Abstract
Several environmental indicators show that climate changed dramatically at the turn of the 19th century, with global warming throughout the 20th century. Among the most used proxies to evaluate long-term changes in climate are trees located at their northern range limit. Several studies have shown enhanced tree regeneration at treeline caused by recent warming, but no data are available on height growth performance of forest trees at treeline before and during the 20th century warmth. In this study, we examined the long-term growth performance of black spruce (Picea mariana [Mill.] B.S.P.) trees located in a lichen-spruce woodland, near the arctic treeline in eastern Canada. The study woodland is an old-growth stand that has escaped fire approximately during the past two millennia. We performed stem analysis on normally developed trees which grew in the woodland over the last centuries. The sampled trees are ramets from long-lived, self-regenerating black spruce clones that are forming small tree islands within the woodland. The co-occurrence of living and dead tree stems inside clones gives the opportunity to evaluate the growth performance of the same genets through time. Height and radial growth of 60 tree stems from 15 old-growth clones were evaluated for a period spanning the last 400 yr. Sampling included the two tallest living stems and the two tallest dead stems of randomly selected clones. No differences in height and radial growth were found among the 30 living stems nor among the 30 dead stems within clones. Living stems were 2 m taller and radial growth was 1.6 times greater than dead stems. Vertical development of stems was divided into two parts according to position of snow cover. Growth of dead and living stems was similar below the snowpack line. However, significant differences were found for growth above snowpack for the two types of stems. Growth above snow cover for all dead stems occurred between the 17th and 19th centuries. In contrast, growth of living stems above snow cover started during the late 19th century. Our data indicate that stems of the same genotype responded directly to climate change, in conjunction with climatic conditions prevailing at the time when they were protruding above the snowpack. Compared to extant trees, significantly smaller trees grew in the woodland during the Little Ice Age. Potential causal factors of differential growth performance through time are discussed.
Introduction
The last millennium was a period of contrasted climatic conditions (CitationLamb, 1977; CitationCrowley, 2000), in particular during the long-enduring cold stage of the Little Ice Age (CitationLamb, 1977; CitationGrove, 1988) which was followed by global warming in most parts of the Northern Hemisphere (CitationJones et al., 1982; CitationFolland et al., 1990; CitationWang and Lewis, 1992; CitationMann and Bradley, 1999). Although the instrumental record of temperature change in high-latitude areas is rather short, weather data indicate that climate warmed by about 0.6°C since the turn of the 19th century (CitationHoughton et al., 2001).
Mounting evidence for large-scale responses of both flora and fauna to recent climate change is being gathered in a variety of ecosystems across the northern hemisphere (CitationSturm et al., 2001; CitationWalther et al., 2002; CitationParmesan and Yohe, 2003). High-resolution proxies like tree-rings are used to evaluate the impact of climate change at the centennial time scale (CitationLaMarche, 1974; CitationEsper et al., 2002). Due to their ability to produce annual growth rings, the ecological responses of trees to changing climate provide reliable records of recent and past environmental conditions. Several studies have documented the impact of warming on tree populations at their range limit, particularly in northern environments where increased tree density and seedling establishment have been reported since the end of the Little Ice Age (CitationKullman, 1986a, Citation1986b, Citation1987; CitationScott et al., 1987; CitationPayette et al., 1989; CitationSzeicz and MacDonald, 1995; CitationMacDonald et al., 1998; CitationSuarez et al., 1999; CitationGervais and MacDonald, 2000; CitationSvein-björnsson, 2000; CitationHofgaard and Wilmann, 2002; CitationLloyd and Fastie, 2002, Citation2003). An increase in radial growth of subarctic conifers over the last 100 yr due to warming has been reported (CitationGarfinkel and Brubaker, 1980; CitationEnright, 1984; CitationFilion et al., 1985; CitationBégin, 1991), but reduced growth also occurred in trees stressed by temperature-induced drought (CitationBarber et al., 2000).
