Abstract
On 11 June 2008 the first author spent 10.66 h on Kasatochi Island and collected 396 terrestrial arthropod specimens estimated to represent a minimum of 58 species. Among these are included the first Alaskan records of the fly genus Lestremia and the ghost moth Sthenopis quadriguttatus (Grote). Also found were a new species of salpingid beetle in the genus Aegialites and sawfly in the genus Pseudodineura. On 10 and 12 August 2009, one year after an eruption that buried the island in ash, the first author spent 15 h sampling terrestrial arthropods. Specimens were also collected on 12–14 June 2009 by other team members. An estimated 17 post-eruption species were documented by the collection of 210 specimens. Evidence of breeding was seen in 4–9 species. Pitfall traps run from 14 June to 10 August 2009 flooded, capturing no arthropods. Fallout collectors representing 1 m2, run during the same period, had four flies and no seeds. The majority of species recovered post-eruption were probably survivors or their offspring, some of which had commenced breeding on rotting kelp and bird carcasses. Of significance as the first post-eruption evidence of multi-trophic level interaction, a fly predator on kelp flies, Scathophaga, and an ichneumonid endoparasite of flies, Phygadeuon, were also present. No phytophagous or fungivorous species were found. Supporting the heterotrophs-first hypothesis of Hodkinson et al. (2002), the current terrestrial ecosystem of Kasatochi is necromass-based rather than plant-based.
Introduction
Opportunities to document the natural growth of an ecosystem from a baseline at or near zero are rare (CitationThornton and New, 2007). Volcanic eruptions allow such successional ecological studies, with the eruptions of Mount St. Helens, Surtsey, and Krakatau being among the better known examples (CitationLindroth et al., 1973; CitationThornton, 1996; CitationDale et al., 2005). These examinations provide rare opportunities to test successional hypotheses that attempt to explain the mechanistic processes of ecosystem assembly (CitationConnell, 1978; CitationMacMahon, 1981). With particular reference to the Aleutian Island chain and the biogeography of Beringia, the eruption of Kasatochi Island may allow a quantification of dispersal rates relevant to CitationLindroth's (1963) work on ground beetles.
The Alaskan arthropod fauna is among the most poorly known of the U.S. states, and most of the Aleutian Islands have never had their arthropod fauna surveyed. In collaboration with the U.S. Fish and Wildlife Service (USFWS) the first author began a large-scale survey of these islands in 2008 that has since yielded data for over 20 islands, including Kasatochi.
Kasatochi is a small stratovolcano among the Andreanof group of the central Aleutian Islands (ca. 52.17°N, 175.51°W). Prior to August 2008 it was 2.6 km east to west, 2.9 km northwest to southeast, totaling about 500 ha and about 314 m in elevation. The center of the island was a caldera with a small lake about 800 m in diameter. The nearest large islands are Atka, 19 km to the southeast, and Great Sitkin, 35.4 km to the southwest. A number of smaller islands (Oglodak, Tagalak, Chugul) lie 19–29 km to the south or southwest. Average maximum daily temperatures for Adak, approximately 72.4 km to the southwest, for May through September 1971–2000 are 7.2, 9.7, 12.1, 12.8, and 11.3 °C (CitationWRCC, 2009). Kasatochi provided breeding habitat for a variety of bird species, including one of the largest auklet (Aethia spp.) colonies in the Aleutians, in addition to a Stellar sea lion (Eumetopias jubatus) rookery, and a small population of harbor seals (Phoca vitulina) (CitationWilliams et al., 2010 [this issue]).
On 7 and 8 August 2008 the island erupted catastrophically, sending a sulfur dioxide cloud over 10,000 m into the air that drifted to the southwest of the island. Most of the island was buried in up to 30 m of ash and a hot (up to 800 °C) pyroclastic flow (CitationMartinsson et al., 2009; CitationDeGange et al., 2010 [this issue]; Waythomas, personal communication). These eruptions extended the shoreline about 400 m, buried the intertidal with ash, and were assumed to have extirpated the terrestrial and nearshore biota. After the eruption interdisciplinary teams were assembled by the USFWS, the U.S. Geological Survey, and the University of Alaska, which included volcanologists, geologists, botanists, ornithologists, soil scientists, marine ecologists, and an entomologist. Four visits to the island were made during 2009 to document post-eruption baseline conditions from which long-term comparisons will be made.
The objective of this study is to document, describe, and analyze the pre- and post-eruption terrestrial arthropod fauna of Kasatochi Island to establish a baseline for future study of the assembly of the island's terrestrial ecosystem. Additionally, we wished to test the hypothesis, based on findings from work done on Krakatau (CitationDammerman, 1948), Mount St. Helens (CitationEdwards and Sugg, 2005), Surtsey (CitationThornton and New, 2007), and a review of many such studies worldwide by CitationHodkinson et al. (2002), that arthropod scavengers, predators, and parasitoids would constitute the primary successional stage of Kasatochi.
