ABSTRACT
This study documents the spatiotemporal distribution of free-living nematodes in glacial-fed river reaches (Möll River, Austrian Alps), which is a widely neglected topic in glacial-fed stream research. Benthic nematodes of the meta- and hypokryal, in a lotic reach within a glacially formed floodplain and in the glacio-rhithral, were investigated in spring, summer, and autumn 2005. The overall nematofauna was diverse and dense with 77 species, H′ of 2.7, and mean abundance of 86 individuals per 10 cm2. Nematode community parameters were significantly influenced by sites, but not by seasons. At the kryal sites, nematode overall mean abundances slightly increased from the metakryal (8 ind. per 10 cm2) to the hypokryal (11 ind. per 10 cm2), but species number decreased (from 15 species to 6 species). Nematodes became more dense and diverse in both the glacio-rhithral (overall mean: 49 ind. per 10 cm2; 52 total species) and the floodplain reach (overall mean: 251 ind. per 10 cm2; 65 total species). Species composition and dominant species differed among the four sites. Deposit feeders represented the dominant feeding type at each site. The lowest maturity index reflected the harsh character of the recently deglacierized meta- and hypokryal sites.
Introduction
Glacial-fed stream reaches constitute one of the main (CitationFüreder et al., 2002) and most sensitive (CitationMcGregor et al., 1995) types of running waters in high-altitude catchments. Glacial reaches are extremely harsh freshwater habitats, where several factors, mainly governed by glacial meltwater, account for a high-stress environment to organisms: low temperatures, channel instability, extreme discharge fluctuations (daily and seasonal), highly varying turbidity, and limited food resources (CitationMilner et al., 2001a; CitationWard, 1994; CitationSteffan, 1971). In general, environmental harshness diminishes downstream with increasing distance from the glacier terminus (CitationBurgherr and Ward, 2001; Milner and Petts, 94). Thus, a longitudinal zonation has been established wherein water temperature acts as the main descriptor for the respective zones: the metakryal with temperatures ≤2°C, the hypokryal with temperatures between 2 and 4°C (both zones: CitationSteffan, 1971), and the glacio-rhithral where temperatures range from 4 to 10°C (CitationFüreder, 1999). Water chemistry and water physics of glacial reaches are generally affected by local and regional settings such as climate and geomorphology.
Typically, the rigorous glacial stream habitats harbor less dense and less diverse benthic biota. Their spatial and temporal variation is well documented in relation to the amelioration of environmental harshness. Due to the harsh conditions at the metakryal, the benthic community is less developed there than in the hypokryal and glacio-rhithral (CitationBrown et al., 2006; CitationIlg and Castella, 2006; CitationFüreder et al., 2005; CitationBurgherr and Ward, 2001; CitationFüreder et al., 2001; CitationLods-Crozet et al., 2001). The metakryon is very similar between glacial rivers and consists mainly of a few individuals in several species of Diamesa. In contrast, the more developed downstream communities differ structurally among glacial rivers. Differences have partly been explained by site-specific habitat characteristics. Confluences of lake outlets and non-glacial tributaries buffering the environmental harshness, for example, support a more developed biota (CitationHieber et al., 2005; CitationKnispel and Castella, 2003; CitationMilner and Petts, 1994). Habitat patches of less harsh environments among glacial floodplains favor benthic productivity (CitationMalard, 2003). More beneficial combinations of abiotic factors in autumn and winter involve seasonal peaks of density and diversity (CitationRott et al., 2006; CitationBürgi et al., 2003, CitationBurgherr and Ward, 2001, CitationFüreder et al., 2001; CitationMilner et al., 2001a; CitationSchütz et al., 2001). Finally, older habitats with long time spans since deglaciation form more stable environments with more highly structured benthic macrofauna communities (CitationMilner and Petts, 1994).
The descriptions above refer mainly to macrofauna, which has been the focus of glacial stream research during the past several decades. Benthic microbiota have gained less attention (CitationRott et al. 2006; CitationHieber et al., 2003; CitationGessner and Robinson, 2003; CitationBattin et al., 2001), and the benthic meiofauna (animals which are intermediate in size between macrofauna and microfauna, especially the smallest visible interstitial benthic or soil invertebrates up to about a millimeter in length) represent the least investigated group, albeit a group that is generally considered important in lotic systems (CitationRobertson et al., 2000). Among this meiofauna, free-living nematodes are abundant and species rich (CitationTraunspurger, 2002). Research on freshwater nematodes in recent years corroborates their importance in freshwater food webs (CitationAbebe et al., 2006). Besides high abundances and high diversity, their importance is also based on their different feeding types and a wide variety of food utilization (CitationBilgrami and Gaugler, 2004), which enables them to influence important ecosystem processes such as decomposition (CitationTraunspurger et al., 1997) and primary production (CitationMontagna, 1995). Nematodes also have abilities to survive freezing (CitationWharton, 2003), desiccation (CitationTreonis et al., 2000; CitationWomersley et al., 1998), and low food resources (CitationPerry and Wright, 1998). Given these abilities, nematode presence in extreme freshwater habitats (reviewed in CitationHodda et al., 2006) does not seem surprising. But in spite of these factors, scarce data exist on nematode distribution in glacial streams, where they could be assumed to be present and, hence, to be of importance.
The objectives of this study were to examine free-living nematodes inhabiting the benthos of four glacially fed stream reaches of the upper Möll River in the Hohe Tauern National Park (Austrian Alps) during June, August, and October 2005. The results show spatial variability of nematofauna attributes (abundances, species distribution and diversity, feeding types, maturity) between the glacial source, a hypokryal reach, a floodplain segment, and a glacio-rhithral reach and the seasonality among them. The conformance of observed nematode patterns with common concepts of spatiotemporal variation in glacial-fed stream biota is verified. Furthermore, nematode maturity is considered as a potential assessment of both habitat succession due to deglaciation (glacial retreat) and biotic stress in glacially fed streams.