The North American forest tundra represents a transition zone between the continuous boreal forest and the treeless tundra (CitationPayette et al., 2001). In this climate-sensitive area, black spruce (Picea mariana [Mill.] B.S.P.) is the most abundant tree species (CitationRowe, 1972; CitationRitchie, 1987). In subarctic Québec, several old-growth lichen-spruce woodlands have escaped fire for many centuries and even millennia (CitationPayette and Morneau, 1993). In absence of fire disturbance, black spruce regeneration occurs mainly by vegetative propagation, producing a complex clonal structure which can become more than 500 yr old (CitationLaberge et al., 2001). The process of continuous recruitment through time leads to the formation of long-lived tree islands composed of mixed-aged living and dead stems belonging to the same genotype (CitationLaberge et al., 2000). Because of their continuous exposure to extreme conditions, subarctic black spruce clones are at their tolerance limit (CitationBégin, 1991; CitationLaberge et al., 2001). Clonal spruces have the capacity to react rapidly to climate changes due to their large phenotypic plasticity. During colder episodes with snowless winters, black spruce maintains a prostrate growth form. However, if conditions improve, black spruce develops a normal arboreal growth form (CitationLavoie and Payette, 1992; CitationPereg and Payette, 1998). These morphological changes are greatly influenced by weather conditions and result mainly from duplication of the main stem axis caused by lethal spring frost of the terminal bud and mechanical defoliation at the snow-air interface (CitationHadley and Smith, 1986; CitationPayette et al., 1996). Stem growth is then fragmented into two vertical components, with the above-snowpack stems exposed to winter conditions and the below-snowpack stems protected by snow. Although the impact of climate on growth of subarctic trees has been outlined in several sites across the circumboreal zone, variations in height and radial growth of forest tree stems from the same genotype, i.e. stems having the same growth potential, have not yet been appreciated. In the absence of external disturbances other than climate change, long-lived tree stems belonging to the same genotype may be useful proxies to evaluate the direct impact of recent climate change on growth performance of forest trees.
The main objective of this study is to document the impact of recent and past climate change on height and radial growth of clonal black spruce trees in an old-growth lichen woodland located near the arctic treeline. Forest trees from the same genotypes were analyzed to evaluate the direct influence of climate on growth while controlling for genetic differences. We hypothesized that stems of the same genotypes that have grown during the 20th century would be taller and larger than those that lived earlier when climate was colder, and that the part of the stem above the snowpack should be the most constrained growth component of the entire stem because of its exposure to winter conditions.
Study Area
We investigated a lichen-spruce woodland located along the Boniface river in subarctic Québec (57°43′N, 76°05′W), 35 km east of Hudson Bay, and 10 km south of the arctic treeline (). The mean annual temperature at the nearest weather station (located in Inukjuak, 135 km northeast) is −7°C, ranging from −26°C in February to 9°C in July. The mean annual frost-free period is about 60 d, with the growing season extending from mid-June to mid-August. Mean annual precipitation totals 550 mm, with 40% falling as snow (CitationEnvironment Canada, 1993). The vegetation is representative of the northern forest tundra with spruce-moss forests in moist protected sites, lichen woodlands on well-drained soils and lichen–heath–dwarf birch (Betula glandulosa Michx.) on wind-exposed, treeless hills (CitationPayette, 1983). Black spruce is the dominant tree species and dwarf birch is the most abundant shrub.
The lichen woodland is located on the slope of a well-drained, low-elevation (130–140 m a.s.l.) drumlin. The woodland is of postfire origin and regenerated about 2000 yr ago according to 14C-dated spruce charcoal sampled at the base of the organic horizon (1950 ± 70 BP [UL-365]) (CitationBégin, 1991). The vegetation is composed of several species of lichens (Cladina spp., Cladonia spp., and Cetraria spp.), shrubs (Betula glandulosa, Ledum groenlendicum Retzius, Empetrum nigrum L., Vaccinium vitis-idaea L., Loiseleuria procumbens [L.] Desv, and Phyllodoce caerulea [L.] Babingt) and mosses (Pleurozium spp., Dicranum spp., and Polytricum spp.) (CitationBégin, 1991). Black spruce is the only tree species growing in the stand, and it regenerates mainly by vegetative propagation, resulting in clones composed of both dead stems in the center and living stems at the periphery.
Methods
SAMPLING
A quadrat of 2500 m2 was used to position three transects (10 m × 50 m) oriented south-north. Little variation in microsite conditions existed in the stand due to homogenous topography and soil conditions. In each transect, 10 spruce clones were selected based on the following criteria. Clonal stems were divided into two groups according to their period of development: the living-stem group was composed of stems currently growing in the stand, and the dead-stem group included stems that have developed in the recent and distant past. Most dead stems were still in standing position at the time of sampling. Only clones with a minimum of two living and two dead stems were selected. To ensure genetic integrity of the clones, only dead and living stems still attached to each other were retained for further sampling and analysis. Selected dead stems had a well-preserved basal trunk to determine the approximate date of onset of vertical growth based on ring counts from wood discs sampled near the ground. Because dead stems may have lost the stem apex after years of exposure, only stems with nontruncated apex and less that 0.5 cm in diameter were selected to avoid bias when comparing height between dead and living stems. Among the 30 clones that satisfied these criteria (10 clones/transect), 15 clones were selected at random (5 clones/transect). In each clone, the two tallest living and dead stems were sampled (n = 4 stems × 15 clones = 60 stems).