Materials and Methods
On 11 June 2008 the first author spent 10.66 h on Kasatochi Island collecting terrestrial arthropods by hand and sweepnet. Nine sites were sampled along a transect running from the westernmost point of the island at sea level to the rim of the caldera at 290 m elevation (). Hand collecting using forceps and aspirator focused on organisms beneath rocks and driftwood but also captured crawling arthropods on trails and vegetation and yielded 208 specimens. Sweeping vegetation with an insect net captured many flies, wasps, and spiders and yielded 189 specimens. All specimens were deposited in the University of Alaska Museum Insect Collection.
Figure 1 Map of pre-eruption terrestrial arthropod sampling sites, 11 June 2008. Each site was georeferenced by the first author using a Garmin GPS following the protocol of CitationChapman and Wieczorek (2006). All Kasatochi specimen records available in the University of Alaska Museum Insect Collection catalog, via Arctos (http://arctos.database.museum), are coded with these geocoordinates and microhabitat information.
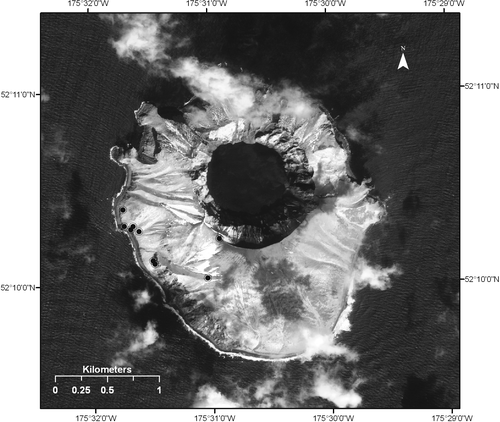
Habitat diversity is low in the Aleutians in general, and Kasatochi Island is no exception. Important microhabitats for terrestrial arthropods in the Aleutians include beach detritus (rotting kelp, driftwood, carcasses), freshwater streams, rocks with bird guano, rocks with plant roots, littoral boulders with cracks, vertebrate nests, and a variety of plant communities (mostly grasses and forbs) including flowers associated with pollinators. The transect sampled on 11 June 2008 lacked freshwater streams, littoral boulders with cracks, vertebrate nests and carcasses; the remaining microhabitats were sampled. See CitationTalbot et al. (2010 [this issue]) for description of the island's flora.
On 14 June 2009 Jeff Williams, a biologist with the USFWS, laid two transects on the west (windward) coast of Kasatochi: one of 10 pitfall traps to catch crawling arthropods and the other of 10 fallout collectors to sample organic matter falling from the sky. The pitfall traps were plastic containers with rainroof covers, 11 cm in diameter, 12.5 cm in depth, 0.95 L in volume, and were spaced about 5 m apart on a transect running uphill at ca. 97° southeast between 52.17152°N, 175.52899°W, 37m elevation, and 52.17147°N, 175.52827°W, 42m elevation. Each was filled one fourth with 100% propylene glycol as a preservative.
The fallout collectors were not traps—they were designed so crawling arthropods could exit the collectors. The walls of the collectors were 3.81 cm wood pieces that formed a frame () inside of which were placed tightly packed golf balls to create dead space allowing arthropods and organic matter to accumulate. There were 10 collectors, each 0.1 m2 in area representing a total of 1 m2 of ground. These fallout collectors were replicas of those used by Edwards and others in their ecological studies of Mount St. Helens (CitationEdwards and Sugg, 2005). The golf balls were painted to match the color of the ash (to avoid attracting arthropods due to the white color) and held off the ground by a piece of tightly stretched mosquito netting that prevented arthropods from escaping or dissolving into the ash below. Whereas traps concentrate organisms over time, these collectors were designed to estimate the amount of organic matter input into 1 m2 of barren ground. The fallout collectors were placed on a straight transect at about 10 m intervals running uphill at ca. 135° southeast from 52.17262°N, 175.53085°W, 12 m elevation, to 52.17196°N, 175.52975°W, 18 m elevation (site D2; ). Pitfall traps and fallout collectors were run continuously from 14 June to 10 August 2009.
Figure 2 Aerial plankton/arthropod biomass fallout collectors built to specifications of CitationEdwards (1986) and CitationEdwards and Sugg (2005).

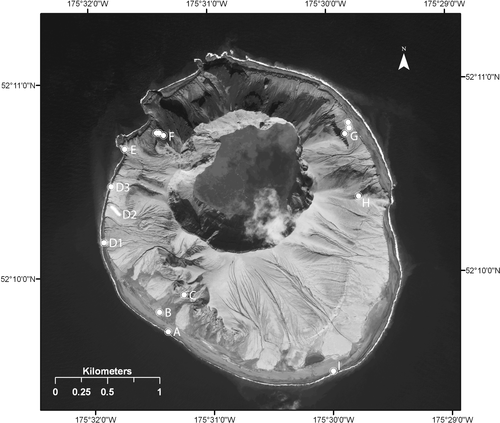
Figure 3 Map of sites post-eruption 12–14 June and 10, 12 August 2009. Sites are coded A–I as referenced in and the text.