Material and Method
Study Site
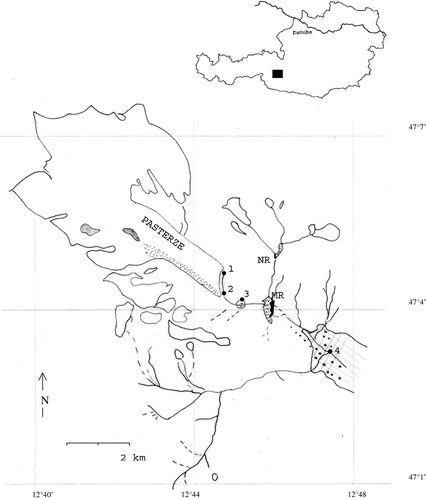
The study site was situated at the headwaters of the Möll River in the Hohe Tauern National Park (Eastern Alps, Austria; ). The Möll River is a tributary of the Drau River, the fourth largest tributary of the River Danube. The total catchment of the 76-km-long Möll River is 1105 km2 with 3% glacier area. The study site catchment is 80 km2, of which 24 km2 are glaciated (at the present covered with glaciers; CitationHasslacher and Lanegger, 1988). The Pasterze Glacier dominates the study site catchment, covering 18 km2 (CitationKellerer-Pirklbauer et al., 2006). The Möll River has its source at the margin of this glacier (2111 m a.s.l.), and flows with a total annual mean discharge of 27 m3 s−1 into the Drau River (655 m a.s.l.). The highest elevation is at 3798 m (Großglockner) for both the total Möll River catchment and the study site catchment; the lowest elevation of the study site is at 1650 m a.s.l.
Figure 1 Location of study site in Austria (▪) and catchment of the four sampling sites (1 = metakryal, 2 = hypokryal, 3 = floodplain reach, 4 = glacio-rhithral). Glaciers = defined white area; supraglacial till = dotted area; rocks amidst glaciers = shaded area; MR, NR = Margaritzen and Naßfeld reservoirs (black bar indicates dams); lake area = wavy lines; vegetation zone (non-pioneer vegetation zone) = checkered area (scrubs, meadows) with large spots as wooded zone. Latitude/longitude numbers are given.
The area of the study site is assigned geologically to the Tauern Window of the Eastern Alps (extensions: 160 km WE, 30 km NS). This window represents a section of the Penninicum that emerges on the surface of the Austroalpine nappes, which overlay the Penninic belt in the Central Eastern Alps. The Penninicum of the Tauern Window consists of orogenically different nappes (CitationKurz et al. 1998), derived from continental (Zentralgneis, Lower Schieferhülle series) or oceanic basement (Upper Schieferhülle) (CitationSelverstone and Hyatt, 2003). One of these nappes, the Glockner nappe, which is equated with the Upper Schieferhülle by CitationSchmid et al. (2004), makes up the underlying geology of the study site. The Glockner nappe comprises an incomplete ophiolitic sequence of serpentinites, ultramafic rocks, greenschists, and amphibolites as oceanic basement and sediments of the Bündnerschiefer-type (CitationSchmid et al., 2004), which covers or intercalates with the basement (CitationDachs et al., 2005).
Within the study site catchment, vegetation is sparse and higher vegetation is mainly concentrated in the surroundings of S4 (), where a mixed forest of larch (Larix decidua), Swiss pine (Pinus cembra), and spruce (Picea abies) makes up the wooded zone extending from about 1600 to 1800 m a.s.l. At tree line (about 1900 m a.s.l.) the forest gives way to alpine vegetation of dwarf shrubs and alpine meadows. Pioneer vegetation on rock surfaces follows the lower vegetation zone in altitude. Forestry and alpine pastoral systems play only a marginal role in land use of the study site catchment.
Four sampling sites (S1 to S4) were established () along the headwaters of the Möll River. S1, the metakryal or glacial source, was on the northeastern margin of the Pasterze tongue. The source gradient was not steep; the river only showed a remarkable incline at about 10 m downstream. S2 was situated 600 m apart from the glacier snout where the stream bed was dominated by heterogeneous moraine debris (ranging from silt to boulders). The character of the glacial tongue differed between S1 and S2: the immediate ice surface at S1 was less covered by glacial debris, whereas there was a thick debris mantle (supraglacial till) covering the tongue at S2.
S3 was situated 1200 m from the glacier source. This site was one of the reaches of a braided lotic system flowing over a former glacial lake basin. This area is in the final stage of a siltation process that can be ascribed to the high, average annual transport of 37,000 m3 sediment in the lake (CitationOEAV, 2004). S3, a channel with a permanent upstream connection to the main channel, was situated at the lower end of the plain of former lake area. S4 is situated 5 km downstream from the source. This site represented a widened channel between narrow, steep canyons up- and downstream. Substrates were heterogeneous at S1, S2, and S4 and consisted of various size fractions from boulders to glacial silt, whereas at S3 the substrate uniformly consisted of glacial silt.
Sampling
Sampling took place in the late mornings on two consecutive days in June (19/20), August (12/13), and October (9/10) 2005 to avoid diurnal variation (S1–S3 were sampled on one day and S4 on the other day). Five samples per date and site (with the exception of site 1 in October, which was dry) were taken from the uppermost sediment surface (0–5 cm) with a PVC corer (diameter 4.0 cm). Water temperature, conductivity, pH, and oxygen were measured with portable fieldmeters (Hanna Instruments; WTW) at each site and date. In the field, benthic samples were immediately preserved with 10% formalin solution stained with rose Bengal. In the laboratory, samples were sieved over 40 µm mesh size (floated several times) and sorted under a dissecting microscope. Due to low densities, macrozoobenthos were not treated separately. Individuals of higher invertebrate groups were counted. Nematodes were separated and treated further according to the protocol of CitationSeinhorst (1962).
Data Analysis
Abundances of higher taxa and nematodes, as well as abundances of nematode feeding types, were expressed as individuals per 10 cm2. Nematode biomass was expressed as µg per 10 cm2. The permanence of appearance of the total nematode fauna and invertebrate taxa was calculated on the basis of percentage of samples containing respective groups, relative to the total sample number (n = 55). Nematodes were classified as deposit feeders, chewers, epistrate feeders (tear-and-swallow feeders on bacteria, unicellular eukaryotes, diatoms and other algae), and suction feeders (CitationTraunspurger, 2002).
Species richness refers to the number of nematode species as a sum for each sample unit (number of samples per site and season), and as a sum for each site and season, respectively. Shannon diversity H′ (on log e base) and Pielou's evenness J′ were calculated with mean abundances of species per sample unit, sites, seasons, and overall nematofauna.
For calculating biomass (CitationAndrássy, 1956; G = l * w 2/1.6 * 106, where G is the weight in µg, w is the maximal body width in µm; l is body length in µm), body length and maximum body width were taken from pictures, either drawn by a camera lucida or photographed by videomicroscopy. For the dominant species, not each juvenile stage was considered separately for calculations, but a mean value was taken from five juveniles. Furthermore, measurements of 10 adult nematodes were taken to calculate average biomass. For rare species every individual was measured.
