Abstract
In polar semidesert communities of northwest Spitsbergen the reproductive potential of keystone vascular plant species, such as Dryas octopetala, is currently being constrained by low summer temperatures, resulting in the infrequent production of viable seeds. This study tests the hypothesis that summer foraging behavior of reindeer (Rangifer tarandus platyrhynchus) may further limit successful seed production due to intense selective grazing pressure on the flowering shoots. Surveys of neighboring coastal tundra areas with contrasting reindeer population densities revealed direct evidence of strong grazing pressure on reproductive shoots of D. octopetala on the Brøgger Peninsula and considerably less floral herbivory on the sparsely reindeer-populated Blomstrand island. Recruitment of Dryas on the Brøgger Peninsula is therefore being hindered by intense selective grazing of flowering shoots by Svalbard reindeer. This situation is not unique to this area of Svalbard and also extends to other species of flowering plants.
Introduction
Long-lived clonal perennial plants are an important component of high arctic vascular flora (CitationCallaghan and Emanuelsson, 1985). Although the recruitment of seedlings may be infrequent or sporadic (CitationFreedman et al., 1982; CitationPhilipp et al., 1990), it is important because genetic recombination might improve survivorship in changing environmental conditions (CitationCrow, 1992; CitationThórhallsdóttir, 1998). There is evidence that genetic variation in arctic populations is higher than previously expected (e.g., CitationBrochmann and Steen, 1999; CitationStenström, 2000). Seed dispersal and seedling establishment also provide opportunities for the colonization of unvegetated or newly available terrain (e.g., following glacier retreat, mass movements, or shifts in river channel position), both important elements of the high arctic environment (CitationCrawford, 1997).
Recent research on polar semidesert communities near Ny-Ålesund (78°55′N, 11°56′E) in northwestern Spitsbergen has demonstrated that the successful production of viable seeds of vascular plants such as Dryas octopetala L. is strongly limited by the availability of thermal energy during short and cold growing seasons (CitationWookey et al., 1993; CitationWookey et al., 1995; CitationWada, 1999). Grazing pressure may also be an important factor in further shaping the population demography of tundra plants (CitationMoen, 1993; CitationVirtanen, 1996; CitationWegner, 1997). Herbivores, such as Svalbard reindeer and barnacle geese, can consume large numbers of reproductive shoots, particularly at the flowering stage (CitationProp et al., 1984; CitationOdasz and Savolainen, 1996; CitationAlsos et al., 1998; CitationWada, 1999). Reindeer and geese graze selectively (CitationProp et al., 1984; Citationvan der Wal and Loonen, 1997; CitationAlsos et al., 1998; CitationMathiesen et al., 2000), and the flowers they eat provide more nutrients and a higher amount of easily ingested sugars than leaves (CitationProp and de Vries, 1993). CitationAlsos et al. (1998) observed that 41% of the diet of female barnacle geese (Branta leucopsis) during the incubation period consisted of “flowers of forbs”—mostly Saxifraga oppositifolia and Silene acaulis. Furthermore, time spent in searching for food increased in proportion to the percentage of flowers in the diet; this pattern indicated that the higher nutritional quality of flowering shoots more than offset the increased time spent searching for them. The ecological implications of floral herbivory are of considerable interest because flowers may form a significant component of the herbivores' summer diet, and the resulting heavy mortality of reproductive shoots could profoundly influence plant demography and community structure with repercussions at the whole-ecosystem level (CitationLouda, 1982).
The principal objective for this study was to quantify the intensity and broad-scale spatial pattern of floral grazing on D. octopetala L. ssp. octopetala Hult. (Rosaceae) by one herbivore, Rangifer tarandus platyrhynchus Vrolik, in an area of northwest Spitsbergen centered around Kongsfjord. The specific objectives were (1) to quantify floral grazing of Dryas by reindeer and relate grazing intensity to reindeer intensity, and (2) to investigate spatial distribution of grazing and investigate connections between timing of grazing and plant phenology. We approached these objectives by using a nested design of study sites and years, with both intensive and extensive monitoring, tagged shoots, and an experiment involving reindeer exclosures.