Specimens were loaned to specialists for verification of identifications () when possible, and many are still in process. Identifications done in-house by Sikes, Slowik, and Fleshman employed the following literature (CitationCrosby and Bishop, 1928; CitationLevi, 1957; CitationHolm, 1960; CitationMillidge, 1976; CitationMillidge, 1983; CitationAnderson and Peck, 1985; CitationHuckett, 1987; CitationMarshall and Richards, 1987; CitationRoberts, 1987; CitationVockeroth, 1987; CitationDondale and Redner, 1990; CitationGoulet and Huber, 1993; CitationMiller, 1999; CitationPeck, 2001; CitationPaquin and Dupérré, 2003; CitationWhitworth, 2006). All specimens will be digitally cataloged into the University of Alaska Museum database, Arctos, which is searchable online at http://arctos.database.museum. As identifications are completed, the database will be updated so the final identifications for all specimens referenced herein will be available online in Arctos for perpetuity (found by searching using the locality name Kasatochi).
Table 1 Pre-eruption taxa based on 396 specimens collected on 11 June 2008 (minus Ixodes) estimated to represent at least 58 species. Microhabitat and geocoordinate data for all specimens below, excepting the Ixodes, will be available in the University of Alaska Museum Insect Collection's database via Arctos (http://arctos.database.museum).
Results
PRE-ERUPTION
The first author collected 396 terrestrial arthropods on 11 June 2008 on a transect from the westernmost beach to the rim of the caldera (). A total of 174 of these specimens have so far been identified to species constituting 33 species. The entire collection of 396 specimens represents an estimated minimum of 58 species.
A number of species in are commonly found on Aleutian islands. However, these results indicate how poorly documented the arthropods of these islands are, and Alaska in general is, that a number of these species are apparent new records for Alaska. The hepialid Sthenopis quadriguttatus is a large moth with a subterranean larva that feeds on roots of Populus and Salix (CitationGross and Syme, 1981). The species is widespread in the boreal forest of North America but has not been reported previously from Alaska. The Kasatochi specimen is a larva taken from under a stone on a ridge at 230 m elevation.
The thrips Apterothrips secticornis is a wingless species, collected from flowers of Lupinus nootkatensis Donn ex Sims, and apparently unrecorded from the Aleutians but known from other mainland Alaskan records (CitationNakahara, 1988; D. Nickle, in litt.). Thrips are very poorly documented in Alaska and we know of only one other, unpublished, record of this order from one other Aleutian island (Amatignak).
The sawfly Pseudodineura is a genus unreported from western North America. There are some Eurasian species and the Kasatochi specimen belongs to an undescribed species most closely related to a group of these (CitationSmith et al., submitted).
The fly genus Lestremia is also a new record for Alaska; however, Lestremia is a common and widespread Holarctic genus so this is not an unusual discovery.
The salpingid beetles, genus Aegialites, belong to an undescribed species as yet known from no other island (V. Gusarov, personal communication). The first author sampled Aegialites on the nearby islands Great Sitkin and Atka, that may turn out to be conspecific. No Aegialites, nor any suitable habitat, was found on Kasatochi in 2009.
Among the spiders Erigone aletris, Aphileta misera, and Walckenaeria spiralis appear to be new records for the Aleutians with the first two species' closest records being Kodiak Island (1450 km distant) and the last species' closest record being Chichagof Island in southeast Alaska (2560 km distant). The remaining spiders in were all reported from Aleutian islands by CitationHolm (1960). Eulaira aletris is a species so far known only from coastal Alaskan records from Attu to southeastern Alaska, and thus is endemic to the state.
POST-ERUPTION
On 10 and 12 August 2009 the first author spent a total of 15 h sampling terrestrial arthropods with help from other team members. Specimens were also collected on 12–14 June 2009 by other team members. A total of 210 post-eruption specimens was collected representing an estimated 17 species (), six of which were found in a single auklet burrow by Jeff Williams.
Table 2 Post-eruption taxa based on 210 specimens estimated to represent 17 species. Sites refer to those indicated on .
Standardized species sampled per unit effort can be used to compare the pre- and post-eruption arthropod species richness of Kasatochi. The first author spent 10.66 h in 2008 which yielded an estimated 58 species (5.4 species h−1) while 15 h in 2009 yielded 9 species, excluding species collected by others (0.6 species h−1), a ninefold difference. If the hours spent and total species obtained by the first author and Jeff Williams are combined (30 h, 14 species), the catch rate is smaller (0.47 species h−1), suggesting a plateau in species accumulated per unit effort was already being reached.