The Maturity Index (CitationBongers, 1990; MI = Σ v i * f i; where v i is the cp value of taxon i and f i is the frequency of that taxon) was used to describe the state of nematode community maturity. To calculate the MI, cp values are assigned to nematodes according to their ecological strategies (see Appendix). The cp values range from cp 1, which characterizes extreme colonizers (small individuals, high productivity, many offspring, able to withstand disturbances and environmental stress), to cp 5, which characterizes extreme persisters (larger nematodes, low productivity, few offspring, susceptible to disturbances and stress).
To avoid unequal cell sizes, data of S1 (10 samples instead of 15) were treated separately. Due to non-parametric (transformed) data, a Mann-Whitney U-Test was used to test seasonal differences between June and August for S1. Data of S2 to S4 were treated together and non-transformed and transformed data were tested for normality (Kolmogorov-Smirnov goodness-of-fit test). Because assumption of normality was not fulfilled, a non-parametric two-way ANOVA—the Scheirer-Ray-Hare extension of the Kruskal-Wallis H-Test (H-SRHE; CitationSokal and Rohlf, 1998) was applied to examine possible influences of place and time factors, and their possible influential interaction on patterns of nematode community parameters. Significance level for statistical tests was set for p-values ≤ 0.05.
The use of the Spearman rank correlation coefficient proved correlations between nematode community parameters of sampling units and water temperature, and between abundances and biomass of the total nematode fauna. Significance level for statistical tests was set for p-values ≤ 0.05. Statistical analyses were conducted with the software package SPSS (Version 11.5.1).
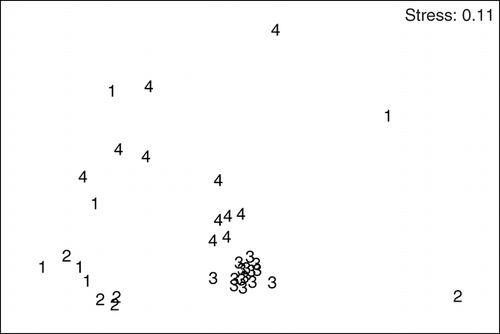
To visualize similarities of species assemblages between samples of the four sites, a plot of non-metric multidimensional scaling (MDS) was used. It was based on a Bray-Curtis similarity matrix calculated with square root transformed species set data of the 55 samples. The plot shows data points (representing each sample) in different (non-metric) distances—points in close proximity represent samples with similar species assemblages. Furthermore, the MDS plots specify a stress factor (based on goodness of fit to regression of distances of similarity data), which reflects the usability of the applied 2-dimensional MDS plot for summarizing the similarity of data. A stress value <0.05 is a perfect graphical summary of data (no misinterpreting of similarity), a value <0.1 also gives a good picture (extreme low chance of misinterpretation), and <0.2 gives a reliable representation where care should only be given to outliers (CitationClarke and Warwick, 1994). MDS was conducted with PRIMER 5 (Version 5.2.0).
Results
Nematodes Versus Benthic Invertebrates
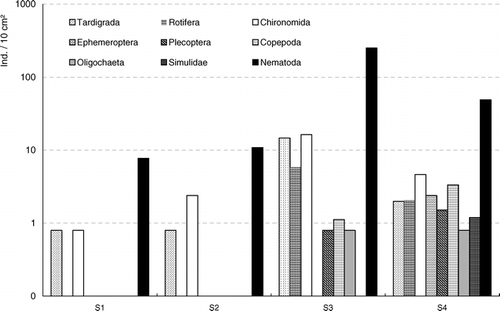
Free-living nematodes made up 91% of the benthic community and were the most abundant group at all study sites (). Chironomids (4%) and tardigrades (2%) were distinctly less abundant. The remaining fauna were extremely scarce (rotifers, copepods, oligochaetes, mayflies, stoneflies, and simulids that ranged from <1% to 1%). Nematodes also showed the highest permanence of appearance (67%), followed by chironomids (45%) and tardigrades (33%). Among the insects, only chironomids were found at all sites.
Nematode Abundance and Biomass
The overall mean of total nematode abundance was 86 individuals per 10 cm2 (a total of 5955 individuals). Spatial mean densities were lowest at S1 and highest at S3; seasonal means were lowest in summer and highest in autumn (). Nematode community parameters varied among and between sites and seasons () and were highly correlated with water temperature (Spearman's ρ = 0.85, p = 0.001). Biomass was strongly correlated with abundances (p < 0.001; Spearman's ρ = 0.95). Hence, it showed similar spatiotemporal patterns as nematode abundance. Only one exception could be observed in June (data not shown). Concerning S2–S4, community patterns varied significantly between sites, but not between seasons. Also, the influence of the interaction of time and space factors was only relevant to the observed distribution of nematode biomass (). At S1, nematode patterns were not significantly different between seasons, with the exception of H′ ().
Table 1 Geographic and abiotic descriptions of the four sampling sites (O2, pH, and water temperature are expressed as ranges of measurements from each date; conductivity was measured once in June).
Table 2 Total nematode species number per site and season, and remaining nematode community parameters (abundances, biomass, H′, J′, and MI) as site and seasonal means with standard deviations (±) for abundances and biomass (abundance as ind. per 10 cm2, biomass as µg per 10 cm2,S = species number, H′ = Shannon's diversity, J′ = Pielou's equitability, MI = maturity index).
Table 3 H-values of the Scheirer-Ray-Hare extension of Kruskal-Wallis H-Test for spatio-temporal differences. Significance is indicated by * for p ≤ 0.05 (significant), *** for p ≤ 0.001 (highly significant), and n.s. for non-significant (A = abundance, B = biomass, S = species number, H′ = Shannon's diversity, MI = maturity index).
Table 4 Z values of the Mann-Whitney U-Test for differences of community parameters at S1 between June and August 2005 (p ≤ 0.05 indicates significance; A = abundance, B = biomass, S = species number, H′ = Shannon's diversity, MI = maturity index).
Nematode Community Composition
The total nematode fauna comprised 77 species at least within 43 genera (Appendix). Overall diversity H′ was 2.7 by an equitability J′ of 0.63. The family Monhysteridae showed high species richness (13 species) and made up more than half of the total nematode community (56%) with two species reaching abundances higher than 10%. The family Plectidae also exhibited high species numbers (14 species), however, this was a comparatively less abundant group (2%). In contrast, the second most abundant family, Mononchidae with 27%, contained just five species. Two of these species were abundant and contributed more than 10% each to the total nematofauna. Other families were less species rich and less abundant.
