depend mostly on their water content. CitationGroulx and Lechowicz (1987) and CitationKärenlampi (1970) found that maximal photosynthesis rates of well-wetted Cladina stellaris are around 1.1–2.2 10−7 g CO2 g−1 s−1, while most vascular plants have rates that are usually higher than 7.5 10−7 g CO2 g−1 s−1 (CitationNiinemets, 1999). Lichens are also poikilohydric plants and they dry out rapidly after rainfall events. Due to this, their photosynthetic capacity depends highly on their relative water contents. In wind tunnel experiments it took only 100 minutes to dry wet Cladina segments to humidity levels that impaired their photosynthetic capacity, even though photosynthesis continued at lower levels until the humidity of lichens was under 10% (CitationLechowicz and Adams, 1974). CitationLechowicz and Adams (1974) showed that the photosynthesis rates per unit mass of lichen segments decline with lichen age (or size) since the inner parts of the lichens have much lower rates of photosynthesis. While these measurements certainly underestimate the time it takes for lichens to dry, since the measurements were done with warm dry air, the data shows that it is not likely that lichens contribute as much to the production of our fell field ecosystems as dwarf shrubs do. However, as lichens have high above-ground biomasses and due to this they contain a significant amount of carbon, they are an important component of these ecosystems.
Usually lichens are slow-growing organisms (e.g. CitationManseau et al., 1996), though this view has been lately questioned (Herder et al., 2003). The results in lichen growth rate vary considerably depending on the habitat and the age of the stand (CitationKumpula et al., 2000; Herder et al., 2003; etc.). The growth rates of Cladina and Cladonia species range between 0.11 and 0.18 g g−1 y−1 in open arctic environments in Scandinavia (CitationCooper and Wookey, 2001; Herder et al., 2003; CitationKärenlampi and Kytöviita, 1988). Herder et al. (2003) also stated that lichens can grow even better in subarctic heathlands than in forests. This variation in lichen growth can be explained by varying moisture conditions, which control the carbon exchange of lichens and due to this, affect greatly the growth capacity of lichens (CitationKärenlampi, 1971). Also the age and condition of the lichen carpet affects the growth rate, as well as the attendance of grazers (Herder, 2003). Herder (2003) as well as CitationKumpula et al. (2000) pointed out that after the grazing is stopped, the growth rate of lichens may be low for even 20 years, because harsh environmental conditions inhibit the recovery of lichen mats. After this initial recovery period, the growth rate of lichens speeds up. Also the work of CitationKumpula et al. (2000) indicated low net growth rates of mature lichen carpets.
When we estimate the total annual biomass production of the site, we have to take into consideration that there is a large, non-estimated amount of production underground due to root growth. We did not measure the root biomass production, thus the actual production at our sites will be superior to the 70 g m−2 y−1 estimated for Värriö fell. The annual production of the lichen component is probably inferior to the production of dwarf shrubs, averaging 5.5 g g−1 m−2 y−1 for the grazed and 40 g g−1 m−2 y−1 for the ungrazed site on Nuortti, when using the lichen growth rate estimate of CitationKärenlampi and Kytöviita (1988). We also acknowledge a certain possibility for a bias in our gas exchange measurements. We did not measure during rain (since our measurement equipment was not rainproof like most photosynthesis equipment are not), but we frequently visited the site on days after rain, or interrupted our measurements for the duration of a rain shower. Therefore, we believe that possible biases are small and our measurements are representative for the conditions during our two field summers.
Ecosystem respiration in the area was low. Its mean values were 0.8–0.9 µmol CO2 m−2 s−1 in both sites. These results are in the lower range of previous measurements in arctic and alpine environments. CitationSolomon and Cerling (1987) estimated soil efflux to be around 1.8 µmol CO2 m−2 s−1 in alpine grassland in the United States in August, whereas CitationBillings et al. (1982), who measured soil respiration in Alaska, observed respiration levels of 2.6 µmol CO2 m−2 s−1. CitationElberling and Brandt (2003) measured soil CO2 flux of 0.2–1.1 µmol CO2 m−2 s−1 in the middle of a growing season in Iceland, depending highly on the soil temperature. Our tundra ecosystem probably has a lower productivity than the sedge-dominated ecosystems in the Rocky Mountains and Alaska. Therefore our respiration rates are lower. The values of CitationGrogan and Jonasson (2005) in a similar tundra ecosystem seem to be very close to our values (with values around 0.9 µmol m−2 s−1 at 15 °C).
Daily average ecosystem respiration and NEE did not correlate with soil moisture, which implies that water is not a restricting factor for the carbon exchange in the study sites, which differs from the results of CitationIlleris and Jonasson (1999) who measured relations of CO2 emissions and moisture conditions in the Abisco area. There was a correlation with ecosystem respiration and soil temperature in our study sites, but not with NEE and soil temperature, which might imply that the soil respiration is more sensitive to the temperature variations than the gas exchange of plants and lichens.