Generalizations can be made about the geographic patterns seen in the taxa recovered post-eruption (). The rotting wrack associated flies (two scathophagid species, one anthomyiid, one sphaerocerid) were all found on the western and southwestern beaches (sites A, C, D3, E; ) where wrack was abundant (). The wrack was dragon kelp, Eularia (Alaria) fistulosa (Postels and Ruprecht), that was presumably the dominant canopy kelp around Kasatochi prior to the eruption (CitationJewett et al., 2010 [this issue]). Wrack was rare to absent along most of the southern, eastern, and northeastern coasts. The coelopid, Coelopa, and sphaerocerid, Thoracochaeta, are known kelp-associated saprophagous flies (CitationThompson and Epler, 2009) and the abundant Scathophaga are known predators of flies in rotting kelp (CitationMarshall and Richards, 1987; CitationVockeroth, 1987; CitationOvchinnikov, 2009). Their abundance indicated they were successfully breeding and a single fly larva was found under wrack at site I (). The unidentified fly pupal exuvia, matching the sphaerocerids in size, found at site A () were attached to the underside of a large piece of driftwood.
Figure 4 Many scathophagid and sphaerocerid flies were on, under, and near wrack of Eularia (Alaria) fistulosa which was abundant on the west coast of Kasatochi (; ; site D3).

The wingless carrion beetle, Lyrosoma opacum, found on bird carcasses in June, in cracks in a cliff face in August, and in great numbers on a single bird carcass in August, were all recovered on the west coast (site G; ) where an extensive cliff face provided some protection from the eruption. This cliff face also contained a spider (Linyphiidae indet.), a centipede, and a dead carabid beetle, in addition to a few surviving plants (CitationTalbot et al., 2010 [this issue]). One Lyrosoma opacum was found under kelp on the south coast (site I; ), a considerable distance from site G. The bird carcass on the beach at site G had over 60 adult Lyrosoma, many of which were teneral, indicating they had recently eclosed from pupae. Only 16 of these were taken as voucher specimens, some for molecular work, leaving the majority alive.
By far the largest single sample of species was recovered from a pre-eruption auklet burrow by Jeff Williams (site F; ) at about 150 m elevation. This burrow had both obligate nest associates (Ixodes ticks and Catops beetles) and other species apparently using the burrow as shelter (Phygadeuon sp. 2 and alyssine braconid wasps, tipulid fly, cybaeid spider). The alyssine braconid wasps and the Phygadeuon wasps are known endoparasites of flies (CitationGoulet and Huber, 1993) and some of the latter wasps parasitize kelp wrack associated flies (A. Bennett, in litt.) while others are known parasites of blow flies (CitationMcClure, 1943).
Beyond these three pockets of arthropod diversity, singletons of highly mobile calliphorid blow fly species were found at sites B and H (). This puts the majority of arthropod diversity on the west and east coasts with very little found in the large ash flow along the south coast. The northwest coast was not sampled but has a number of rock outcroppings and a Stellar sea lion rookery that was active in 2009. Examination of one of the rock outcroppings (site E; ) failed to find any suitable microhabitats for arthropods. All cracks were filled with ash mud. The only evidence of arthropod life were the remains of dead barnacles.
Of the 210 post-eruption specimens, 50 were found dead. Of particular note are 46 flies (45 Scathophaga sp. and 1 Scathophagidae indet.) that were found dead but undamaged in a tight group in a crevice on pre-eruption rocks at 52.17736°N, 175.52910°W (site E; ). A lack of decomposition indicated these flies had died recently. The other four specimens found dead, two scathophagid flies in the fallout collectors, one carabid beetle in a cliff-face crack, and one moth on the beach, were badly decomposed.
The transect of pitfall traps had no arthropods. Despite rainroof covers, the traps had suffered flooding during the two months they were active. The surface of Kasatochi is rapidly eroding and much is either sloping, barren fields of ash, or gullies cut into this surface, making placement of pitfall traps difficult.
The fallout collectors also suffered from flooding and some were partially filled due to mud flow. Some had also been attacked by gulls, which had removed some of the golf balls. Two dead and decomposing flies and two live flies were recovered from these collectors. No seeds were found. This equates to an input of 6.6 × 10−2 flies m−2 day−1 and given that the island was 685.3 hectares on 13 September 2009 (Scott et al. [this issue]) the entire island would have a fallout input of 452,298 flies day−1 during the summer months (assuming an unrealistically uniform distribution of flies).
Discussion
Based on more extensive pitfall and Malaise trap sampling done on other islands during 2008 and 2009, the total of 58 pre-eruption species is probably less than half the full pre-eruption terrestrial arthropod fauna. Most certainly present pre-eruption but unsampled were soil mites, bristletails, and springtails among many additional fly, wasp, and beetle species. In contrast, the post-eruption sampling was more intense (more people, total hours, traps, etc.) and the number of microhabitats for arthropods was greatly reduced so the 17 species estimated is probably relatively close to the actual number making the current fauna about 15% or less of the size of the original. However, of these 17 species only four to possibly nine were found in sufficient numbers to suggest they were breeding. The ixodid ticks and leiodid beetles are dependent on the nesting sea birds that failed to nest successfully in 2009 so these species may have difficulty persisting over the next few years. With the exception of Scathophaga, which are known predators on kelp flies, and Phygadeuon, which are known parasitoids of kelp and carrion flies among others, the remaining predators, two spider species and one centipede, were singletons. The species that seemed to be reproducing were all decomposers feeding on bird carcasses (agyrtid beetles, calliphorid flies) or rotting kelp (remaining flies) or predators or parasitoids on these decomposers (scathophagids, ichneumonids). No plant-dependent arthropod taxa were found post-eruption. Interestingly, the pre-eruption fauna included two parasitoids (Pimpla and Ichneumon) of Lepidoptera (A. Bennet, in litt.). No living phytophagous insects nor their parasitoids were found post-eruption.