A detailed morphological description of the selected clones and stems was made before sampling. Clone description included height, diameter, and number of living and dead stems. Stem description included total height, diameter, foliage quality (density, coloration) and distribution, number and height of leaders, and growth form. Snowpack thickness was inferred based on height of the erosional level along the stem axis. Snow and ice-crystal abrasion, low temperature, and high wind at the snow-air interface cause stem anomalies, which represent long-term adaptations to the snow environment and indicate the average snow thickness (CitationLavoie and Payette, 1992).
Stem cross-sections were taken every 25 cm in dead stems and every 50 cm in living stems, in both cases beginning at the stem base. A shorter sampling interval was used for dead stems because of their smaller size. Wood samples were air-dried and sanded until xylem cells were clearly visible under a binocular microscope at 40×. For each cross-section, tree-rings were counted and cross-dated using diagnostic light rings (CitationFilion et al., 1986). Cross-sections were checked for anomalous ring growth, missing rings, and incomplete rings. None of these anomalies were recorded in all the analyzed cross-sections. Actual dates of tree death are underestimated for most of the stems because of erosion and decomposition of the outermost rings. Although the number of years missing is unknown, this underestimation of mortality dates is insignificant because stems mortality extend over a period of about 200 yr. Tree-ring width of each basal cross-section was measured along two opposed radii with a Velmex micrometer (± 0.002 mm) avoiding reaction wood or branch traces when present. The average of the two measured radii was calculated to obtain 30 series for both dead and living stems. All series were checked with the COFECHA program (CitationHolmes, 1983). Five-year running means were calculated for the 30 series to smooth year-to-year variability and maintain low frequency variations (CitationFritts, 1976). The average of the 30 series of the two stem groups was used to build dead- and living-stem chronologies.
DATA ANALYSES
Height growth curves of the 60 sampled stems showed a similar height growth pattern that was divided into three different growth stages. Stage I corresponds to stem development beneath the snowpack, stage II to stem development above the snowpack until reduction of height growth rate or stem death occurred, and stage III (only dead stems) is the period of time from the onset of decrease of height growth and stem death. The four growth parameters analyzed were height, ring width, height growth rate, and duration of growth stages. Total stem height was measured in the field. Mean ring width of the three growth stages were measured for each stem. Height growth rates were calculated by performing linear regressions of each height growth curve. The slope of the regression (cm yr−1) corresponds to the height growth rate. Although sampling has shortened the lifespan of the living stems, comparison between the two stem groups was done as if growth of the living stems was terminated. Because the stems have developed from the ground and snow cover at different periods of time, beginning and end of growth stages as well as growth parameters have been determined for each stem independently with their respective height growth characteristics and snow-cover estimation.
An ANOVA was performed on each parameter to verify if growth of living stems within a clone was significantly different from growth of living stems of the other clones. There were no significant differences in the four parameters between the living stems of a clone and those of the other clones (0.02 > F 1,28 < 0.9; 0.3 > P < 0.8). The same result was found for the dead stems (0.0005 > F 1,28 < 3.7; 0.07 > P < 0.9). This indicates that genotypic variability within the two stem groups was controlled. The average of each parameter was calculated for the 30 living stems and the 30 dead stems. Because data were normally distributed, paired t-tests were used to compare the average of each parameter between living and dead stems. We used paired t-tests because we have compared means of each parameter of stems from the same genotype over a different growth period.
Results
STEM MORPHOLOGY
Fourteen out of the 30 living stems showed a normal, arboreal growth form; the upper part (above snowpack) of 14 stems was slightly damaged, and two other stems had a skirted growth form (that is, with a lower leafy and densely branched part, a damaged mid-part and a moderately leafy and branched upper part). Because dead stems were branchless, stem shape was used as a proxy for past growth forms. Twenty-six out of the 30 dead stems had a bottle-shape form (that is, with a large diameter below the snowpack line and becoming abruptly narrow above the snowpack line). The other four stems showed a normal shape with a gradual decrease in diameter from the base towards the apex. The number of leaders at the snow-air interface was 0.4 ± 0.07 and 1.16 ± 0.21 (mean ± SE) for living and dead stems, respectively. The inferred mean snow thickness was 0.95 m ± 0.04 for living stems and 0.77 m ± 0.04 for dead stems.
POPULATION STRUCTURE
The initial period of vertical development of living and dead stems () ranged between 1616 and 1897, and 1476 and 1701, respectively. Growth above snowpack of living and dead stems occurred between 1772 and 1948, and 1527 and 1789, respectively. Death of the upper stem part of dead stems occurred between 1693 and 1888, whereas mortality of whole dead stems extended from 1732 to 1956 with an increase between 1825 and 1900. Mean age of living and dead stems was 240.4 ± 11.9 yr and 242.2 ± 9.1 yr, respectively.
