Abstract
A description is presented of the nearshore benthic community of Kasatochi Island 10–12 months after a catastrophic volcanic eruption in 2008. The eruption extended the coastline of the island approximately 400 m offshore, mainly along the south, southeast, and southwest shores, to roughly the 20 m isobath. Existing canopy kelp of Eualaria (Alaria) fistulosa, as well as limited understory algal species and associated fauna (e.g., urchin barrens) on the hard substratum were apparently buried following the eruption. Samples and observations revealed the substrate around the island in 2009 was comprised almost entirely of medium and coarse sands with a depauperate benthic community, dominated by opportunistic pontogeneiid amphipods. Comparisons of habitat and biological communities with other nearby Aleutian Islands, as well as with the Icelandic volcanic island of Surtsey, confirm dramatic reductions in flora and fauna consistent with an early stage of recovery from a large-scale disturbance event.
Introduction
A wide array of biological and physical processes are recognized as influential in the dynamics of nearshore marine communities (CitationDayton, 1971; CitationSousa, 2001). Examples of biological factors include competition, predation, herbivory, and succession (CitationPaine, 1974; CitationEstes and Palmisano, 1974; CitationEstes and Duggins, 1995). Examples of physical factors include wave energy, sunlight, water temperature, and salinity (CitationDayton and Tegner, 1984; CitationDayton et al., 1992). Disturbance, either physical or biological, is a dominant influence in marine ecosystems, and a rich literature describes the effects of various sources and types of disturbance on community structure (e.g., CitationSousa, 2001; CitationPaine and Levin, 1981). The frequency of disturbance events is generally inversely related to the magnitude of the disturbance, i.e., large disturbance events are relatively rare (CitationSousa, 2001). As a consequence, much of the research describing community responses to disturbance focuses on relatively small-scale disturbance events (CitationMichener and Haeuber, 1998). Occasionally, disturbance events result in the complete elimination of all organisms in and on the substrate (CitationWhite and Pickett, 1985). Examples of these types of events include landslides and volcanic events (CitationTownsley et al., 1962; CitationGulliksen et al., 1980; CitationOkey, 1997). Because of the rarity and unpredictable nature of disturbance events, relatively little work has been conducted to explore the physical and biological processes responsible for recovery of marine communities following catastrophic disturbance.
On 7–8 August 2008 the eruption of Kasatochi volcano in the eastern Aleutian Islands completely covered the island and nearshore marine habitats with pyroclastic flows and volcanic ash (CitationScott et al., 2010 [this issue]). Observations in the months following the eruption suggested that all terrestrial plant and animal life was eliminated. The effect of the eruption on the intertidal and nearshore subtidal marine communities was expected to be similarly widespread.
Little preexisting shallow (<20 m), nearshore benthic data exists around Kasatochi Island. However, numerous nearshore dive studies have been conducted at other locations in the Aleutians, primarily focusing on the flora and fauna of hard substrates (CitationEstes et al., 1989, Citation2004; CitationVicknair, 1997; CitationKonar, 2000; CitationReisewitz et al., 2006). The recent decline of sea otters (Enhydra lutris) throughout the Aleutian archipelago has caused a shift in the rocky nearshore community, from one dominated by kelps to one dominated by sea urchins (Strongylocentrotus polyacanthus) (CitationEstes et al., 2010). Presumably, urchins also dominated around Kasatochi prior to the 2008 eruption.
Here we report results of a 2009 reconnaissance study to assess the acute effects of the eruption on the nearshore benthic community. There were two primary objectives: (1) characterize the nearshore sediment and benthic community following the 2008 eruption; and (2) provide a comparison of the post-eruption community with data from nearby islands without recent volcanic eruptions. We initially planned to sample the intertidal zone, but finding no organisms there, all sampling was directed to assess the subtidal benthos.
Materials and Methods
STUDY AREA
Kasatochi Island (52.17°N, 175.51°W) is located 80 km northeast of Adak, Alaska, and is part of the Andreanof Islands in the central Aleutians (). The island lies approximately 20 km north of Atka and Fenimore passes, two of several passes which transition between the North Pacific Ocean and the Bering Sea. Prior to the 2008 eruption Kasatochi was a 2.9 km by 2.6 km island volcano (CitationScott et al., 2010 [this issue]). Beds of canopy kelp, Eualaria fistulosa, were evident around Kasatochi Island, except along the northern shore, before the 2008 eruption (J. Williams and W. Pepper, Alaska Maritime National Wildlife Refuge, personal communication, 2009). The status of understory kelps prior to 2008 is unknown, but presumably similar to nearby islands. The eruption extended the coastline of the island, mainly to the east, south, and west sides, burying the kelp beds and associated understory flora and fauna by volcanic flows and ash.