The cluster of dead scathophagid flies lacking evidence of decomposition suggests they were poisoned or gassed. Starvation would probably not have killed such a large group simultaneously. Jeff Williams found two dead adult fork-tailed stormpetrels on the southwest point and thinks they succumbed to gas based on their condition and proximity to several small heat vents. The island continues to degas sulfur (SO2/H2S) and probably carbon dioxide.
SURVIVORS VERSUS IMMIGRANTS
There are three means of dispersal that would bring immigrants to Kasatochi: air, oceanic drift, and vertebrate transport. The Aleutian islands show ample evidence of all three modes with vertebrate transport being the least obvious and probably the least important. Wingless beetles, pseudoscorpions, bristletails, springtails, mites, opilionids, and centipedes are common especially along beaches and these animals are most likely dispersed via drift. Spiders, wasps, moths, winged beetles, true bugs, and flies form the majority of island species and are most likely dispersed via air.
Although not conclusive, the evidence suggests most or all of the 2009 arthropods were survivors or their descendants. Given the 2008 sampling was incomplete, the presence of species post-eruption absent from the 2008 list should not be taken as evidence of immigration. Wingless species such as the centipede, carrion beetle, ticks, and spiders are the least likely to be immigrants, although the spiders are more capable of airborne dispersal (via ballooning) than the others. The ticks rely on their host seabirds for dispersal. The carrion beetles, centipedes, and one of the spiders were found in protected cracks and crevices of a large cliff face that also bore surviving plants, so these animals are almost certainly survivors. The auklet nest arthropods included two wasp species in multiple numbers whose degree of association with birds nests is currently unknown. Their presence, along with the tipulid fly, in the burrow cannot be explained beyond the burrow providing shelter from harsh external conditions. The species most likely to be immigrants are the kelp and blow flies, which are strong fliers; however, these species could also be survivors. Future examination of genetic markers between 2009 and 2008 samples and inter-island comparisons should provide additional evidence. Approximately 19 plant species apparently survived the eruption, most as root masses in protected areas (CitationTalbot et al., 2010 [this issue]).
Current conditions influencing the assembly of Kasatochi's ecosystem may differ significantly from historical. It is uncertain how old the pre-eruption ecosystem was, or how old the island itself is. Prior eruptions of Kasatochi are not known with certainty. Some evidence suggests there may have been multiple eruptions since the 1700s (CitationCoats, 1950), and prior to this date stretching back to Pleistocene times when the Bering land bridge was present, there may have been multiple eruptions, or none. Thus, it is uncertain if any of the pre-2009 arthropod fauna may have dispersed to Kasatochi during periods of lower sea level when ground travel between adjacent islands was possible. Additionally, the island's ecosystem was not pristine, having had a population of introduced foxes from 1927 to 1985 (J. Williams, personal communication).
COMPARISON WITH SURTSEY
Surtsey, being a northern island (63°N), has great relevance to Kasatochi. Unlike Kasatochi, Surtsey lacked a pre-eruption island, having erupted from the ocean between 1963 and 1964. By 1967 Surtsey had 63 terrestrial arthropod species (CitationLindroth et al., 1973) although the vast majority were thought to be temporary inhabitants with true breeding populations composed of shore flies, particularly the heleomyzid Leria modesta Meigen ( = Heleomyza modesta). By 1975 only three insect species were thought to have colonized Surtsey: a fly, a midge, and a collembolan, all associated with littoral carcasses (CitationThornton and New, 2007). The nearest islands to Surtsey are 14.5–19 km to the northeast with the mainland of Iceland only 32 km to the northeast. Kasatochi is relatively more isolated than Surtsey and it will be valuable to compare the gradual ecological development of Kasatochi with the now 46-year record of Surtsey.
AERIAL PLANKTON AND HETEROTROPHS AS PRIMARY SUCCESSIONAL PLAYERS
There is extensive evidence that a large biomass of arthropods is airborne during the summer months. For example, CitationHeydemann (1967) estimated 4.5 billion insects per summer day disperse over the North Sea from a 30-km-wide coastal strip. The ecological importance of this phenomenon was investigated repeatedly by Edwards (e.g. CitationEdwards and Banko, 1976; CitationEdwards, 1986) and others (e.g. CitationAshmole and Ashmole, 1988) who focused on the fallout of aerial arthropod plankton in unfavorable, nutrient-poor habitats such as alpine zone snow fields and volcanic ash fields. The quantity, energetic and nutrient inputs from the arthropod fallout has been shown to contribute significantly to these barren ecosystems, providing a resource base that sustains numerous other species. Edwards estimated fallout inputs of approximately 1 kg ha−1 per season of dry weight arthropod biomass. Contrary to the classical concepts of primary succession (e.g. CitationClements, 1916) that emphasize plants as pioneers with herbivores following and predators, parasites, and decomposers arriving later, work on aerial plankton fallout after the eruption of Mount St. Helens demonstrated the reverse pattern held (CitationEdwards, 1986; CitationEdwards and Sugg, 2005). Edwards found the initial colonizers were predators and scavengers surviving on arthropod fallout that formed the basis of these new food webs before plants established and herbivores began feeding.