In regard to the total fauna, Eumonhystera filiformis was the most abundant species, followed by E. hungarica, Mononchus sandur, and M. truncatus (see Appendix). These four species represented 61% of the total fauna. Altogether, nine species made up 81% of the total nematode fauna (Appendix). Besides the four dominant species, only E. dispar and E. vulgaris contributed more than 5% of the fauna. Other taxa were less abundant: nine taxa showed percentages between one and three, and the remaining taxa showed percentages less than one. Females represented the main portion of the fauna (about 73%), and juveniles contributed about 27% to the total fauna. The most juveniles were of the species E. filiformis (7%), M. truncatus (6%), M. sandur (5%), E. hungarica (2%), and E. vulgaris (1%). Rare males (0.2%) were mainly found within more abundant species such as M. sandur (4), M. truncatus (1), E. filiformis (1), E. hungarica (1), and E. dispar (1), but also within the Secernentea.
Each site was characterized by different nematode species assemblages that significantly varied both in species number, diversity H′ and evenness J′ (), and in dominant species (Appendix). Correlations were found between water temperature and both species richness and diversity H′ (Spearman's ρ = 0.79, p = 0.004** for species richness and Spearman's ρ = 0.73 and p = 0.01 for diversity H′). Species richness and diversity were lowest at S2, diversity was highest at S4, and species richness was highest at S3. The equitability between species was highest at S4 and lowest at S2, reflecting the unbalanced abundances between observed species. Samples of S1 and S2 were more similar than were the samples of S1 and S3; S3 harbored the most self-contained assemblage and S4 the most heterogeneous one ().
Figure 3 Non-metric multidimensional scaling (MDS) of the four sampling sites; 1 to 4 represent the four sampling sites (S1–S4) and symbols represent single samples of respective sites. Relative distances based on Bray-Curtis similarity matrix of species set data.
Hofmaenneria hazenensis and E. longicaudatula were dominant at S1 with nearly equal portions. E. hungarica and H. hazenensis dominated S2, also with nearly equal portions. E. filiformis clearly dominated S3, but E. hungarica, M. sandur and M. truncatus also were abundant. S4 was dominated by E. barbata, followed by E. vulgaris and E. hungarica, and T. allophysis. The appearance of some of the site-dominant species was restricted to certain sites; for example, no individuals of E. filiformis and E. barbata occurred at S1 and S2, and E. vulgaris and E. filiformis were not found at S2. In addition, certain species that were dominant at one site were not found in appreciable numbers at other sites; for example, T. allophysis and E. barbata dominated S4, but were extremely rare at S3, and M. truncatus was dominant at S3 and was nearly missing at S4. Ethmolaimus pratensis had approximately similar relative abundances at S3 and S4.
Feeding Types and Maturity
Deposit feeders were the predominant feeding type (63%), followed by chewers (30%). Suction and epistrate feeders were less abundant with 3.8% and 3.3%, respectively. The mean site densities of deposit feeders ranged from 15 ind. per 10 cm2 at S1 in June to 189 ind. per 10 cm2 at S3 in October. Densities of chewers peaked at S3 in August (90.3 ind. per 10 cm2) and reached a minimum at S3 in October (81.4 ind. per 10 cm2). Suction and epistrate feeders were often not present in the samples. Both feeding types had their maxima at S3 in October (14.2 ind. per 10 cm2, suction feeders; 14.5 ind. per 10 cm2, epistrate feeders). The distribution of each feeding type was not significantly influenced by sites (S2–S4), by seasons, or by an interaction of these factors (H-SRHE: p > 0.05). Feeding types also did not vary significantly between seasons at S1 (Mann Whitney U-Test; p > 0.05).
The overall MI was 2.7 and varied between and among sites and seasons (). Site, but not season or an interaction of both factors, influenced significantly the distribution of the MI (). According to the densities of Eumonhystera and Plectus, MI was low at S1; according to Eumonhystera densities at S2 and at S4, the MI was also low there. Highest values of MI at S3 were due to the high abundances of Mononchus species.
Discussion
General Aspects
In early research on glacial streams, they were considered extreme but simple systems, where only few adaptive organisms can survive. Today, glacial streams are known to harbor a diverse and complex benthos influenced by highly spatiotemporally controlled hydrological pathways. Several groups of organisms, however, remain neglected in this research, among them nematodes. Free-living nematodes could be considered to be of minor importance in glacial streams based on poor qualitative and quantitative data from inadequate sampling that described a species-poor and sparse benthic nematofauna in glacial catchments (CitationSnook and Milner, 2001; CitationAltherr, 1976; CitationBretschko, 1969; CitationTilzer, 1968). The present results, however, show a completely different picture of the nematodes here.
The spatiotemporal abiotic characteristics of the four investigated glacial stream reaches (representative for typical glacial catchment sectors) are in good accordance with previous observations (CitationFüreder et al., 2001; CitationLods-Crozet et al., 2001; CitationMilner et al., 2001b; CitationRobinson et al., 2001) and common concepts (CitationMilner et al., 2001a; CitationMilner and Petts, 1994; CitationWard, 1994) in glacial stream research. Nonetheless, the nematofauna characteristics in the Möll River differ from previous findings in that: nematodes dominated the benthic fauna in each reach, nematode abundances were higher than those of other invertebrates (e.g. CitationBurgherr et al., 2003; CitationWard and Uehlinger, 2003, CitationFüreder et al., 2003, CitationBurgherr and Ward, 2001; CitationLods-Crozet et al., 2001), nematode diversity was higher than that of any other benthic group (both in total and in individual reaches) (CitationRott et al. 2006; CitationBurgherr et al., 2003; CitationFüreder et al., 2002, Citation2001; CitationSnook and Milner, 2001), and, finally, the relatively abundant and diverse metakryal nematodes go against the typical assumptions in glacial stream research that metakryal reaches are nearly uninhabited by invertebrates (CitationWard, 1994).
Nematode spatiotemporal distribution patterns agree well with those of other glacial stream biota (CitationBurgherr et al., 2003; CitationRobinson et al., 2002; CitationBurgherr and Ward, 2001; CitationMilner et al., 2001a; CitationMilner and Petts, 1994; CitationWard, 1994). Specifically, the increase of abundances and diversity from the source to the glacio-rhithral, but also to the floodplain reach, the distinct reduction of abundance and diversity in the main channel (S1, S2, S4) in summer due to glacial ablation, and the species- and individual-rich recolonization at S4 again in autumn are consistent with known trends in glacial benthic communities. But besides the unexpectedly high abundances and diversity of nematodes, other uncommon features included the decrease of community parameters (abundances, diversity, maturity) from the meta- to the hypokryal, the desiccated autumnal metakryal (when parts of the glacier collapsed and diverted the river), and the non-existent autumnal hypokryal nematofauna (probably due to extreme discharge in autumn).