HEIGHT GROWTH
A difference of 2.06 m (P < 0.001) was found between mean height of living stems (4.63 ± 0.14 m) and mean height of dead stems (2.57 ± 0.09 m). Although negative and positive trends in age-height relationships were observed for living and dead stems, respectively, the two relations were not significant indicating that there is no age effect on height reached by stems (). Because the 60 studied stems showed a similar height growth pattern whatever their initial year of development, mean height growth curves of living and dead stems were calculated to illustrate the three growth stages (). Each point of the height growth curves represents the mean initial year of development for each sampled level. The confidence interval (95%) of the dead-stem curve increased above the 2-m stem-height level because fewer stems were used in the calculation of the mean. During a period of 200 yr (1615–1815), extreme values of living stems overlapped those of dead stems. During stage I, trees showed slow vertical growth during the development of the lower stem part. During stage II, stems experienced faster growth beginning with growth of the upper stem part above the snowpack. Finally, stage III corresponded to the period when height growth showed a marked reduction before stopping. On average, stage I of living and dead stems lasted 112.3 ± 9.1 yr and 69.5 ± 5.8 yr, and stage II lasted 128.1 ± 9.1 yr and 90.9 ± 11.1 yr, respectively. Stage III spanned 81.7 ± 8.3 yr (dead stems only). Only mean duration of stage I differed significantly between both groups of stems (P < 0.001) ().
Mean height growth rate was calculated for living and dead stems according to the respective growth stages of each stem (). There were no significant differences in height growth increment between living (0.94 ± 0.08 cm yr−1) and dead stems (1.28 ± 0.18 cm yr−1) during stage I (P = 0.18). However, in stage II, living stems showed a higher height growth rate (3.76 ± 0.17 cm yr−1) than dead stems (2.01 ± 0.14 cm yr−1; P < 0.001). Height growth rate of dead stems during stage III was 0.20 ± 0.08 cm yr−1. Within a stem group, mean height growth rate between stages I and II differed significantly in living and dead stems (P < 0.001), with mean height growth rate during stage II being 2 and 4 times greater than during stage I for dead and living stems, respectively. Significant differences in mean height growth rate of dead stems were also found between stages II and III (P < 0.001), as mean height growth rate during stage III was particularly small.
RADIAL GROWTH
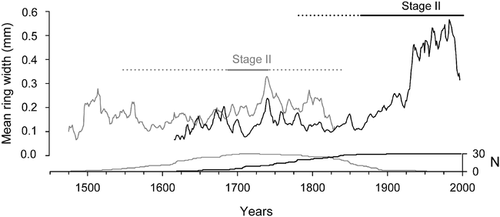
Mean ring width of living and dead stems was not significantly different during stage I (0.12 ± 0.01 mm yr−1 and 0.14 ± 0.01 mm yr−1, respectively; P = 0.2), whereas the difference was significant during the second stage of their development (0.37 ± 0.03 mm yr−1 and 0.23 ± 0.01 mm yr−1, respectively; P < 0.001) (). Tree ring growth curves were divided into two periods (). First, synchronous growth was recorded between the two stem groups from the 1600s to the 1800s. During this period, tree ring width of living stems was narrower than tree ring width of dead stems. Second, radial growth of living stems increased during the early 20th century and climaxed during the late 20th century, whereas ring width of dead stems dropped dramatically in the early 1800s and remained low until death.
Discussion
The analysis of the 60 clonal stems from 15 clones was used to evaluate differences in black spruce growth parameters over the past 400 yr in a lichen woodland located near the arctic treeline. Based on our data, genotypic diversity does not seem to cause differential growth among clones for living and dead stems. Living stems were taller and larger than dead stems of the same genotypes. Height growth curves indicated that all sampled stems followed a similar height growth pattern whatever their period of development. The growth performance of black spruce forest trees from the same genotypes has changed significantly before and after the late 19th century corresponding to the break point between the Little Ice Age and the 20th century warmth. The factors responsible for the contrasted growth between dead and living stems are probably associated with changing climatic conditions.
However, other controlling factors where involved when all the stems were of small size, that is, when stem height was lower than thickness of the snow cover (stage I). Smaller but similar mean height growth rates of living and dead stems during stage I, whatever the time period, may be attributed to light competition due to overcrowding within clones. Stage I begins when clonal stems emerge from the ground and lasts until they reach the snow-air interface. This could be attributed to the snow cover that protects small stems from winter-exposed conditions (CitationPayette et al., 1996; CitationPereg and Payette, 1998). However, the time necessary to reach the snow-air interface was significantly greater for the living stems (about 100 yr) than for the dead stems (about 70 yr). Between the mid-1700s and the mid-1800s most dead stems, which were growing above the snow cover at that time, likely reduced light reaching the developing living stems that were below the snow cover.