TRANSECTS
Six primary transects that radiated from the caldera to the shore of Kasatochi Island were established for terrestrial sampling. These transects were extended into the nearshore marine environment where sampling/observations occurred between 10 and 20 m depths (). Benthic samples were collected by divers at 10 m depths along secondary transects (see Subtidal Sampling); underwater videos were recorded at 10–20 m depths (see Appendix).
Figure 2 Transects and locations where sampling occurred via divers at 10 m and where observations were made with a drop camera at >10 m, June and August 2009.
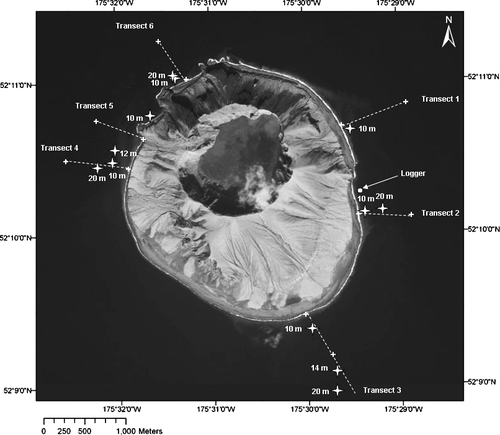
For sampling at the 10 m depth, a skiff began at the shore end of the primary transect and ran a compass bearing offshore along the transect until the 10 m depth was located with the aid of a portable depth sounder and GPS. Each site was marked with an anchor and surface float. Other observations along the transect beyond 10 m were accessed from the M/V Tiglax. Actual locations where sampling/observations were made were often not on the target transect lines because of the difficulty divers had in holding their position in the current. The 10 m site on Transect 5 was moved to an embayment to the north because of rough weather.
SUBTIDAL SAMPLING
The following subtidal sampling protocol, previously used in the Aleutian Islands by CitationJewett et al. (2008), was implemented. At the anchor a diver extended a 25 m tape parallel to shore along the 10 m (±2 m) depth contour to establish the secondary transect. Once the tape was in place, a diver slowly swam back toward the anchor over the tape while video recording approximately a 1 m swath (1 × 25 m) to characterize the habitat (see Underwater Video).
Following video recording, samples were collected at three random points along the secondary transect. At each sampling point, a set of 3 nested quadrats (1 × 1 m, 50 × 50 cm, and 25 × 25 cm in size) were placed on the offshore side of the transect. Within each 1 m2 quadrat, a 50 × 50 cm (0.25 m2) and a 25 × 25 cm (0.06 m2) quadrat were placed for algal and invertebrate sampling, respectively. The abundance and percent cover of algae and macroinvertebrates were recorded within the 1 × 1 m (1 m2) quadrat. A diver-operated airlift capped with a 0.5 mm mesh bag was used to collect all invertebrates found within the 25 × 25 cm quadrat. In soft substrates, sediment was removed down to a depth of 10 cm. Floral and faunal samples were preserved in 10% buffered formalin, labeled, and taken back to the laboratory where invertebrates were sorted, identified, and counted.
Whenever soft sediment (e.g., sand) was encountered at a site, an additional 1 × 1 m quadrat was placed on the inshore side of the tape at the first sampling point along the transect. A single 125 mL sediment sample was taken to a sediment depth of 5 cm from an undisturbed portion of the 1 m2 quadrat for the purpose of determining the surficial sediment grain size at each site. Sediment samples were collected before the macrobenthic samples to ensure no disturbance to the fine sediments. Samples were frozen at −20 °C prior to analysis at the University of Alaska Fairbanks. Grain size was analyzed according to CitationFolk (1980).
The post-eruption Kasatochi benthic community and sediments were compared to two communities previously sampled from nearby Atka and Amlia islands in 2006 and 2007; sampling methods were comparable (Jewett, unpublished AKMAP data).