Due to different sampling methods used in the present (small area sampling) and previous studies, comparisons of the macrozoobenthos (MZB) data should be made with care. A purely qualitative comparison of major MZB taxa reveals some resemblance between the Möll River and other running waters for the metakryal (solely chironomids), the glacial floodplain, and the glacio-rhithral reach (CitationWard and Uehlinger, 2003; CitationMalard, 2003). In contrast, the hypokryal macrofauna in other rivers have been richer than that of the Möll River. The extreme discharge throughout the study might have restricted the development of a more diverse fauna in the Möll River hypokryal.
The less developed macrofauna found in glacial river systems, in comparison to those of non-glacial systems, may be explained by environmental stress, in which water temperature and low food resources are among the crucial factors (e.g. CitationWard, 1994; CitationMilner and Petts, 1994). Water temperature seems to be the major driving force for the glacier–near-stream reaches, because they have widely harbored only certain cold-adapted groups such as the genus Diamesa. Among nematodes, cold tolerance/resistance is widespread and might be advantageous in glacial rivers with respect to the above-mentioned differences between macrofauna and nematodes.
In general, there is no evidence for consistent influence of water temperature on nematode abundance and species number on river bed surfaces (CitationBarbuto and Zullini, 2005; CitationBeier and Traunspurger 2003a, Citation2003b; CitationSnook and Milner, 2001; CitationSchmid-Araya, 1994; CitationEder, 1983). Abundance and species number may not necessarily be the highest at warmer temperatures, and they may not necessarily be lowest at lower temperatures. Nevertheless, the positive correlation of abundances with water temperature in the Möll River, and the abundances of nematodes at S1, S2, and S4 within the lower range among lotic nematodes (compare to survey in CitationTraunspurger, 2002), indicate potential local effects (at least indirectly) of water temperature. This effect might be less pronounced for nematode diversity, because it was similar between low-altitude, probably summer warm reaches (CitationBarbuto and Zullini, 2005) and the meta- and hypokryal and lies within the mean ranges for lotic nematodes (see CitationTraunspurger, 2002) at the summer cold glacio-rhithral.
As in the case of low temperatures, nematode diversity is not solely affected by glacial influence. Non-glacially influenced, high-altitude sites (2140 and 2050 m a.s.l.) have similar nematode diversity as the meta- and hypokryal, and species number was even lower at a 1460 m a.s.l. site (CitationOcaña and Picazo, 1991) than at the Möll River glacio-rhithral (1650 m a.s.l.). Comparing high altitude as well as other lotic reaches, nematode species nearly exclusively restricted to glacial reaches are apparently absent in the Möll River sites, with the exception of Hofmaenneria hazenensis, which has only been previously reported from the Canadian Arctic (CitationMulvey, 1969). H. hazenensis might be among the extreme cold stenotherm species capable of existing in environments such as the kryal of glacial streams. Eumonhystera barbata, found in abundance in the Möll glacio-rhithral, seems to be restricted to mainly high-altitude river reaches (CitationOcaña and Picazo, 1991), whereas closely related species such as E. vulgaris and E. hungarica, present in nearly all of the Möll River reaches, seem to tolerate a wide variety of habitats ranging from high- to low-altitude rivers (CitationBeier and Traunspurger, 2003a, Citation2003b; CitationOcaña and Picazo, 1991; CitationEder, 1983).
Although no distinct glacial nematofauna (beside H. hazenensis) seems to characterize the two most extreme, near-glacier sites, one community trait, namely nematode maturity, does. These are extremely young freshwater habitats, because deglaciation took place around 2001 (K. Lieb, personal communication). The lowest MI values characterize a “young” nematode community inhabiting a place in an early stage of succession (CitationEttema and Bongers, 1993; CitationBongers, 1990). Abundant r-strategists (Monhysterida, Plectida) inhabiting S1 and S2 caused the low MI. They seem to be able to cope with the biotic stress on the kryal reaches. The slight decrease of maturity from the source to the hypokryal might identify S2 as being more unstable than S1, which represents a low-gradient, constrained reach between bedrock and glacier tongue. The highest MI at S3 indicates that the habitat stability there has enabled a more mature nematode community to develop, and intermediate maturity at S4 might reflect the combination between frequent destabilization, but sufficient resources.
Site-Specific Aspects
At the meta- and hypokryal, potential food resources at the base of food webs (e.g. bacteria, algae, benthic organic matter) are generally low. The living and dead food web compartments, however, have seasonal peaks affected by seasonal snow and glacial melt. The melt waters release organic and inorganic nutrients as well as particulate organic matter to the near-glacier sites (CitationTockner et al., 2003; CitationZah and Uehlinger, 2001). Algae, bacteria, and fungi benefit from these resources (CitationRott et al. 2006; CitationBattin et al. 2004; CitationGessner and Robinson, 2003) and directly or indirectly from each other (CitationWetzel, 2001). Because these organisms can cope with extreme conditions, primary production and decomposition, and the interaction between them, can already take place close to the glacier. The dominant deposit feeders at the meta- and hypokryal are generally considered to be closely tied with production and decomposition processes, and their diverse June patterns in the Möll River kryal sites might have responded to spring peaks in various inputs and diverse auto- and heterotrophic production. The more developed nematode community at the metakryal probably reflects the more retentional character of the low-gradient source. The poor nematode fauna at both kryal sites in summer reflects the extreme discharge due to glacial ablation and concurrent thinning of stream biota and their resources.
Unfortunately, little information is available on the biotic components of peculiar reaches such as represented by S3. In principle, finer sediments positively affect bacteria, clear water favors algae (potentially harmful UV radiation ignored), and both groups are positively driven by higher water temperature, lower velocity, and high oxygen content. These abiotic characteristics were represented at S3. Together with enhanced nutrient inputs from the inflows and imports from the shrub-vegetated adjoining hillside, these features might have favored consistently high auto- and heterotrophic production throughout the study. This interpretation is supported by a constantly dense nematode fauna. Although the sidearm appeared to be rather uniform, without macroscopically distinct habitat patches, numerous nematode species occurred there, suggesting a high variability of microhabitat patches. The dominant deposit feeders typically had brown-green gut contents, suggesting algal feeding. Whether a less dense diatom biota, for example, determined the low abundances of the diatom-feeding Chromadoridae, remains speculative. Potential interactions between the abundant predatory mononchs and other nematodes, which they most likely prey on, also remain unknown, because the presumptive prey was not visibly reduced.