Q10 values were about the same for both the grazed and ungrazed plots. The Q10 values were similar to the values reported elsewhere in the literature, but substantially lower than the values of CitationGrogan and Jonasson (2005) who worked in a similar ecosystem in eastern Sweden. We argue, based on the tables of CitationGrogan and Jonasson (2005), that their ecosystem might have had higher soil fertility and a higher proportion of deciduous plants. Their work took place during the first half of the summer. Since Q10 values from chamber measurements integrate vegetation development and responses of vegetation to temperature changes, their Q10 values might have been lower if their measurements would have covered the whole growing season, which was the case in our measurements.
We do not comment on possible differences in the yearly carbon balance of the fell, since our data was measured during the summer only. Previous research found that winter respiration might continue and contribute to the carbon balance of tundra in a significant way. Although respiration rates during winter are low, respiration does not stop at wintertime and as winter in these latitudes is long, even low respiration rates can contribute substantially to the annual carbon balance (CitationKähkönen et al., 2001). The effect of microbial respiration under snow is particularly important in springtime before the snowmelt, when the microbial activity contributes significantly to the annual carbon exchange. For example, CitationSommerfeld et al. (1993) discovered that alpine soils have a positive flux of 0.13–0.16 µmol CO2 m−2 s−1 during spring time before the snow melt, and CitationFahnestock et al. (1998), who measured soil efflux in dry tundra heath in Alaska, found carbon exchange to be less that 0.16 µmol CO2 m−2 s−1 before the snowmelt. Also, CitationGrogan and Jonasson (2005) found that CO2 emissions during winter in Abisko, Sweden, were significant due to the length of the snow-covered period.
Respiration declined rapidly after clipping and trenching and reached a minimum of about half of the initial rate. Then the respiration of the clipped plots increased slowly. Our interpretation of the data is that the initial decrease is caused by the death of roots. The minimum values of soil respiration after clipping are, therefore, an estimate of the heterotrophic soil respiration. The final increase results from the start of the decomposition of the root litter. Previous studies have shown that soil respiration is responsible for about 50–70% of the total ecosystem respiration in open arctic or alpine environments (CitationWohlfahrt et al., 2005; CitationGrogan and Jonasson, 2005). Our results fit into that range. Even though soil microbe numbers are high in alpine soils (CitationKörner, 1999), growth and activity of soil microbes in arctic and alpine environments are slow due to the cold temperatures (CitationRosswall et al., 1975). Therefore, decomposition is slower in alpine soils than in lowland ecosystems (CitationRosswall et al., 1975; CitationBerg et al., 1975). In our study site it took at least two months before the post-clipping soil respiration peaked. The last measurement was two months after the cutting, so it is possible that the highest point of microbial respiration would have been even later. This shows that considerable delays between litter input and peak respiration are likely in these arctic ecosystems.
In summary, grazing decreased substantially the biomass and cover of lichens. Carbon exchange of the vegetation and soil to the atmosphere did not change due to grazing, although the above-ground biomass was reduced to about half compared to the ungrazed site. The reason is that the ecosystem carbon exchange is dominated by the dwarf shrub component of the system. Lichens, while reaching high biomasses due to their longevity, are relatively unproductive and do not affect ecosystem carbon exchange very much. However, it would be important to analyze their role in ecosystem water and energy exchange, since they seemed to affect soil energy balance and in this way the soil temperature.
Acknowledgments
We thank European Union–funded CARBOMONT project (contract number EVK2-CT-2001-00125), University of Helsinki, Treeline project and Finnish Cultural Foundation. We also thank the staff of Värriö research station and Pepe Hari for all their help and support during the measurements and the writing process. Finally, we thank Mauri Nieminen from the Game and Fisheries research institute for consultation. F. Berninger had also some helpful discussions with Martin Lechowicz on lichen photosynthesis.
References Cited
- Berg, B. , L. Kärenlampi , and A. K. Veum . 1975. Comparisons of decomposition rates measured by means of cellulose. In Wielgolaski, F. E. , editor. Fennoscandian Tundra Ecosystems, Part 1 Berlin Springer-Verlag. 261–267.
- Bernes, C. 1996. Valoa ja kaamosta—Arktinen ympäristö Pohjoismaissa Copenhagen Pohjoismaiden ministerineuvosto.
- Billings, W. D. , J. O. Luken , D. A. Mortensen , and K. M. Peterson . 1982. Arctic tundra: a source or sink for atmospheric carbon dioxide in a changing environment. Oecologia 53:7–11.