No such direct control existed during stem development above the snow cover (stage II). By selecting the two tallest dead and living stems of each clone, we have chosen the most performing stems of each stem group, whatever their period of growth above the snow cover. Stems of the dead-stem group, which were distributed more at the center of the clones than at their periphery, as it is the case for most living stems, have not suffered from spatial competition because they were the most competitive of all stems: if any competition existed it was against the smaller stems. Also, no spatial interference existed between the two stem groups during stage II: living stems developed when most stems of the other group where dying or were already dead. What would be the other mechanisms responsible for the contrasted growth pattern between the two stem groups? As stated earlier, soil conditions and vegetation composition and cover are homogenous throughout the stand (CitationBégin, 1991). Ground vegetation in spruce clones is remarkably sparse, with only lichen patches and scattered shrubs, and dominated by wood and needle litter. The organic horizon beneath the clone surface also testifies to similar plant composition during stand development as it is largely dominated by spruce remains.
The growth of taller and larger tree stems during the 20th century may be attributed to increased temperature as reported across the northern hemisphere (CitationLamb, 1977; CitationJones et al., 1982; CitationBradley, 1988; CitationOverpeck et al., 1997; CitationTett et al., 1999). The same pattern of growth changes from the Little Ice Age to Present has been reported across the circumboreal zone (CitationKullman, 1986a, Citation1986b, Citation1998; CitationPayette et al., 1989; CitationSzeicz and MacDonald, 1995; CitationMacDonald et al., 1998; CitationPereg and Payette, 1998). Potential causal factors likely to explain the main differences in height and radial growth of trees growing in the same forest environment for several centuries may be associated with changing tree vigor at the stand scale. Although the arboreal growth form of black spruce has been maintained throughout the woodland history from the 16th century to Present, lower growth performance of trees living during the Little Ice Age was caused probably by reduced photosynthate availability, as tree vigor depends largely on meristematic activity (CitationRobichaud and Methven, 1991). Bud mortality and attrition of foliage above the snowpack due to mechanical loss of needles during winter (CitationHadley and Smith, 1986; CitationPayette et al., 1996; CitationBégin and Filion, 1999; CitationLaberge et al., 2001) were probably more frequent before the 20th century, thus reducing the photosynthetic biomass necessary for greater height and radial growth. The marked differences in growth performance between the two stem groups are best explained by survival of a larger needle and bud biomass above the snow cover during the 20th century despite a greater ratio of respiration to photosynthesis in the taller, extant trees. Whereas the ratio of foliage biomass to non-productive biomass remains rather stable in long-lived stunted black spruce surviving below the snowpack each winter (CitationLaberge et al., 2000), greater loss of foliage above the snow cover in stems growing during the Little Ice Age likely explains their lower growth performance.
Stage III was characterized by the decrease or cessation of stem height growth as a result of stem degeneration. The latter generally begins in the upper stem and continued according to a basipetal gradient. Death of the upper stems occurred in the 1800s, whereas the lower stems below snowpack remained alive and continued their radial development until the late 19th century. As a result, stems developed a bottle-shape growth form as observed in most of the dead stems. Although none of the living stems showed this type of growth form, the upper part of 14 living stems were slightly damaged, and two stems were skirted, an indication of the beginning of the degradation process. Height growth decrease of dead stems began around 1750, and was likely attributed to their exposure to harsh winter conditions. However, radial growth of stems below the snow cover only began to decrease in the early 19th century.
Because this woodland environment is located in a marginal area for tree growth, one can expect that the living trees also will be affected by progressive attrition of foliage with time, in a pattern similar to that experienced by the dead trees. Until then, greater winter survival of needles and buds is still promoting fast growth. According to their growth form and the relatively small number of leaders at the snow-air interface, living stems seem to have gone through the interface without major difficulties. Living stems began to develop above the snow cover at the end of the 19th century. The stems were likely exposed to milder winter and warmer summer conditions that enhanced growth. As a result, height and radial growth increased much more during stage II for this group. In contrast, the development above the snow cover of dead stems coincided with the beginning of the Little Ice Age. Their smaller height growth rate and tree ring width were likely due to colder summers and sustained exposure to harsh winter conditions of the upper stems that are conducive to traumatic events, including mechanical defoliation and branch or meristem death (CitationBégin and Filion, 1999).