Study Area
FIELDWORK AREA
All the fieldwork was carried out on the Brøgger Peninsula and nearby coastal areas of Kongsfjord, northwest Spitsbergen, Svalbard, latitude 78°56′N, 15–65 m a.s.l. (). The areas chosen for study were not within the boundaries of geese breeding areas, nor were they commonly visited by geese during the study period. Reindeer densities on the Brøgger Peninsula were at maximum 0.89 individuals km−2 (CitationAanes et al., 2000). The reindeer were released on the Brøgger Peninsula in 1978, have no natural predators, and have behaved typically for an introduced population, with rapid population increase and sudden decline (CitationAanes et al., 2000): neither the reindeer population nor the vegetation communities on the Brøgger Peninsula can be assumed to be in equilibrium. Reindeer can walk freely around the peninsula, although because they are nonmigratory, their seasonal and temporal foraging patterns are highly constrained.
At all sites in this study, Dryas octopetala is the vascular plant species with the highest-percentage cover (see , ). The communities were analogous to the “Polar Willow–Mountain Avens” Ridge vegetation described by CitationRønning (1996), within the D. octopetala zone (CitationBrattbakk, 1986), and the “northern arctic-tundra zone” defined by CitationElvebakk (1997).
SITES
Site A consisted of two locations within 500 m of one another 3 km to the west of Ny-Ålesund on the Brøgger Peninsula and less than 50 m a.s.l. (demarcated as Site A in ). Both were on gentle ridges, one (A1) on a low bluff above the Bayelva River (78°55.89′N, 11°49.92′E) and the other (A2) on a low ridge south of a small lake, Trehyrningenvatnet (78°56.03′N, 11°48.92′E).
Site B was established on a southwest-facing raised beach sequence above Jakobskjelda (79°58.00′N, 12°1.70′E) at 15 m a.s.l. on Blomstrand-halvøya (“halvøya” means peninsula, but recent retreat of the ice of Blomstrand glacier has shown that it is in fact an island, and it will hereafter be refered to as such). This island has a recent history of low reindeer density compared to Brøgger Peninsula, although no formal reindeer counts have been made (R. Aanes, pers. comm., 1999). An estimation of 0.1 individuals km−2 has been made (based on the authors' observations of two animals on the island). This site had a greater coverage of foliose lichens of the genus Cetraria than the Brøgger Peninsula (), which is indicative of less intense grazing pressure (CitationCooper and Wookey, 2001).
Site C consisted of a polar semidesert community on a southwest-facing ridge (78°52.10′N, 11°40.00′E), 1 km from the sea at Leinstranda, on the south coast of the Brøgger Peninsula. The site was 65 m a.s.l., with a study area of approx. 50 × 50 m.
Methods
Exclosures and open plots were used in order to quantify the floral grazing by reindeer (Sites A1 and A2). Extensive monitoring plots on two areas of different reindeer intensity were used to quantify the effect of reindeer intensity on floral grazing intensity (Sites A and B). Tagged reproductive shoots were used to follow the fate of individual buds and relate grazing events to phenological events. Presence of reindeer at Site C was indicated using “trampiometers”: thin vertical wires that were flattened by trampling. The exclosures and extensive monitoring plots were used in 1997, tagged shoots and trampiometers in 1999.
EXPERIMENTAL EXCLOSURES
At Sites A1 and A2, 6 plots were set up systematically in linear sequence, at 5-m intervals. Each plot measured 2.5 × 2.5 m, with a central monitoring area of 1 m2 and a 0.75-m-wide buffer zone. Subsequently 3 of the plots were randomly assigned as either (1) “open” plots to which reindeer had open access, or (2) exclosures from which reindeer were barred by the erection of temporary wooden barriers. The exclosures were erected on 26 June 1997 and removed on 23 July 1997, leaving no damage to the vegetation communities or soils. Exclosures were designed so that neither adult reindeer nor calves would be able to gain access, but they were completely open to birds.
The phenology of flowering shoots of D. octopetala was determined for all shoots within a 1-m2 central area within each plot, as described in CitationWookey et al. (1995) immediately after the exclosures were put in place; subsequently every second day until 7 July; and thereafter every third day until 22 July. The plots were monitored on 11 occasions during the 25 d. In addition, the central areas were repeatedly searched for signs of herbivory, e.g., severed peduncles of flowers, with or without additional signs of leaf herbivory, and the vicinity was checked for fresh feces of reindeer and of important grazing birds such as barnacle geese (Branta leucopsis). The reproductive shoots were classified into particular phenological and herbivory categories, i.e., buds, flowers, senesced flowers, severed peduncles, and aborted or damaged flowers.