- Brown, R. T. and P. Mikola . 1974. The influence of fruticose soil lichens upon the mycorrhizae and seedling growth of forest trees. Acta Forestalia Fennica 141:5–22.
- Chapin III, F. S. and R. W. Ruess . 2001. The roots of the matter. Nature 411:749–752.
- Cooper, E. J. and P. A. Wookey . 2001. Field measurements of the growth rates of forage lichens, and the implications of grazing by Svalbard reindeer. Symbiosis 31:173–186.
- Elberling, B. and K. K. Brandt . 2003. Uncoupling of microbial CO2 production and release in frozen soil and its implications for field studies of arctic C cycling. Soil Biology & Biochemistry 35:263–272.
- Fahnestock, J. T. , M. H. Jones , P. D. Brooks , D. A. Walker , and J. M. Welker . 1998. Winter and early spring CO2 efflux from tundra communities of northern Alaska. Journal of Geophysical Research 103:D22 29023–29027.
- Gaudinski, J. B. , S. E. Trumbore , E. A. Davidson , and S. H. Zheng . 2000. Soil carbon cycling in a temperate forest: radio-carbon based estimates of residence times, sequestration rates and partitioning of fluxes. Biogeochemistry 51:33–69.
- Grogan, P. and S. Jonasson . 2005. Temperature and substrate controls on intra-annual variation in ecosystem respiration in two subarctic vegetation types. Global Change Biology 11:465–475.
- Groulx, M. and M. J. Lechowicz . 1987. Net photosynthetic recovery in subarctic lichens with contrasting water relations. Oecologia 71:360–368.
- Den Herder, M. 2003. Impacts of ungulates in boreal forest and subarctic tundra ecosystems in Finland. University of Joensuu, Research Notes 152 pp.
- Den Herder, M. , M-M. Kytöviita , and P. Niemelä . 2003. Growth of reindeer lichens and effects of reindeer grazing on ground cover vegetation in a Scots pine forest and a subarctic heathland in Finnish Lapland. Ecogeography 26:3–12.
- Hobbie, S. E. 1992. Effects of plant species on nutrient cycling. Trends in Ecology & Evolution 7:336–339.
- Högberg, P. , A. Nordgren , N. Buchmann , A. F. S. Taylor , A. Ekblad , M. N. Högberg , G. Nyberg , M. Ottosson-Löfvenius , and D. J. Read . 2001. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792.
- Illeris, L. and S. Jonasson . 1999. Soil and plant CO2 emission in response to variations in soil moisture and temperature and to amendment with nitrogen, phosphorus, and carbon in northern Scandinavia. Arctic, Antarctic, and Alpine Research 31:264–271.
- Jernsletten, J-L. L. and K. Klokov . 2002. Kestävä Porotalous [in Finnish] Tromssa, Norway Gjøvik Trykkeri AS.
- Kähkönen, M. A. , C. Wittmann , J. Kurola , H. Ilvesniemi , and M. S. Salkinoja-Salonen . 2001. Microbial activity of boreal forest soil in a cold climate. Boreal Environment Research 6:19–28.
- Kärenlampi, L. 1970. Distribution of chlorophyll in the lichen Cladonia alpestris . Reports of Kevo Subarctic Research Station 7:1–8.
- Kärenlampi, L. 1971. Studies on the relative growth rate of some fruticose lichens. Reports of Kevo Subarctic Research Station 7:33–39.
- Kärenlampi, L. and M-M. Kytöviita . 1988. Kuinka nopeasti jäkälä kasvaa? [in Finnish]. Poromies 55:4–7.
- Körner, C. 1999. Alpine plant life Berlin Springer-Verlag.
- Kumpula, J. , A. Colpaert , and M. Nieminen . 2000. Condition, potential recovery rate, and productivity of lichen ( Cladonia spp.) ranges in Finnish reindeer management area. Arctic 53:152–160.
- Kumpula, J. , H. Norberg , and M. Nieminen . 2004. Kesälaidunnuksen vaikutukset poron ravintokasveihin—Kesälaitumet ja porojen kunto [in Finnish]. Kala-ja Riistaraportteja 319:46.
- Lechowicz, M. J. and M. S. Adams . 1974. Ecology of Cladonia lichens: II comparative ecology of C. rangiferina , C. mitis and C. uncialis. . Canadian Journal of Botany 52:411–422.
- Lindroth, A. , A. Grelle , and A. S. Moren . 1998. Long-term measurements of boreal forest carbon exchange reveal large temperature sensitivity. Global Change Biology 4:443–450.
- Maa-ja Metsätalousministeriö, 1999. Porolukujen tarkistamistyöryhmän esitys suurimmiksi sallituiksi eloporoluvuiksi [in Finnish]. Työryhmämuistio 20:29.