UNDERWATER VIDEO
Diver video (1–2 minutes) from the 10 m transects were intended to provide semiquantitative information on habitat characteristics and distribution of major algal and macrofauna taxa. To assist with characterizing the habitat, another diver either recorded on a slate or collected representatives of the dominant mature algae and macrofauna from 1 m on either side of the transect (2 × 25 m). A Canon™ PowerShot S80 digital camera was used for the video as well as occasional still photos.
A Splashcam™ drop camera deployed from the M/V Tiglax was used to characterize the benthic substrate and biotic community at the 20 m depth along the six primary transects. Video segments of 2–13 minutes in duration were recorded on a DVD while the ship drifted over the site. Additional drop camera recordings were occasionally made along the primary transects at depths between 10 and 20 m.
WATER TEMPERATURES
As part of the annual monitoring within the Alaska Maritime National Wildlife Refuge, near-bottom water temperatures were recorded hourly at approximately 7–9 m depths along the east side of Kasatochi Island during late May to early September, 1996–2009 (J. Williams, Alaska Maritime National Wildlife Refuge, personal communication, 2009). Between 1996 and 2007 the Onset Corporation HOBO® Optic StowAway® Tidbit® was used; the comparable Onset Corporation HOBO® water temp Pro v2 was used in 2008 and 2009.
STATISTICAL ANALYSES
Comparisons were made between (1) water temperatures and years, sediment grain size (percent) and study sites, and (2) amphipod abundance (percent) and sites using ANOVAs. All percentages were arcsine transformed prior to the ANOVA testing. Statistical significance was set at p ≤ 0.05.
Results
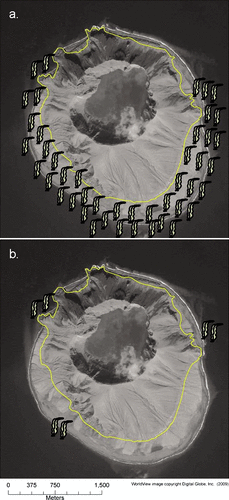
Limited pre-eruption information on the nearshore marine community at Kasatochi exists. Interviews with personnel and scientists who worked around Kasatochi from the M/V Tiglax attest that before the 2008 eruption beds of canopy kelp, Eualaria fistulosa, were evident, except along the northern shore where slope and water depths were too great for suitable habitat for large algal species (). Aerial reconnaissance in the spring of 2009 determined that the eruption extended the coastline of the island about 400 m, mainly along the south, southeast, and southwest shores, to roughly the 20 m isobath (CitationScott et al., 2010 [this issue]). This expansion of volcanic material covered the kelp beds and associated benthic community that previously occurred there. Surveys from the M/V Tiglax in August 2009 found kelp canopy only visible at the water surface in small patches at three locations, off the northeast, northwest, and southwest sides of the island ().
Figure 3 (a) Generalized pre-2008 eruption distribution of canopy kelp (mainly Eualaria fistulosa) around Kasatochi Island (J. Williams and W. Pepper, personal communication, 2009). The 18 April 2009 photo shows the shoreline expansion from the pre-eruption boundary of the island (yellow line). (b) Distribution of canopy kelp (mainly Eualaria fistulosa) around Kasatochi Island one year after the August 2008 eruption.
HABITAT CHARACTERIZATION
Video recordings (by diver and drop camera) made within the 20 m isobath revealed little hard substrate on a largely featureless bottom (Appendix). At 10 m, sand with some gravel and scattered boulders typified the surficial substrate. At 20 m, sand waves with coarser sand on the wave crests were evident on Transects 2 and 5, but fines, i.e., mud and fine sand were more prevalent on Transects 1, 2, 4, and 6. Soft sediment comprised 100% of the substrate within most of the 1 × 1 m quadrats, except along Transect 3 where boulders and cobbles were encountered.
Sediment samples were collected by divers at 10 m depth on five of the six subtidal transects; Transect 1 was not sampled for sediment due to rough seas. Sediment grain size on Transects 2–6 was predominantly medium and coarse sands (0.25–1.0 mm) (A). There was no significant difference in the grain size composition (gravel, sand, mud) at the five sampling sites (ANOVA: df = 4, F = 0.012; p = 0.99).