MONITORING PLOTS ON THE BRØGGER PENINSULA AND ON BLOMSTRAND ISLAND
Five 10-m2 plots were established within 1 km of the experimental exclosures at Site A on the Brøgger Peninsula on 2 July 1997 and on a raised beach on Blomstrand (Site B, ) on 4 July 1997. In these plots a complete survey was made of reproductive shoots of D. octopetala and all signs of herbivory noted. The measurements were repeated on 14 and 15 July 1997 for Brøgger and Blomstrand, respectively, and the extent of herbivory during the intervening period was quantified. A small PVC tube (15 mm o.d.) inserted at the center of each plot with <100 mm protruding above the ground surface enabled accurate relocation for repeat measurements. These markers would have been visible to reindeer at close range, but it is unlikely that they influenced foraging, and there were no signs of biting or other disturbance to the markers. The extensive monitoring plots were not associated with the presence of any exclosures or other artificial structures.
TAGGED PLANTS AND REINDEER PRESENCE
At Site C, 20 reproductive shoots of Dryas on each of 10 mats were randomly selected and marked using a small piece of split plastic drinking straw placed around the peduncle, under the bud. The 200 tagged shoots were observed daily from 6 July to 31 August 1999, and any changes in phenology and herbivory were recorded. At the same time, biweekly records of reindeer visitation were made using an arrangement of 360 trampiometers placed around the site. These consisted of a piece of vertical wire (ca. 25 mm high) wound around a nail placed in the ground (CitationBayfield, 1971). Flattened wires indicated reindeer visitation to the site, since the only people in the area were the four fieldworkers, who knew the position of the wires and avoided walking on them. Each time the wires were checked, any that had been flattened were raised again.
STATISTICAL ANALYSES
Data from the exclosure experiment at Site A were changed into percentages of total reproductive shoots found within particular phenological and herbivory categories. Prior to analysis, percentage data were arcsin transformed. Treatment effects on each category were then analysed by repeated-measures ANOVA with exclosure (open versus exclosed) and site (A1 and A2) as the independent (treatment) variables. Herbivory results (arcsin-transformed percentage values) from the extensive monitoring plots at Sites A and B were analyzed by t-test for independent samples, comparing A and B, and homogeneity of variances was checked by Levene's test (STATISTICA®, 1997).
Results
DENSITY OF FLOWERING SHOOTS
The densities of flowering shoots averaged 33 (±8.89), 24 (±8.18), and 30.8 (±6.41) m−2 (mean ± SE) at the A1 and A2 plots and extensive surveys at Brøgger (near Site A), respectively. These figures compare well with previous values of 30.3 m−2 for a nearby polar semidesert site in 1993 (CitationWookey et al., 1995). Flower densities for the extensive surveys on Blomstrand (B) were 25.0 (±2.92) m−2. No such data exist for the tagged Dryas mats.
REINDEER PRESENCE
No numerical data exist for reindeer presence in sites A and B during the study period. Casual observations indicate that they were present in the general area throughout this time. No fresh reindeer droppings were found inside the exclosures, thus showing that the barricades were reindeer-proof. No fresh goose droppings were found at any of the sites, emphasizing that geese are not the main herbivores in these areas. Flattening of trampiometers at site C between 28 June and 31 August 1999 indicated that reindeer were present at this site at least once every week during this period ().
GRAZING OF REPRODUCTIVE SHOOTS
Exclosures in Site A
and present summary results of the repeated-measures ANOVA for the exclosure experiment. There was no effect on the phenological categories for either location (plots at A1 or A2) or treatment (with or without exclosures), so data from the plots at A1 and A2 are combined. Reindeer consumed 54.4% of all reproductive shoots of D. octopetala in the open plots and none in the exclosures, a significant difference (P = 0.005, F = 15.01, df = 1). At Site A, the number of shoots eaten was positively correlated (r = 0.669) with the total number of reproductive shoots, although this correlation was not statistically significant (P = 0.146; df [model, residual] = 1,4; F = 3.243). The herbivory percentage was weakly negatively correlated (r = −0.283) with the total number of shoots (N = 6), although this, again, was not statistically significant (P = 0.587; df = 1,4; F = 0.348). The efficiency of floral grazing was therefore not constant at different densities of flowers. A significant percentage (16.7%) of the flowering shoots that were grazed showed signs of associated leaf herbivory (P = 0.013, F = 10.08, df = 1).
Extensive Surveys at Sites A and B
At least 63.2% of all flowering shoots was removed from the extensive monitoring plots at A (coefficient of variation, V = 25.6%) compared with only 7.3% at B (V = 179.9%) (). Results from a t-test on the arcsin-transformed percentage herbivory data at the close of monitoring (14 and 15 July, respectively, at the Brøgger and the Blomstrand plots) revealed that these differences between the sites were highly significant (P < 0.001, T = 6.72 and df = 8). The reduction in total scored reproductive shoots between 2 and 14 July 1997 at the Brøgger plots () probably represents shoots that had been consumed by reindeer but were difficult to find, e.g., peduncles bitten very close to the basal rosette of leaves.