Our 2009 work on Kasatochi was designed to quantify the arthropod fallout using the same methods as at Mount St. Helens (CitationEdwards, 1986; CitationEdwards and Sugg, 2005). CitationCrawford et al. (1995) reported during a 125-day field season in 1985 an average rate of spider arrival on the Pumice Plain of Mount St. Helens of 0.84 spiders m−2 day−1. Spiders made up about 23% of the arthropods sampled (CitationEdwards and Sugg, 2005), putting the total arthropod input above 3.65 animals m−2 day−1. This rate is 55 times greater than the rate of arthropod input onto Kasatochi, reflecting the greater isolation of Kasatochi from source populations. The aerial fallout sampled on Kasatochi could be entirely from surviving and breeding populations rather than extra-island influx.
Perhaps due to the remote and isolated location of Kasatochi the arthropod fallout does not seem as significant an allochthonous input as it was in Mount St. Helens. Our results nevertheless agree with those of CitationEdwards (1986) in contradicting the classic view of succession based on plant colonists and emphasize the often overlooked importance of decomposers in early ecosystem assembly. CitationDammerman (1948) documented the same pattern—Krakatau's first successional sequence began with scavengers. Surtsey's terrestrial arthropod fauna, even after 12 years, was composed of only three species, all associated with littoral carcasses (CitationThornton and New, 2007). The value of decomposing wrack as an energy and nutrient source for terrestrials systems was recently studied by CitationOrr et al. (2005) in British Columbia, who estimated depositions of up to 140 Mg (dry mass) km−1 daily, during summer months. CitationHodkinson et al. (2002) reviewed literature on primary succession which led them to propose a general rule that a largely unrecognized heterotrophic phase precedes community assembly by autotrophs. In many cases this phase facilitates autotroph establishment via fertilization of soils. Some plants did survive the eruption on Kasatochi (CitationTalbot et al., 2010 [this issue]) but there was no evidence of herbivory aside from one observation of a single uncaptured fly resting on the flower of a Ranunculus on 10 August 2009. Plants are currently not the basis of Kasatochi's fledgling terrestrial ecosystem. This role is held by the necromass of kelp and bird carcasses that appear to constitute a larger and more actively exploited energy source for consumers (and indirectly for predators and parasitoids) than the plants.
Acknowledgments
We thank those who provided determinations: A. Bennett (Ichneumonidae), D. Nickle (Thripidae), B. Fleshman (Araneae), M. Bowser (Opiliones), R. Davidson (Carabidae), C. W. O'Brien (Curculionidae), H. Douglas (Elateridae), V. Gusarov (Salpingidae), A. Davies (Staphylinidae), M. Thayer (Staphylinidae), A. Smetana (Staphylinidae), J. Klimaszewski (Staphylinidae), T. Whitworth (Calliphoridae), W. Mathis, C. F. Thompson, S. Peek (Diptera), J. Forteir (Braconidae), D. Smith and H. Goulet (Tenthredinidae), D. Wagner (Hepialidae), L. Bonato (Geophilomorpha), M. Zapparoli (Lithobiida), S. Jewett (algae), M. Yoder (Diapriidae). We also thank Vernon Byrd and Jeff Williams of the U.S. Fish and Wildlife Service for their help in obtaining funding and transportation aboard the USFWS's vessel the M/V Tiglax. Kelly May and Mary Wyatt, specimen preparators and volunteers in the University of Alaska Museum, are thanked for their efforts. A. DeGange is also thanked for his role in organizing research efforts, workshops, and this special issue devoted to Kasatochi. Additional support is acknowledged from Alaska EPSCoR NSF award #EPS-0701898, National Science Foundation BRC award # DBI-0847904, the state of Alaska, and the North Pacific Research Board (Project #923). We thank two anonymous reviewers for their helpful comments. This is contribution 256 of the North Pacific Research Board.
References Cited
- Anderson, R. S. and S. B. Peck . 1985. The carrion beetles of Canada and Alaska (Coleoptera: Silphidae and Agyrtidae). In. The Insects and Arachnids of Canada, Part 13. Ottawa Research Branch Agriculture Canada, Publication 1778. 121 pp.
- Ashmole, N. P. and M. J. Ashmole . 1988. Insect dispersal on Tenerife, Canary Islands: high altitude fallout and seaward drift. Arctic and Alpine Research 20 1:1–12.
- Chapman, A. D. and J. Wieczorek . (eds.). 2006. Guide to best practices for georeferencing. Copenhagen Global Biodiversity Information Facility. 90 pp.