- Manseau, M. , J. Huot , and M. Crête . 1996. Effects of summer grazing by caribou on composition and productivity of vegetation: community and landscape level. Journal of Ecology 84:503–513.
- Nieminen, M. 1983. Neuvostoliiton porotalous ja porotutkimus [in Finnish]. Poromies 6:32–44.
- Niinemets, Ü 1999. Components of leaf dry mass per area thickness and density alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytologist 144:35–47.
- Oksanen, J. 2005. Multivariate Analysis of Ecological Communities in R: vegan tutorial (http://cc.oulu.fi/∼jarioksa/opetus/metodi/).
- Oksanen, L. 1978. Lichen grounds of Finnmarksvidda, northern Norway, in relation to summer and winter grazing by reindeer. Reports from the Kevo Subarctic Research Station 14:64–71.
- Oksanen, L. and R. Virtanen . 1995. Topographic, altitudinal and regional patterns in continental and suboceanic heath vegetation of northern Fennoscandia. Acta Botanica Fennica 153:1–80.
- Olofsson, J. , H. Kitti , P. Rautiainen , S. Stark , and L. Oksanen . 2001. Effects of summer grazing by reindeer on composition of vegetation, productivity and nitrogen cycling. Ecography 24:13–24.
- Pastor, J. , B. Dewey , R. J. Naiman , P. F. McInnes , and Y. Cohen . 1993. Moose browsing and soil fertility in the boreal forests of Isle Royale National Park. Ecology 74:467–480.
- Pinheiro, J. , D. Bates , S. DebRoy , and D. Sarkar . 2007. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–83.
- R Development Core Team 2007. R: a language and environment for statistical computing. Vienna, Austria R Foundation for Statistical Computing.
- Ricklefs, R. E. 1990. Ecology New York W. H. Freeman and Company.
- Rosswall, T. , A. K. Veum , and L. Kärenlampi . 1975. Plant litter decomposition at Fennoscandian tundra sites. In Wielgolaski, F. E. , editor. Fennoscandian Tundra Ecosystems, Part 1 Berlin Springer-Verlag. 268–278.
- Schlesinger, W. H. , J. F. Reynolds , G. L. Cunningham , L. F. Huenneke , W. M. Jarrell , R. A. Virginia , and W. G. Whitford . 1990. Biological effects of global desertification. Science 247:1043–1048.
- Solomon, D. K. and T. E. Cerling . 1987. The annual carbon dioxide cycle in a montane soil: observations, modeling, and implications for weathering. Water Resources Research 23:2257–2265.
- Sommerfeld, R. A. , A. R. Mosier , and R. C. Musselman . 1993. CO2, CH4 and N2O flux through a Wyoming snowpack and implications for global budgets. Nature 361:140–142.
- Stark, S. , R. Strömmer , and J. Tuomi . 2002. Reindeer grazing and soil microbial processes in two suboceanic and two subcontinental tundra heaths. Oikos 97:69–78.
- Turi, J. M. 2002. The world reindeer livelihood—Current situation, threats and possibilities. In Kankaanpää, S. , L. Müller-Wille , P. Susiluoto , and M-L. Sutinen , editors. Northern timberline forests: environmental and socio-economic issues and concerns Jyväskylä Gummerrus Kirjapaino Oy. 70–75.
- Väre, H. , R. Ohtonen , and K. Mikkola . 1996. The effect and extent of heavy grazing by reindeer in oligotrophic pine heaths in northeastern Fennoscandia. Ecography 19:245–253.
- Warenberg, K. , Ö Danell , E. Gaare , and M. Nieminen . 1997. Porolaidunten kasvillisuus [in Finnish] Helsinki, Finland Pohjoismainen Porontutkimuselin and A/S Landbruksforlaget.
- Wielgolaski, F-E. 2002. Nordic mountain birch forests. In Kankaanpää, S. , L. Müller-Wille , P. Susiluoto , and M-L. Sutinen , editors. Northern timberline forests: environmental and socio-economic issues and concerns Jyväskylä Gummerrus Kirjapaino Oy. 76–90.
- Wohlfahrt, G. , M. Bahn , A. Haslwanter , C. Newesely , and A. Cernusca . 2005. Estimation of daytime ecosystem respiration to determine gross primary production of a mountain meadow. Agricultural and Forest Meteorology 130:13–25.
- Zimov, S. A. , V. I. Chuprynin , A. P. Oreshko , F. S. Chapin III , J. F. Reynolds , and M. C. Chapin . 1995. Steppe-tundra transition: A herbivore-driven biome shift at the end of the Pleistocene. The American Naturalist 146:765–794.