A positive relationship (r = 0.648, P = 0.237) was seen between herbivory and shoot density on Blomstrand (Site B) but a weak negative relationship (r = −0.341, P = 0.574) on Brøgger (Site A) (N = 5). These results warrant a more thorough investigation into the effect of flower density on herbivore resource use.
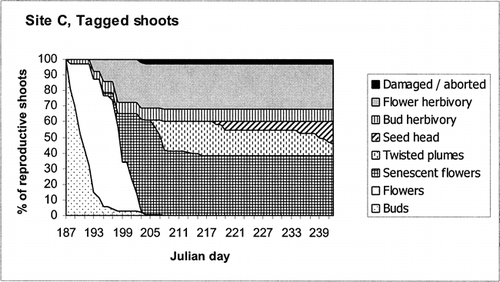
Tagged Plants in Site C
The phenological stages and floral grazing throughout the 55-d observational period are shown in . Out of 200 reproductive shoots, 73 (36.5%) were consumed: 7.5% as buds, 29% as flowers, and 1% in the post-flowering stage. The majority of the grazed flowers or buds were bitten off very close to the top of the peduncle, leaving the split plastic straw in place. Three percent of the shoots were immature buds or aborted flowers. Therefore, at least 39.5% of shoots failed to produce seeds. Twenty-two percent of the shoots became ripe seed heads, ready for dispersal by the end of August 1999, and a further 38% were not yet ripe by that time.
TIMING OF GRAZING
At Site C reindeer principally grazed Dryas flowers 5 (±3) d old, ranging from the first to the thirteenth day after their petals opened (N = 58). No flower grazing occurred until day 193 (12 July), when 72% of the tagged flowers were opened (). After day 203 (22 July) there were no additional open tagged flowers, and the reindeer did not graze on the tagged shoots, despite their regular presence in the area for the remainder of the monitoring period as shown by the trampiometers (see ). Grazing on the plots at Site A lagged at least 8 d behind the start of flowering. The timing of grazing may possibly be linked to maximum nectar content, since this increases with sunshine (CitationProp et al., 1984).
SPATIAL DISTRIBUTION OF FLORAL HERBIVORY
A large geographical variation in floral grazing pattern was seen on the tagged shoots at Site C. Tagged reproductive shoots were initially uniformly distributed between Dryas mats. At the end of summer, some mats had received no floral herbivory, whereas others had up to 18 shoots (90%) grazed. On both the Brøgger Peninsula and Blomstrand island, the grazed flowers did not have an even distribution between the plots, with extreme variation in spatial grazing pattern on Blomstrand: 2 out of 5 plots had no measurable herbivory. A very sparse reindeer population may thus leave “windows of opportunity” for whole patches to escape herbivory and to set seed.
At a grazing event, the number of flowers grazed averaged 61% (±34) of the available opened tagged flowers on that mat. At the time of the grazing event 90% (±15) of all the tagged flowers that were grazed on one mat were grazed in one grazing event. Out of 10 marked Dryas mats, 5 had no flowers grazed at all (though a few buds were grazed), and the others had between 15 and 80% of the shoots grazed in the opened flower phase. Three mats had all the flowers grazed in one grazing event, while 2 had them grazed on two occasions.
Discussion
FLORAL HERBIVORY
The data shown here demonstrate the severity and magnitude of floral herbivory on Polar Willow–Mountain Avens Ridge vegetation (CitationRønning, 1996) on the Brøgger Peninsula. Such impacts are easily overlooked on superficial inspection of the landscape, since severed peduncles are difficult to see without close examination of the vegetation. It has been noted previously that “evidence of herbivore pressure on plants tends to be cryptic” (CitationLouda, 1982), and this applies strongly to small-stature tundra species. Evidence of flower herbivory associated with some removal of leaf tips around the basal rosette of leaves demonstrated that reindeer can also remove shoots very close to the base of the peduncle, thus exacerbating the problem of herbivory detection. A substantial difference in the intensity of herbivory at nearby locations was identified, possibly due to differences in topography, visibility, accessibility to the reindeer, and/or phenological differences.