- Clements, F. E. 1916. Plant succession. An analysis of the development of vegetation. Washington, D.C Carnegie Institution of Washington publication, Issue 242. 512 pp.
- Coats, R. R. 1950. Volcanic activity in the Aleutian Arc. U.S. Geological Survey Bulletin B-0974B:35–49.
- Connell, J. H. 1978. Diversity in tropical rain forests and coral reefs. Science 199:1302–1310.
- Crawford, R. L. , P. M. Sugg , and J. S. Edwards . 1995. Spider arrival and primary establishment on terrain depopulated by volcanic eruption at Mount St. Helens. American Midland Naturalist 133:60–75.
- Crosby, C. R. and S. C. Bishop . 1928. Revision of the spider genera Erigone, Eperigone , and Catabrithorax (Erigonidae). New York State Museum Bulletin No. 278:3–74.
- Dale, V. H. , F. J. Swanson , and C. M. Crisafulli . (eds.). 2005. Ecological Responses to the 1980 Eruption of Mount St. Helens. New York Springer. xx +. 342 pp.
- Dammerman, K. W. 1948. The fauna of Krakatau, 1883–1933. Verhandelingen Koniklijke Nederlansche Akademie van Wetenschappen, Afdeling Natuurkunde II 44:1–594.
- DeGange, A. R. , G. V. Byrd , L. R. Walker , and C. F. Waythomas . 2010. Introduction—The impacts of the 2008 eruption of Kasatochi Volcano on terrestrial and marine ecosystems in the Aleutian Islands, Alaska. Arctic, Antarctic, and Alpine Research 42:245–249.
- Dondale, C. D. and J. H. Redner . 1990. The wolf spiders, nurseryweb spiders and lynx spiders of Canada and Alaska (Araneae: Lycosidae, Pisauridae, Oxyopidae). In. The Insects and Arachnids of Canada: Part 17. Ottawa Agriculture Canada, Publication 1856. 383 pp.
- Edwards, J. S. 1986. Derelicts of dispersal: arthropod fallout on Pacific northwest volcanoes. In Danthanarayana, W. (ed.). Insect Flight: Dispersal and Migration. Berlin/Heidelberg/New York Springer. 196–203.
- Edwards, J. S. and P. C. Banko . 1976. Arthropod fallout and nutrient transport: a quantitative study of Alaskan snowpatches. Arctic and Alpine Research 8:237–245.
- Edwards, J. S. and P. M. Sugg . 2005. Arthropods as pioneers in the regeneration of life on the pyroclastic-flow deposits of Mount St. Helens. In Dale, V. H. , F. J. Swanson , and C. M. Crisafulli . (eds.). Ecological Responses to the 1980 Eruption of Mount St. Helens. New York Springer. 127–138.
- Goulet, H. and J. T. Huber . 1993. Hymenoptera of the World: an Identification Guide to Families. Ottawa Centre for Land and Biological Resources Research. 668 pp.
- Gross, H. L. and P. D. Syme . 1981. Damage to aspen regeneration in northern Ontario by the ghost moth, Sthenopis quadriguttatus . Canadian Forest Service Research Notes l 4:30–31.
- Heydemann, B. 1967. Der Überflug von Insekten über Nord-und Ostee nach Untersuchungen auf Feuerschiffen. Deutsche Entomologische Zeitschrift 14:185–215.
- Hodkinson, I. D. , N. R. Webb , and S. J. Coulson . 2002. Primary community assembly on land—The missing stages: why are the heterotrophic organisms always there first? Journal of Ecology 90:569–577.
- Holm, Å 1960. On a collection of spiders from Alaska. Zoologiska Bidrag från Uppsala 33:109–134.
- Huckett, H. C. 1987. Anthomyiidae. In McAlpine, J. F. , B. V. Peterson , G. E. Shewell , H. J. Teskey , J. R. Vockeroth , and D. M. Wood . (eds.). Manual of Nearctic Diptera 2 Agriculture Canada, Research Branch, Monograph,. 28:675–1332.
- Jewett, S. C. , J. L. Bodkin , H. Chenelot , G. G. Esslinger , and M. K. Hoberg . 2010. The nearshore benthic community of Kasatochi Island, one year after the 2008 volcanic eruption. Arctic, Antarctic, and Alpine Research 42:315–324.
- Levi, H. W. 1957. The spider genera Enoplognatha, Theridion, and Paidisca (Araneae: Theridiidae). Bulletin of the American Museum of Natural History 112 1:1–123.
- Lindroth, C. H. 1963. The Aleutian islands as a route for dispersal across the North Pacific. Pacific Basin Biogeography. A Symposium. Honolulu Bishop Museum Press. 121–131.
- Lindroth, C. H. , H. Andersson , H. Bödvarsson , and S. H. Richter . 1973. Surtsey, Iceland. The development of a new fauna, 1963–1970. Terrestrial invertebrates. Copenhagen Entomologica Scandinavica, Supplement 5.