Glacio-rhithral reaches have distinct characteristics that differ from the meta- and hypokryal in being more stable (decreasing glacial influence) and having more food resources due to enhanced auto- and allochthonous production (CitationRott et al. 2006; CitationZah and Uehlinger, 2001). As a consequence, the glacio-rhithral sector is richer in individuals and species than the kryal sectors (CitationBurgherr et al., 2003; CitationBurgherr and Ward, 2001; CitationFüreder et al., 2001; CitationMilner et al., 2001b). Nevertheless, glacial melt can decimate the glacio-rhithral populations in summer, but the benthic biota generally recovers and is abundant and diverse in autumn again (also assumed as response to enhanced autumnal leaf fall). As already mentioned, the glacio-rhithral nematodes in the Möll River follow the longitudinal and the seasonal patterns common for glacio-rhithral invertebrates. The summer community was extremely less developed, but the nematode community indicates already sufficient nutrition in spring, and increased food resources in autumn. The relative variation of feeding types, and to a lesser extent of species among feeding types, between spring and autumn might reflect a change between and among autho- and allochthonous resources.
Given our lack of knowledge of the autecology of freshwater nematode species, it is not possible to determine which ecological factors are primarily responsible for controlling species dominance, abundance, and numbers at each site and season. Therefore, we also do not know which ecosystem processes in glacial streams are actually affected by nematodes. Furthermore, nematodes may be part of the indifferent food which is ingested by glacial stream macroinvertebrates (CitationFüreder et al. 2003). In general, resource composition affects nematode community composition and vice versa (CitationMontagna, 1995). But see below for some potential relationships between nematodes and food web components.
All nematode feeding types observed in the Möll River sites, especially the deposit feeders, are potential consumers of detritus associated with bacteria, fungi, protists, and microphytobenthos (MPB), chewers and suction feeders might also feed on other metazoans. Detritus and MPB, important benthic carbon regulators, are qualitatively and quantitatively unevenly distributed in space and time in glacial stream reaches. Nonetheless, their longitudinal and seasonal variation (CitationRott et al. 2006; CitationGessner and Robinson, 2003; CitationBattin et al. 2001) might directly determine the nematodes' spatial and temporal patterns. For freshwaters, a close spatial relationship has been established between algae (e.g. diatoms) and nematodes as potential algal feeders (CitationGreenwood et al., 1999). In addition, bacterial-feeding nematodes have been observed to enhance bacterial activity (CitationTraunspurger et al., 1997).
Nematode feeding modes are better studied in the marine environment. Correlations between predatory nematodes and other nematodes have been observed (CitationDanovaro and Gambi, 2002), but chewers normally considered to be predatory can also use MPB (CitationMoens et al., 2002). Deposit feeders consume blue-green algae (CitationJensen, 1987), and nematode grazing rates increase in response to increased microphytobenthic production (CitationMontagna et al. 1995). Nematode species selectively feed on certain algae (CitationTrotter and Webster, 1984) and also on certain particulate organic matter (CitationRiera and Hubas, 2003). In both marine and terrestrial studies, increased mineralization (and therefore increased nutrient availability for primary producers) are well-known positive indirect effects of nematode bacterial-feeding behavior.
Conclusions
This first survey of the spatiotemporal distribution of lotic, free-living nematodes in a glacial catchment demonstrates that nematodes are an abundant and diverse group in the benthos of glacial stream reaches. Results have revealed that only certain nematode species seem to be able to cope with harsh conditions in kryal reaches and that nematode species composition differs between the four differently structured reaches. The dominant spatiotemporal appearance of deposit feeders suggests that they play an important role in the ecological processes at the base of benthic food webs in glacial-fed reaches. Due to abundances, diversity, and feeding types, nematodes might be essential contributors to ecological processes in glacial streams, and therefore, to the ecosystem functioning of these sensitive systems. Hence, food webs in glacial-fed reaches are not restricted only to the micro- and macrobiota.
The distributional patterns of nematode communities in these various reaches fit the general patterns observed in the other kinds of benthic fauna in glacial-fed systems, that is, increasing taxa richness and abundance along the longitudinal and the lateral (floodplain) aspect. The spatial patterns reflect habitat improvement and alteration, particularly with respect to downstream increases and changes in food resources. Based on the extremely high species richness and abundance, the floodplain reach can be seen as a biological hot spot (diversity and productivity) in the headwaters of the Möll River. Finally, this study demonstrates that the relative maturity of nematode communities can be used to assess glacial stream habitats in regard to both time since deglaciation and environmental stress.
Acknowledgments
I thank Cornelia Lunder for her ongoing assistance and support in the field. I also thank Johann Flöckner and Michael Lunder for their help with field work. Two anonymous reviewers and the editors are thanked for their helpful and constructive comments. This study was financed by an ecological research grant from the Großglocknerhochalpenstr. AG.
References Cited
- Abebe, E. , I. Andrássy , and W. Traunspurger , editors. 2006. Freshwater nematodes: ecology and taxonomy Wallingford CABI Publishing. 752 pp.
- Altherr, E. 1976. Nématodes des eaux stygorhitrales des Alpes autrichiennes. Revue Suisse de Zoologie 83:779–847.
- Andrássy, I. 1956. Die Rauminhalts- und Gewichtsbestimmungen der Fadenwürmer (Nematoden). Acta Zoologica Academiae Scientiarum Hungaricae 2:1–15.
- Barbuto, M. and A. Zullini . 2005. The nematode community of two Italian rivers. Nematology 7:667–675.
- Battin, T. J. , A. Wille , B. Sattler , and R. Psenner . 2001. Phylogenetic and functional heterogeneity of sediment biofilms along environmental gradients in a glacial stream. Applied Environmental Microbiology 67:799–807.
- Battin, T. , A. Wille , A. Psenner , and A. Richter . 2004. Large-scale environmental controls on microbial biofilms in high-alpine streams. Biogeosciences 1:159–171.
- Beier, S. and W. Traunspurger . 2003a. Seasonal distribution of free-living nematodes in the stream Krähenbach, a fine-grained submountain carbonate stream in southwest Germany. Nematology 5:113–126.
- Beier, S. and W. Traunspurger . 2003b. Seasonal distribution of free-living nematodes in the stream Körsch, a coarse-grained submountain carbonate stream in southwest Germany. Nematology 5:481–504.
- Bilgrami, A. L. and R. Gaugler . 2004. Feeding behaviour. In Gaugler, R. and A. L. Bilgrami , editors. Nematode behaviour Wallingford CABI Publishing. 91–126.
- Bongers, T. 1990. The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83:14–19.
- Bongers, T. and M. Bongers . 1998. Functional diversity of nematodes. Applied Soil Ecology 10:239–251.
- Bretschko, G. 1969. Zur Hydrobiologie zentralalpiner Gletschabflüsse. Verhandlungen der Deutschen Zoologischen Gesellschaft 1968:741–750.