Conclusion
The long-lived, clonal forest trees from the same genotypes have experienced differential growth performance during the last 400 yr, coinciding with changing climatic conditions from the Little Ice Age to Present. During the Little Ice Age, vertical stem growth was restricted to a maximum height of 2.57 m on average, which was 1.8 times lower than current stem height during the 1900s. The smaller dead stems had the potential to grow for a longer period of time and at a faster rate above the snow cover similar to current growth of living stems from the same genet. Their lower performance may be attributed to environmental constraints causing greater needle loss above the snow cover due to more severe winter conditions before the 20th century. The growth of taller and larger tree stems during the 20th century may be attributed to increased temperature as reported across the Northern Hemisphere.
FIGURE 2. Lifespan of (a) the 30 dead stems and (b) the 30 living stems. Thicker lines correspond to lifespan of the upper part of stems (above snow cover)
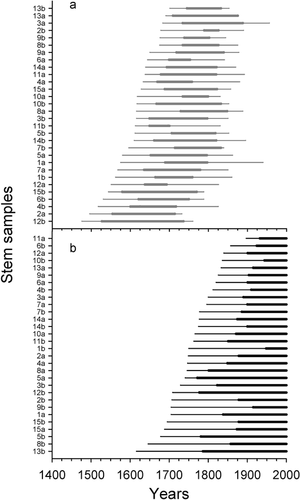
FIGURE 3. Age-height relationships of living stems (black dots; y = 5.42 − 0.0032x) and dead stems (open dots; y = 2.02 + 0.0021x)
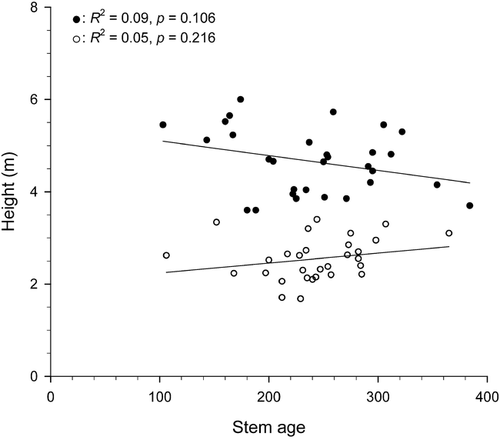
FIGURE 4. Mean years of initial development of the 30 living stems (black squares) and the 30 dead stems (black dots) at each sampled level. Shaded areas correspond to the confidence interval (95%) for each curve. Horizontal lines a and b correspond to inferred snowpack line for dead and living stems, respectively. Line c corresponds to beginning of height growth reduction. Extreme values of the mean height growth curves for living (dash lines) and dead (dotted lines) stems show the range of height growth
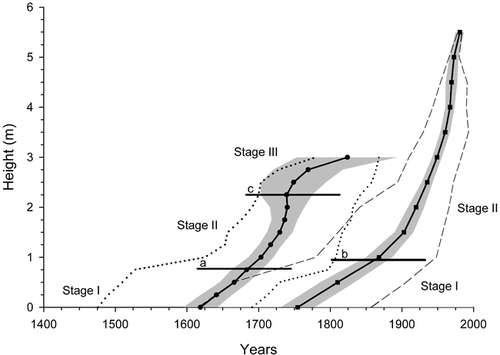
FIGURE 5. (a) Mean duration (+ SE), (b) mean height growth rate (+ SE) and (c) mean ring width (+ SE) of each growth stage (I, II, III) of the 30 living (white bars) and 30 dead (black bars) stems. (*) Significant difference (P < 0.001)
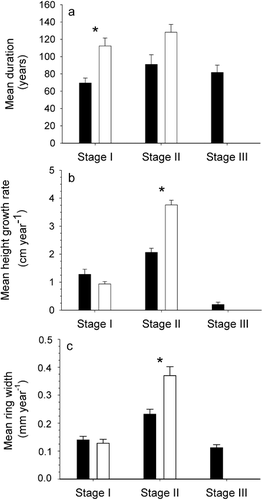
FIGURE 6. Tree-ring chronologies of dead (gray line) and living (black line) stems. Mean duration (± extreme values) of stage II is shown by gray line (± gray dotted line) for dead stems and black line (± black dotted line) for living stems. The number of stems used to construct the two chronologies is shown below the two curves
Acknowledgments
This study has been financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), the Ministère de la Recherche, de la Science et de la Technologie du Québec (FCAR program), and the Canadian Department of Indian and Northern Affairs. We thank M. Beauchemin, A. Delwaide, and S. Champagne for field and laboratory assistance. We are grateful to Peter M. Brown and anonymous referees for useful and constructive comments.