- MacMahon, J. A. 1981. Successional processes: Comparisons among biomes with special reference to probable roles of and influences on animals. In West, D. C. , H. H. Shugart , and D. B. Botkin . (eds.). Forest Succession, Concepts and Application. New York Springer-Verlag. 277–304.
- Marshall, S. A. and O. W. Richards . 1987. Sphaeroceridae. In McAlpine, J. F. , B. V. Peterson , G. E. Shewell , H. J. Teskey , J. R. Vockeroth , and D. M. Wood . (eds.). Manual of Nearctic Diptera 2 Agriculture Canada, Research Branch, Monograph,. 28:675–1332.
- Martinsson, B. G. , C. A. M. Brenninkmeijer , S. A. Carn , M. Hermann , K-P. Heue , P. F. J. van Velthoven , and A. Zahn . 2009. Influence of the 2008 Kasatochi volcanic eruption on sulfurous and carbonaceous aerosol constituents in the lower stratosphere. Geophysical Research Letters 36:article L12813. doi:10.1029/2009GL038735.
- McClure, H. E. 1943. Aspection in the biotic communities of the Churchill Area, Manitoba. Ecological Monographs 13 1:1–35.
- Miller, J. A. 1999. Revision and cladistic analysis of the erigonine spider genus Sisicottus (Araneae, Linyphiidae, Erigoninae). Journal of Arachnology 27 3:553–603.
- Millidge, A. F. 1976. Re-examination of the erigonine spiders “ Micrargus herbigradus ” and “ Pocadicnemis pumila ” (Araneae: Linyphiidae). Bulletin of the British Arachnological Society 3 6:145–155.
- Millidge, A. F. 1983. The erigonine spiders of North America. Part 6. The genus Walckenaeria Blackwall (Araneae, Linyphiidae). Journal of Arachnology 11 3:105–200.
- Nakahara, S. 1988. A new synonym and revised status in Apterothrips (Thysanoptera: Thripidae). Proceedings of the Entomological Society of Washington 90 4:508–509.
- Orr, M. , M. Zimmer , D. E. Jelinski , and M. Mews . 2005. Wrack deposition on different beach types: spatial and temporal variation in the pattern of subsidy. Ecology 86 6:1496–1507.
- Ovchinnikov, A. N. 2009. The ovipositor morphology in the members of the family Scathophagidae (Diptera) with reference to their biology. Entomological Review 89 6:623–636.
- Paquin, P. and N. Dupérré . 2003. Guide d'identification des Araignées (Araneae) du Québec. Fabreries Supplément 11:251.
- Peck, S. B. 2001. Leiodidae Fleming, 1821. In Arnett Jr, R. H. and M. C. Thomas . (eds.). American Beetles, Volume 1: Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. Boca Raton CRC Press. 250–258.
- Roberts, M. J. 1987. The Spiders of Great Britain and Ireland. Volume II. Descriptions of Species—Linyphiidae, Check List of the British Spiders. Colchester, England Harley Books. 204 pp.
- Smith, D. R. , H. Goulet , and D. S. Sikes . submitted. A new Pseudodineura Konow (Hymenoptera: Tenthredinidae) from Kasatochi Island, Alaska. Proceedings of the Entomological Society of Washington.
- Talbot, S. S. , S. L. Talbot , and L. R. Walker . 2010. Post-eruption legacy effects and their implications for long-term recovery of the vegetation on Kasatochi Island, Alaska. Arctic, Antarctic, and Alpine Research 42:285–296.
- Thompson, F. C. and J. H. Epler . 2009. Diptera (flies) of Attu: the first assessment of the entomofauna of the last place on earth. Studia dipterologica 15:267–275.
- Thornton, I. W. B. 1996. Krakatau. The Destruction and Reassembly of an Island Ecosystem. Cambridge, Massachusetts Harvard University Press.
- Thornton, I. W. B. and T. R. New . 2007. Island Colonization: The Origin and Development of Island Communities. Cambridge and New York Cambridge University Press. 287 pp.
- Vockeroth, J. R. 1987. Scathophagidae. In McAlpine, J. F. , B. V. Peterson , G. E. Shewell , H. J. Teskey , J. R. Vockeroth , and D. M. Wood . (eds.). Manual of Nearctic Diptera 2 Agriculture Canada, Research Branch, Monograph,. 28:675–1332.
- Whitworth, T. 2006. Keys to the genera and species of blow flies (Diptera: Calliphoridae) of America north of Mexico. Proceedings of the Entomological Society of Washington 108:689–725.
- Williams, J. C. , B. A. Drummond , and R. T. Buxton . 2010. Initial effects of the August 2008 volcanic eruption on breeding birds and marine mammals at Kasatochi Island, Alaska. Arctic, Antarctic, and Alpine Research 42:306–314.
- WRCC 2009. Historical Climate Information, Western U.S. Historical Summaries, NCDC 1971–2000 Monthly Normals—Adak, AK. Available from http://www.wrcc.dri.edu/. Reno, Nevada: Western Regional Climate Center (accessed 21 November 2009).