EXTRAPOLATION TO LANDSCAPE SCALE
In supporting the observations of CitationWada (1999) this paper also highlights the potential difficulty of extrapolation from a single survey plot to larger units of the landscape. This sampling problem in relation to landscape pattern requires further emphasis in future work on ungulate foraging. Earlier work on reindeer and other arctic ungulates also points to highly selective use of the landscape mosaic to exploit the spatially and temporally changing availability of “high-quality” forage (CitationWhite, 1983; CitationJefferies et al., 1992, Citationvan der Wal et al., 2000), thus making sampling strategies challenging. Our observations and those of CitationWada (1999) at the fine scale of individual Dryas patches are exciting because they suggest that reindeer focus on “flower-dense” patches, but further study is needed to demonstrate this relationship conclusively. Since N = 5 at the extensive plots at Sites A and B, there was, in practice, insufficient replication to demonstrate density dependency of herbivory, although at Site B the results indicate that this was the case. This connection has additional implications for pollen flow via insect pollinators (CitationKrupnick et al., 1999; CitationWada, 1999) and thus for the potential reproduction success of remaining flowering shoots (CitationTotland, 1994).
REINDEER POPULATION INTENSITY LINKED WITH FLORAL HERBIVORY
The extensive plot survey showed that during summer 1997 there was a large difference between the percentage floral herbivory in two areas with contrasting reindeer densities. Data do not exist for the number of reindeer on the north compared to the south of the Brøgger Peninsula during 1996–1999. These reindeer are nonmigratory but are free to walk around the whole peninsula, although they tend to have a home range and remain within a local area (CitationHenriksen, 2001). The herbivory data from the tagged plants at Site C (36.5% grazed) in 1999 were similar to those found by CitationWada (1999) in 1996 (33%), despite being recorded in different years and on opposite sides of the peninsula. Warmer weather results in more flowering and a higher proportion of flowers in the diet of geese (CitationAlsos et al., 1998). The warmer summer of 1997 compared to 1996 might explain the differences in percentage herbivory in these relatively close locations; however, the differences may be due to regional or annual differences in flowering or grazing intensity or to differences in methodology.
The data presented from 2 yr and 3 sites reveal extreme selectivity in summer grazing patterns by Svalbard reindeer. Further work is required to evaluate the nutritional significance of this grazing pattern for the herbivore and also in terms of the allocation patterns of carbon and nutrients within the plants themselves. It is conceivable that floral herbivory exerts little long-term damage to the mineral nutrient and carbon economy of established Dryas clones in this environment, bearing in mind that production of viable seeds may often be temperature-constrained during “normal” summer conditions (CitationWookey et al., 1995; CitationWada, 1999). Since herbivory appears to be most intense during floral display, and thus prior to the major allocation of mineral nutrients and carbon to developing embryos within the gynoecium, it could be argued that the loss of resources to herbivory is insignificant compared to the normal costs of unsuccessful reproductive effort (see CitationBloom et al., 1985). Sexual reproduction is, in any case, a “high-risk” strategy in high arctic plant populations that, according to CitationPhilipp et al. (1990), already balance upon a “reproductive knife edge.” Data from this project cannot be used to assess the impact of reindeer upon the overall growth and nutrient economy of established D. octopetala plants in the Kongsfjord area. However, our results do reinforce the view that Dryas may be persisting largely clonally in areas grazed by reindeer and/or with unfavorable summer temperatures for seed development. An ongoing study (CitationCooper, 2002) aims to further quantify the effect of grazing on the seedbank of northwest Svalbard.
The importance of herbivory for plant reproductive output and recruitment has been described previously (see CitationLouda, 1982; CitationPettersson, 1991; CitationKrupnick et al., 1999), although principally in connection with the impacts of invertebrate herbivores and not in an arctic setting. By contrast, the debate on interactions between arctic ungulates and vegetation communities has lacked an emphasis on the demography of plant populations and has focused instead upon questions of net primary productivity, carrying capacity, and community composition (see CitationManseau et al., 1996). The data presented here suggest that more attention could usefully be directed toward studies of selective seasonal grazing patterns, set within the context of landscape and vegetation heterogeneity, and their impacts upon plant demography.
Conclusions
This study reveals direct evidence of strong grazing pressure by Svalbard reindeer on reproductive shoots of D. octopetala, with 36 to 65% of flowers removed on the Brøgger Peninsula, which had a relatively high reindeer intensity. There was considerably less floral herbivory (7%) on the sparsely populated Blomstrand island. The reindeer came regularly to the study areas, tracked the phenology of the flowers, and initiated grazing on average 5 d after the flowers opened. Flowers greater than 13 d old were not grazed. Many of the flowers on a particular Dryas mat were grazed on the same day, and the grazing had a patchy spatial distribution. The implications are that floral foraging imposes a significant further limitation on seed production and may therefore limit seedling recruitment of Dryas on the Brøgger Peninsula.