References Cited
- Barber, V. A. , G. P. Juday , and B. P. Finney . 2000. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 405:688–673.
- Bégin, C. 1991. Analyse architecturale et dendroécologique d'une pessière à lichens à la limite des forêts. Ph.D. thesis, Université Laval, Québec, Canada. 159 pp.
- Bégin, C. and L. Filion . 1999. Black spruce ( Picea mariana ) architecture. Canadian Journal of Botany 77:664–672.
- Bradley, R. S. 1988. The explosive volcanic eruption signal in northern hemisphere continental temperature records. Climatic Change 12:221–243.
- Crowley, T. J. 2000. Causes of climate change over the past 1000 years. Science 289:270–277.
- Enright, N. J. 1984. Principal components analysis of tree-ring/climate relationships in white spruce ( Picea glauca ) from Schefferville, Canada. Journal of Biogeography 11:353–361.
- Environment Canada, 1993. Canadian Climate Normals, Québec, 1961–1990, Canada. Atmospherique Environment Service, Environment Canada, Ottawa, Ontario, Canada.
- Esper, J. , E. R. Cook , and F. H. Schweingruber . 2002. Low-frequency signals in long tree-ring chronologies for reconstructing past temperature variability. Science 295:2250–2253.
- Filion, L. , S. Payette , and L. Gauthier . 1985. Analyse dendroclimatique d'un krummholz à la limite des arbres, Lac Bush, Québec nordique. Géographie Physique et Quaternaire 39:221–226.
- Filion, L. , S. Payette , L. Gauthier , and Y. Boutin . 1986. Light rings in subarctic conifers as a dendrochronological tool. Quaternary Research 26:272–279.
- Folland, C. K. , T. R. Karl , and K. Y A. Vinnikov . 1990. Observed climate variations and change. In Houghton, J. T., Jenkins, G. J., and Ephraums, J. J. (eds.), Climate Change. The IPCC (Intergovernmental Panel on Climate Change) Scientific Assessment. Cambridge: Cambridge University Press, 195–238.
- Fritts, H. C. 1976. Tree Rings and Climate. New York: Academic Press. 567 pp.
- Garfinkel, H. L. and L. B. Brubaker . 1980. Modern climate-tree-growth relationships and climatic reconstruction in sub-arctic Alaska. Nature 286:872–874.
- Gervais, B. R. and G. M. MacDonald . 2000. A 403-year record of July temperatures and treeline dynamics of Pinus sylvestris from the Kola Peninsula, northwest Russia. Arctic and Alpine Research 32:295–302.
- Grove, J. M. 1988. The Little Ice Age. London: Methuen. 498 pp.
- Hadley, J. L. and W. K. Smith . 1986. Wind effects on needles of timberline conifers: seasonal influence on mortality. Ecology 67:12–19.
- Hofgaard, A. and B. Wilmann . 2002. Plant distribution pattern across the forest-tundra ecotone: The importance of treeline position. Ecoscience 9:375–385.
- Holmes, R. L. 1983. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bulletin 44:69–75.
- Houghton, J. T. , Y. Ding , D. J. Griggs , M. Noguer , P. J. van der Linden , X. Dai , K. Maskell , and C. A. Johnson . 2001. Climate Change 2001: The Scientific Basis: The IPCC (Intergovernmental Panel on Climate Change) Scientific Assessment. Cambridge: Cambridge University Press. 892 pp.
- Jones, P. D. , T. M L. Wigley , and P. M. Kelly . 1982. Variations in surface air temperature. Part 1. Northern Hemisphere 1881–1980. Monthly Weather Review 110:59–70.
- Kullman, L. 1986a. Late Holocene reproductional patterns of Pinus sylvestris and Picea abies at the forest limit in central Sweden. Canadian Journal of Botany 64:1682–1690.
- Kullman, L. 1986b. Recent tree-limit history of Picea abies in the southern Swedish Scandes. Canadian Journal of Forest Research 16:761–771.
- Kullman, L. 1987. Little Ice Age decline of a cold marginal Pinus sylvestris forest in the Swedish Scandes. New Phytologist 106:567–584.
- Kullman, L. 1998. Tree-limits and montane forests in the Swedish Scandes: sensitive biomonitors of climate change and variability. Ambio 27:312–321.
- Laberge, M-J. , S. Payette , and J. Bousquet . 2000. Life span and biomass allocation of stunted black spruce clones in the subarctic environment. Journal of Ecology 88:584–593.
- Laberge, M-J. , S. Payette , and N. Pitre . 2001. Development of stunted black spruce ( Picea mariana ) clones in the subarctic environment: a dendro-architectural analysis. Ecoscience 8:489–498.