- Brown, L. E. , A. M. Milner , and D. M. Hannah . 2006. Stability and persistence of alpine stream macroinvertebrate communities and the role of physicochemical habitat variables. Hydrobiologia 560:159–173.
- Burgherr, P. and J. V. Ward . 2001. Longitudinal and seasonal distribution patterns of the benthic fauna of an alpine glacial stream (Val Roseg, Swiss Alps). Freshwater Biology 46:1705–1721.
- Burgherr, P. , B. Klein , C. T. Robinson , and K. Tockner . 2003. Surface zoobenthos. In Ward, J. V. and U. Uehlinger , editors. Ecology of a Glacial Flood Plain Dordrecht Kluwer Academic Publishers. 153–173.
- Bürgi, H. R. , P. Burgherr , and U. Uehlinger . 2003. Aquatic flora. In Ward, J. V. and U. Uehlinger , editors. Ecology of a glacial flood plain Dordrecht Kluwer Academic Publishers. 139–151.
- Clarke, K. R. and R. M. Warwick . 1994. Change in marine communities: an approach to statistical analysis and interpretation Plymouth Plymouth Marine Laboratory. 144 pp.
- Dachs, E. , W. Kurz , and A. Proyer . 2005. Alpine eclogites in the Tauern Window. Mitteilungen der Österreichischen Mineralogischen Gesellschaft 150:199–226.
- Danovaro, R. and C. Gambi . 2002. Biodiversity and trophic structure of nematode assemblages in seagrass systems: evidence for a coupling with changes in food availability. Marine Biology 141:667–677.
- Eder, R. 1983. Nematoden aus dem Interstitial der Donau bei Fischamend (Niederösterreich). Archiv für Hydrobiologie/Suppl 68:100–113.
- Ettema, C. H. and T. Bongers . 1993. Characterization of nematode colonization and succession in disturbed soil using the Maturity Index. Biology and Fertility of Soils 16:79–85.
- Füreder, L. 1999. High alpine streams: cold habitats for insect larvae. In Margesin, L. and F. Schinner , editors. Cold adapted organisms. Ecology, physiology, enzymology and molecular biology Berlin Springer. 181–196.
- Füreder, L. , C. Schütz , M. Wallinger , and R. Burger . 2001. Physico-chemistry and aquatic insects of a glacier-fed and a spring-fed alpine stream. Freshwater Biology 46:1673–1690.
- Füreder, L. , C. Vacha , K. Amprosi , S. Bühler , C. M. E. Hansen , and C. Moritz . 2002. Reference conditions of alpine streams: physical habitat and ecology. Water, Air and Soil Pollution: Focus 2:275–294.
- Füreder, L. , C. Welter , and J. K. Jackson . 2003. Dietary and stable isotope (δ13C, δ15N) analyses in alpine stream insects. International Review of Hydrobiology 88:314–331.
- Füreder, L. , M. Wallinger , and R. Burger . 2005. Longitudinal and seasonal pattern of insect emergence in alpine streams. Aquatic Ecology 39:67–78.
- Gessner, M. O. and C. T. Robinson . 2003. Aquatic hyphomycetes in alpine streams. In Ward, J. V. and U. Uehlinger , editors. Ecology of a glacial flood plain Dordrecht, Boston, London Kluwer Academic Publishers. 123–137.
- Greenwood, J. L. , T. A. Clason , R. L. Lowe , and S. E. Belanger . 1999. Examination of endopelic and epilithic algal community structure employing scanning electron microscopy. Freshwater Biology 41:821–828.
- Hasslacher, P. and C. Lanegger . 1988. Österreichisches Gletscherbachinventar Innsbruck Österreichischer Alpenverein. 177 pp.
- Hieber, M. , C. T. Robinson , and U. Uehlinger . 2003. Seasonal and diel patterns of invertebrate drift in different alpine stream types. Freshwater Biology 48:1078–1092.
- Hieber, M. , C. T. Robinson , U. Uehlinger , and J. V. Ward . 2005. A comparison of benthic macroinvertebrate assemblages among different types of alpine streams. Freshwater Biology 50:2087–2100.
- Hodda, M. , A. Ocaña , and W. Traunspurger . 2006. Nematode from extreme freshwater habitats. In Abebe, E. , I. Andrássy , and W. Traunspurger , editors. Freshwater Nematodes: Ecology and Taxonomy Wallingford CABI Publishing. 179–210.
- Ilg, C. and E. Castella . 2006. Patterns of macroinvertebrate traits along three glacial stream continuums. Freshwater Biology 51:840–853.
- Jensen, P. 1987. Feeding ecology of free-living aquatic nematodes. Marine Ecology Progress Series 35:187–196.
- Kellerer-Pirklbauer, A. , M. Avian , and G. K. Lieb . 2006. Supraglacial till on Pasterze Glacier, Austria: spatial distribution, characteristics and significance on change of height. 3rd General Assembly of the European Geosciences Union (EGU) Vienna, Austria.
- Knispel, S. and E. Castella . 2003. Disruption of a longitudinal pattern in environmental factors and benthic fauna by a glacial tributary. Freshwater Biology 48:604–618.
- Kurz, W. , F. Neubauer , J. Genser , and E. Dachs . 1998. Alpine geodynamic evolution of passive and active continental margin sequences in the Tauern Window (Eastern Alps, Austria, Italy): a review. Geologische Rundschau 87:225–242.
- Lods-Crozet, B. , E. Castella , D. Cambin , C. Ilg , S. Knispel , and H. Mayor-Simeant . 2001. Macroinvertebrate community structure in relation to environmental variables in a Swiss glacial stream. Freshwater Biology 46:1641–1661.
- Malard, F. 2003. Interstitial fauna. In Ward, J. V. and U. Uehlinger , editors. Ecology of a glacial flood plain Dordrecht Kluwer Academic Publishers. 175–198.
- McGregor, G. , G. E. Petts , A. M. Gurnell , and A. M. Milner . 1995. Sensitivity of alpine stream ecosystems to climate change and human impacts. Aquatic Conservation: Marine and Freshwater Ecosystems 5:233–247.
- Milner, A. M. and G. E. Petts . 1994. Glacial rivers: physical habitat and ecology. Freshwater Biology 32:295–307.
- Milner, A. M. , J. E. Brittain , E. Castella , and G. E. Petts . 2001a. Trends of macroinvertebrate community structure in glacier-fed rivers in relation to environmental conditions: a synthesis. Freshwater Biology 46:1833–1847.
- Milner, A. M. , R. C. Taylor , and M. J. Winterbourne . 2001b. Longitudinal distribution of macroinvertebrates in two glacier-fed New Zealand rivers. Freshwater Biology 46:1765–1775.