FIGURE 1. Map of the study areas in the Kongsfjord region of northwestern Spitsbergen, Svalbard. Work conducted at the sites was as follows: Site A (Brøgger Peninsula)—reindeer exclosure experiment, extensive monitoring plots; Site B (Blomstrand)—extensive monitoring plots; Site C (Brøgger Peninsula)—tagged reproductive shoots and trampiometers
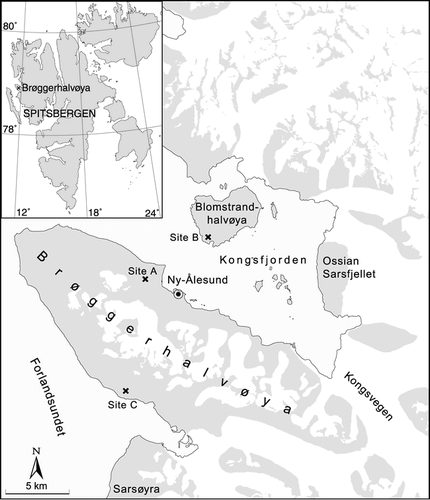
FIGURE 2. Percentages of reproductive shoots within defined categories during the reindeer exclosure experiment on the Brøgger Peninsula (Site A) from 27 June to 22 July 1997 (corresponding to Julian dates 178 to 203). Fig. 2(a) shows data from the exclosures (protected from reindeer); Fig. 2(b) shows data from the plots open to reindeer
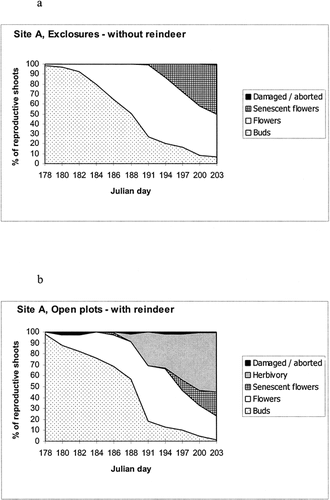
FIGURE 3. Numbers of reproductive shoots (m−2) within defined categories at the extensive monitoring plots on the Brøgger Peninsula (Site A, Fig. 3a) and on Blomstrand island (Site B, Fig. 3b) on the dates shown (n = 5 replicate plots). The transformations between these dates are also indicated. The coefficiants of variation for herbivory categories were 25.6% and 179.9% for the Brøgger Peninsula and Blomstrand island plots, respectively
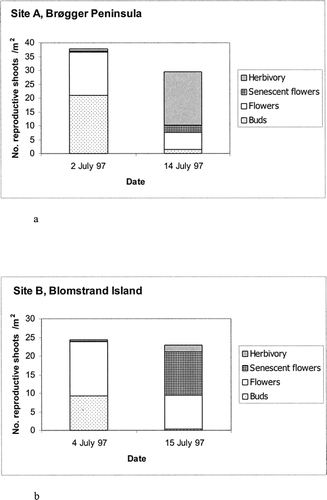
Table 1a. Species cover data, expressed as percentage of total, for Site A plots with experimental exclosures (A1 and A2) on the Brøgger Peninsula (7 July 1997) and for Site B plots with extensive monitoring on Blomstrand Island (15 July 1997). Measurements at each location are based upon 4 10-m line transects, with recording every 100 mm (first hit only) to give a total of 400 observations (and therefore resolution down to 0.25% cover). Standard error of the mean is in parenthesis. Locations of Sites A and B are given in
Table 1b. Species cover data, expressed as percentage of total, for Dryas mats at Site C, representative of plots with tagged reproductive shoots. Ten point frame quadrats of size 0.5 × 0.5 m were placed over representative Dryas mats spread throughout the study site on 28 June 1999. Data from first hits only were used to give a total of 1000 observations. Standard error of the mean is in parenthesis. Location of Site C is given in
Table 2. Reindeer presence shown by trampiometers (weekly) and grazing events at site C in 1999. One or more flattened nails suggest the presence of reindeer at the site. Two hundred Dryas buds were tagged at the begining of week 26. No recording was carried out in week 33
Table 3. Summary statistics of the repeated-measures ANOVA for the reindeer exclosure experiment at Site A. Response variables tested were the percentage (arcsin transformed) of total flowering shoots that could be classified within the different categories
Acknowledgments
The authors would like to thank the Ny-Ålesund Large Scale Facility (LSF) scheme and the Commission of the European Communities TMR program for the opportunity for P. A. W to work at Ny-Ålesund during 1997. Funding was provided for E. J. C. from the Norwegian Research Council “ALV” program, project number 121507/720. Technical and logistic support was provided by the staff of the Norwegian Polar Institute, the UK Base Manager Nick Cox (NERC), and Kings Bay Kull Compani AS. We are grateful to Mats Fröberg, Leidulf Lund, Helga Perander, Michelle Simey, and Fiona Smith for help and support in the field and to Bernt Bye for drawing the map. We thank I. G. Alsos, J. A. Baddeley, S. J. Coulson, R. M. M. Crawford, D. Gwynn-Jones, L. Nilsen, and N. Wada for comments that improved the paper.