- LaMarche, V. C. 1974. Paleoclimatic inferences from long tree-ring records. Science 183:1043–1048.
- Lamb, H. H. 1977. Climate, Present, Past and Future. Volume 2, Climatic History and the Future. London: Methuen. 835 pp.
- Lavoie, C. and S. Payette . 1992. Black spruce growth forms as a record of a changing winter environment at treeline, Quebec, Canada. Arctic and Alpine Research 24:40–49.
- Lloyd, A. H. and C. L. Fastie . 2002. Spatial and temporal variability in tree growth and climate response of treeline trees in Alaska. Climatic Change 52:481–509.
- Lloyd, A. H. and C. L. Fastie . 2003. Recent changes in treeline forest distribution and structure in interior Alaska. Ecoscience 10:176–185.
- MacDonald, G. M. , R. A. Case , and J. M. Szeicz . 1998. A 538-year record of climate and treeline dynamics from the lower Lena river region of northern Siberia, Russia. Arctic and Alpine Research 30:334–339.
- Mann, M. E. and R. S. Bradley . 1999. Northern hemisphere temperatures during the past millennium: inferences, uncertainties, and limitations. Geophysical Research Letters 26:759–762.
- Overpeck, J. , K. Hughen , D. Hardy , R. Bradley , R. Case , M. Douglas , B. Finney , K. Gajewski , G. Jacoby , A. Jennings , S. Lamoureux , A. Lasca , G. MacDonald , J. Moore , M. Retelle , S. Smith , A. Wolfe , and G. Zielinski . 1997. Arctic environmental change of the last four centuries. Science 278:1251–1256.
- Parmesan, C. and G. Yohe . 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42.
- Payette, S. 1983. The forest tundra and present tree-lines of the Northern Québec-Labrador Peninsula. In Morisset, P. and Payette, S. (eds.), Proceeding of the Northern Québec Tree-line Conference. Nordicana, Québec, 3–23.
- Payette, S. , A. Delwaide , C. Morneau , and C. Lavoie . 1996. Patterns of tree stem decline along a snow-drift gradient at treeline: a case study using stem analysis. Canadian Journal of Botany 74:1671–1683.
- Payette, S. , L. Filion , A. Delwaide , and C. Bégin . 1989. Reconstruction of tree-line vegetation response to long-term climate change. Nature 341:429–432.
- Payette, S. , M-J. Fortin , and I. Gamache . 2001. The subarctic forest-tundra: the anatomy of a biome in a changing climate. BioScience 51:709–719.
- Payette, S. and C. Morneau . 1993. Holocene relict woodlands at the eastern Canadian treeline. Quaternary Research 39:84–89.
- Pereg, D. and S. Payette . 1998. Development of black spruce growth forms at treeline. Plant Ecology 138:137–147.
- Ritchie, J. C. 1987. Postglacial Vegetation of Canada. Cambridge: Cambridge University Press. 178 pp.
- Robichaud, E. and I. R. Methven . 1991. Tree vigor and height growth in black spruce. Trees 5:158–163.
- Rowe, J. S. 1972. Les régions forestières du Canada. Ottawa, Canada: Publication no. 1300, Canadian Forest Service. 172 pp.
- Scott, P. A. , C. V. Bentley , D. C F. Fayle , and R. I C. Hansell . 1987. Crown forms and shoot elongation of white spruce at the treeline, Churchill, Manitoba, Canada. Arctic 46:316–323.
- Sturm, M. , C. Racine , and K. Tape . 2001. Increasing shrub abundance in the Arctic. Nature 411:546.
- Suarez, F. , D. Binkley , M. W. Kaye , and R. Stottlemyer . 1999. Expansion of forest stands into tundra in the Noatak National Preserve, northwest Alaska. Ecoscience 6:465–470.
- Sveinbjörnsson, B. 2000. North American and European treelines: External forces and internal processes controlling position. Ambio 29:388–395.
- Szeicz, J. M. and G. M. MacDonald . 1995. Recent white spruce dynamics at the subarctic alpine treeline of north-western Canada. Journal of Ecology 83:873–885.
- Tett, S. F B. , P. A. Stott , M. R. Allen , W. J. Ingram , and J. F B. Mitchell . 1999. Causes of twentieth-century temperature change near the earth's surface. Nature 399:569–572.
- Walther, G-R. , E. Post , P. Convey , A. Menzel , C. Parmesan , T. J C. Beebee , J-M. Fromentin , O. Hoegh-Guldberg , and F. Bairlein . 2002. Ecological responses to recent climate change. Nature 416:389–395.
- Wang, K. and T. J. Lewis . 1992. Geothermal evidence from Canada for a cold period before recent climatic warming. Science 256:1003–1005.