- Moens, T. , C. Luyten , J. J. Middelburg , P. M. J. Herman , and M. Vincx . 2002. Tracing organic matter sources of estuarine tidal flat nematodes with stable carbon isotopes. Marine Ecology Progress Series 234:127–137.
- Montagna, P. A. 1995. Rates of metazoan meiofaunal microbivory: a review. Vie Milieu 45:1–9.
- Montagna, P. A. , G. F. Blanchard , and A. Dinet . 1995. Effect of production and biomass of intertidal microphytobenthos on meiofaunal grazing rates. Journal of Experimental Marine Biology and Ecology 185:149–165.
- Mulvey, R. H. 1969. Soil-inhabiting nematodes of the orders Araeolaimida, Chromadorida, Enoplida and Monhysterida from the Canadian High Arctic. Canadian Journal of Zoology 47:365–382.
- Ocaña, A. and J. S. Picazo . 1991. Study on nematode species encountered in the Monachil River (Granada Spain): response to organic pollution. Verhandlungen der Internationalen Vereinigung für Limnologie 24:2729–2737.
- OEAV (ed.) 2004. Naturkundlicher Führer zum Nationalpark Hohe Tauern, Bd. 2, Gletscherweg Pasterze Innsbruck Österreichischer Alpenverein. 121 pp.
- Perry, R. N. and D. J. Wright . 1998. The physiology and biochemistry of free-living and plant-parasitic nematodes Wallingford CABI Publishing. 438 pp.
- Riera, P. and C. Hubas . 2003. Trophic ecology of nematodes from various microhabitats of the Roscoff Aber Bay (France): importance of stranded macroalgae evidenced through δ13C and δ15N. Marine Ecology Progress Series 260:151–159.
- Robertson, A. L. , S. D. Rundle , and J. M. Schmid-Araya . 2000. An introduction to a special issue on lotic meiofauna. Freshwater Biology 44:1–4.
- Robinson, C. T. , U. Uehlinger , and M. Hieber . 2001. Spatio-temporal variation in macroinvertebrate assemblages of glacial streams in the Swiss Alps. Freshwater Biology 46:1663–1672.
- Robinson, C. T. , K. Tockner , and P. Burgherr . 2002. Seasonal patterns in macroinvertebrate drift and seston transport in streams of an alpine glacial flood plain. Freshwater Biology 47:985–993.
- Rott, E. , M. Cantonati , L. Füreder , and P. Pfister . 2006. Benthic algae in high altitude streams of the Alps—A neglected component of the aquatic biota. Hydrobiologia 562:195–216.
- Schmid, S. M. , B. Fügenschuh , E. Kissling , and R. Schuster . 2004. Tectonic map and overall architecture of the Alpine orogen. Eclogae Geologicae Helvetiae 97:93–117.
- Schmid-Araya, J. M. 1994. Spatial and temporal distribution of micro- meiofaunal groups in an alpine gravel stream. Verhandlungen der Internationalen Vereinigung für Limnologie 25:1649–1655.
- Schütz, C. , M. Wallinger , R. Burger , and L. Füreder . 2001. Effects of snow cover on the benthic fauna in a glacier-fed stream. Freshwater Biology 46:1691–1704.
- Seinhorst, J. M. 1962. On the killing, fixation and transferring to glycerine of nematodes. Nematologica 8:29–32.
- Selverstone, J. and J. Hyatt . 2003. Chemical and physical responses to deformation in micaceous quartzites from the Tauern Window, Eastern Alps. Journal of Metamorphic Geology 21:335–345.
- Snook, D. L. and A. M. Milner . 2001. The influence of glacial runoff on stream macroinvertebrate communities in the Tallion catchment, French Pyrénées. Freshwater Biology 46:1609–1623.
- Sokal, R. R. and F. J. Rohlf . 1998. Biometry New York W. H. Freeman and Company. 887 pp.
- Steffan, A. W. 1971. Chironomid (Diptera) biocoenoses in Scandinavian glacier brooks. The Canadian Entomologist 103:477–486.
- Tilzer, M. 1968. Zur Ökologie und Besiedlung des hochalpinen hyporheischen Interstitials im Arlberggebiet (Österreich). Archiv für Hydrobiologie 65:253–308.
- Tockner, K. , R. Illi , F. Malard , and U. Uehlinger . 2003. Nutrient dynamics. In Ward, J. V. and U. Uehlinger , editors. Ecology of a glacial flood plain Dordrecht Kluwer Academic Publishers. 91–107.
- Traunspurger, W. 2002. Nematoda. In Rundle, S. D. , A. L. Robertson , and J. M. Schmid-Araya , editors. Freshwater meiofauna: biology and ecology Leiden Backhuys Publishers. 63–104.
- Traunspurger, W. , M. Bergtold , and W. Goedkoop . 1997. The effects of nematodes on bacterial activity and abundance in a profundal freshwater sediment. Oecologia 112:118–122.
- Treonis, A. M. , D. H. Wall , and R. A. Virginia . 2000. The use of anhydrobiosis by soil nematodes in the Antarctic Dry Valleys. Functional Ecology 14:460–467.
- Trotter, D. B. and J. M. Webster . 1984. Feeding preferences and seasonality of free-living marine nematodes inhabiting the kelp Macrocystis integriflia . Marine Ecology Progress Series 14:151–157.
- Ward, J. V. 1994. Ecology of alpine streams. Freshwater Biology 32:277–294.
- Ward, J. V. and U. Uehlinger . 2003. Ecology of a glacial floodplain Dordrecht Kluwer Academic Publishers. 306 pp.
- Wetzel, R. 2001. Limnology San Diego Saunders College Publishing. 1006 pp.
- Wharton, D. A. 2003. The environmental physiology of Antarctic terrestrial nematodes: a review. Journal of Comparative Physiology and Biochemical Systemic and Environmental Physiology 173:621–628.
- Womersley, C. Z. , D. A. Wharton , and L. M. Higa . 1998. Survival biology. In Perry, R. N. and D. J. Wright , editors. The physiology and biochemistry of free-living and plant-parasitic nematodes Wallingford CABI Publishing. 271–302.
- Zah, R. and U. Uehlinger . 2001. Particulate organic matter inputs to a glacial stream ecosystem in the Swiss Alps. Freshwater Biology 46:1597–1608.
Appendix
Nematode species and their percentage distribution (+ = species <1%). Cp‐values according to CitationBongers and Bongers (1998), values in brackets are not used for calculation. S1–S4 = collection sites. Σ = species' portion of the overall nematofauna.