References Cited
- Aanes, R. , B-E. Sæther , and N. A. Øritsland . 2000. Fluctuations of an introduced population of Svalbard reindeer: the effects of density dependence and climatic variation. Ecography 23:437–443.
- Alsos, I. G. , A. Elvebakk , and G. W. Gabrielsen . 1998. Vegetation exploitation by barnacle geese Branta leucopsis during incubation on Svalbard. Polar Research 17:1–14.
- Bayfield, N. G. 1971. A simple method for detecting variations in walker pressure laterally across paths. Journal of Applied Ecology 8:533–535.
- Bloom, A. J. , F. S. Chapin III , and H. A. Mooney . 1985. Resource limitation in plants—an economic analogy. Annual Reviews in Ecology and Systematics 16:363–392.
- Brattbakk, I. 1986. Vegetasjonsregioner—Svalbard og Jan Mayen:. Nasjonalatlas for Norge (National Atlas for Norway), Kartblad (map sheet) 4.1. 3.
- Brochmann, C. and S. Steen . 1999. Sex and genes in the flora of Svalbard—implications for conservation biology and climate change. Det Norske Vitenskaps-Akademi. I. Matematisk Naturvitenskapelig Klasse, Skrifter, Ny serie, 38: 33–72.
- Callaghan, T. V. and U. Emanuelsson . 1985. Population structure and processes of tundra plants and vegetation. In White, J. (ed.), The Population Structure of Vegetation. Dordrecht: Junk, 399–439.
- Cooper, E. J. 2002. Does reindeer floral herbivory affect the germinable seed bank on Svalbard?. In Cho, D.-S. (ed.), Proceedings of the VIII INTECOL International Congress of Ecology, “Ecology in a changing world,” August 11–18, Seoul, Korea. VIII INTECOL Congress Committees, 307: 50–51.
- Cooper, E. J. and P. A. Wookey . 2001. Field measurements of the growth rates of forage lichens and the implications of grazing by Svalbard reindeer. Symbiosis 31:173–186.
- Crawford, R. M M. 1997. Natural disturbance in high arctic vegetation. In Crawford, R.M.M. (ed.), Disturbance and Recovery in Arctic Lands. Dordrecht: Kluwer Academic Publishers, 47–62.
- Crow, J. F. 1992. An advantage of sexual reproduction in a rapidly changing environment. Journal of Heredity 83:169–173.
- Elvebakk, A. 1997. Tundra diversity and ecological characteristics of Svalbard. In Wielgolaski, F. E. (ed.), Ecosystems of the World, 3: Polar and Alpine Tundra. Amsterdam: Elsevier Science, 347–359.
- Freedman, B. , N. Hill , J. Svoboda , and G. Henry . 1982. Seed bank and seedling occurrence in a high arctic oasis at Alexandra Fjord, Ellesmere Island, Canada. Canadian Journal of Botany 60:2112–2118.
- Henriksen, S. 2001. Does availability of resources influence grazing strategies in female Svalbard reindeer?. Cand. Scient. Thesis. Norges Teknisk-Naturvitenskapelige Universitet.
- Jefferies, R. L. , J. Svoboda , G. Henry , M. Raillard , and R. Ruess . 1992. Tundra Grazing Systems and Climatic Change. In Chapin, F. S., III, Jefferies, R. L., Reynolds, J. F., Shaver, G. R., and Svoboda, J. (eds.), Arctic Ecosystems in a Changing Climate. San Diego: Academic Press, 391–412.
- Krupnick, G. A. , A. E. Weis , and D. R. Campbell . 1999. The consequences of floral herbivory for pollinator service to Isomeris arborea . Ecology 80:125–134.
- Louda, S. M. 1982. Limitation of the recruitment of the shrub Haplopappus squarrosus (Asteraceae) by flower- and seed-feeding insects. Journal of Ecology 70:43–53.
- Manseau, M. , J. Huot , and M. Crête . 1996. Effects of summer grazing by caribou on composition and productivity of vegetation: community and landscape level. Journal of Ecology 84:503–513.
- Mathiesen, S. D. , W. Sørmo , ØE. Haga , H. J. Norberg , T. H A. Utsi , and N. J C. Tyler . 2000. The oral anatomy of arctic ruminants: coping with seasonal changes. Journal of Zoology (London) 251:119–128.
- Moen, J. 1993. Herbivory and plant community structure in a subarctic altitudinal gradient. Ph.D. thesis. Department of Ecological Botany, University of Umeå, Sweden. 153 pp.
- Odasz, A. M. and O. Savolainen . 1996. Genetic variation in populations of the arctic perennial Pedicularis dasyantha (Scrophulariaceae), on Svalbard, Norway. American Journal of Botany 83:11 1379–1385.
- Pettersson, M. W. 1991. Flower herbivory and seed predation in Silene vulgaris (Caryophyllaceae): effects of pollination and phenology. Holarctic Ecology 14:45–50.
- Philipp, M. , J. Böcher , O. Mattsson , and S. R J. Woodell . 1990. A quantitative approach to the sexual reproductive biology and population structure in some arctic flowering plants: Dryas integrofolia , Silene acaulis and Ranunculus nivalis . Meddelelser om Grønland (Bioscience) 34:1–60.
- Prop, J. and J. de Vries . 1993. Impact of snow and food conditions on the reproductive performance of barnacle geese, Branta leucopsis . Ornis. Scandinavica 24:110–121.
- Prop, J. , M. R. van Erden , and R. H. Drent . 1984. Reproductive success of the barnacle goose, Branta leucopsis , in relation to food exploitation on the breeding grounds, western Spitsbergen. Norsk Polarintitutt Skrifter 181:87–117.
- Rønning, O. I. 1996. The Flora of Svalbard. Polarhåndbok No. 10. Oslo: Norsk Polarinstitutt. 184 pp.
- Stenström, A. 2000. From pollination to variation—reproduction in arctic clonal plants and the effects of simulated climate change. Ph.D. thesis, Botanical Institute, Göteborg University, Sweden. 148 pp.
- Thórhallsdóttir, T. E. 1998. Flowering phenology in the central highland of Iceland and implications for climatic warming in the Arctic. Oecologia 114:43–49.
- Totland, Ø 1994. Influence of climate, time of day and season, and flower density on insect flower visitation in alpine Norway. Arctic and Alpine Research 26:66–71.
- van der Wal, R. , N. Madan , S. van Lieshout , C. Dormann , R. Langvatn , and S. D. Albon . 2000. Trading forage quality for quantity? plant phenology and patch choice by Svalbard reindeer. Oecologia 123:108–115.
- van der Wal, R. and M. J J. E. Loonen . 1997. Goose droppings as food for reindeer. Canadian Journal of Zoology 76:1117–1122.
- Virtanen, R. 1996. Arctic and Oroarctic vegetation patterns in Northern Europe as a consequence of topography, climate, bedrock conditions and grazing. Ph.D. thesis, Department of Biology, Oulu University, Finland. 30 pp.
- Wada, N. 1999. Factors affecting the seed-setting success of Dryas octopetala in front of Brøggerbreen (Brøgger Glacier) in the high arctic, Ny-Ålesund, Svalbard. Polar Research 18:261–268.
- Wegner, C. 1997. Responses to plant-herbivore interactions in Svalbard reindeer forage plants. Ph.D. thesis, Tromsø University, Norway. 37 pp.
- White, R. G. 1983. Foraging patterns and their multiplier effects on the productivity of northern ungulates. Oikos 40:377–384.
- Wookey, P. A. , A. N. Parsons , J. M. Welker , J. A. Potter , T. V. Callaghan , J. A. Lee , and M. C. Press . 1993. Comparative responses of phenology and reproductive development to simulated environmental change in subarctic and high arctic plants. Oikos 67:490–502.
- Wookey, P. A. , C. H. Robinson , A. N. Parsons , J. M. Welker , M. C. Press , T. V. Callaghan , and J. A. Lee . 1995. Environmental constraints on the growth, photosynthesis and reproductive development of Dryas octopetala at a high arctic polar semi-desert, Svalbard. Oecologia 102:478–489.