Table 1 Comparison of sediment grain size composition (percent) <20 m depths on five transects off (A) Kasatochi Island, 2009, and (B) other islands in the Andreanof Islands, 2007 (Jewett, unpublished; CitationJewett et al., 2008).
Benthic organisms (infauna and epifauna) were rarely observed by divers or recorded on videos (Appendix). No holes in or mounds on the soft substrate, indicative of macrobenthic invertebrates, and no large epifaunal invertebrates were observed on the soft substrate. Divers observed a few live barnacles on Transect 6 and a few juvenile flatfishes on Transects 2 and 6. One large yellow Irish lord, Hemilepidotus jordani, was recorded with the drop camera at 14–20 m on Transect 3.
Divers found few living representatives of the mainly smaller species of algae on secondary transects at 10 m depth. Although no algae were encountered within any of the 1 × 1 m quadrats, specimens were visible along the transects on scattered boulders partially buried in the sand. Juvenile brown alga of the family Laminariaceae, Saccharina subsimplex (formerly Laminaria bongardiana), dominated on Transect 4. Some fertile plants were observed. Thick diatom mats with occasional filamentous green alga, Urospora spp., were on Transects 2 and 5 (Appendix). No sea urchins were observed.
At 14–20 m depths along Transect 3, the drop camera recorded small brown algae of the family Alariaceae, mainly Eualaria fistulosa, with some Cymathaere triplicata, Alaria marginata, and Laminaria longipes, as well as several clumps of Saccharina subsimplex and the red alga Porphyra spp. Some fertile Laminaria longipes were observed. Saccharina subsimplex was also noted at 12 m on Transect 4. Most of the algae were recently settled, usually less than 2 m in length. In contrast, no algae were observed at 20 m on Transects 1, 2, 4, 5, and 6 (Appendix). Again, no sea urchins were observed.
Additional features that reflected eruption disturbance included dead barnacles on boulders at 10 m on Transects 4 and 6. Also, on Transect 4, at 12 m, the drop camera recorded bubbles flowing from the sand at four points during a nearly 3-minute drift.
The 13-year record (1996–2009, excluding 2008) of summer bottom water temperatures around Kasatochi Island indicated that temperatures gradually warmed from ~4.5 to ~7.0 °C between late May and early September. Temperatures were not obtained for 2008 because the data logger was buried by the eruption. Mean weekly seabed temperatures were statistically similar among years (ANOVA: df = 12, F = 1.655, p = 0.08). Thus, temperatures for 2009, within a year after the 2008 eruption, were not different than other years.
MACROBENTHIC FAUNA
Benthic samples were collected at 10 m on five of the six subtidal transects; Transect 1 was not sampled due to rough seas. All sampling was predominantly on medium and coarse sands. A depauperate macrobenthic fauna typified all five sites with average abundance values of 8, 0, 352, 389, and 789 individuals m−2 on Transects 2, 3, 4, 5, and 6, respectively (A). Amphipods were the most abundant organisms, although some polychaetes, cumaceans, and barnacles were found. Amphipods occurred at three sites where they accounted for between 51 and 89% of the total abundance (A). Amphipods belonged to six families and there was no significant difference in abundance of the distinct families between the three sites where amphipods occurred (; ANOVA: df = 2, F = 0.011, p = 0.98). The dominant amphipod family Pontogeneiidae, composed of Pontogeneia ivanovi, P. makarovi, and Pontogeneia sp., comprised 27 to 81% of the total site abundance ().
Table 2 Comparison of macrobenthic abundance and dominant taxa from soft substrates at <20 m depths off (A) Kasatochi Island in 2009 and (B) other islands in the Andreanof Islands, 2007 (Jewett, unpublished; CitationJewett et al., 2008).
Table 3 Comparison of amphipod taxa at 10 m sites on transects where amphipods dominated.
Because no pre-eruption information on the macrobenthic community at Kasatochi was available for comparison with 2009 data, comparisons were made with data from Atka and Amlia islands (not shown on ) in the Andreanof Islands group, with similar depths and soft sediments as sampled at post-eruption Kasatochi. Depths at the two sites were 9 and 14 m, respectively. Although the sediments at these two sites were mostly sands, they were generally less coarse and finer sands than at Kasatochi (B).
Regarding Objective 2, the faunal abundance at the Atka and Amlia sites were an order of magnitude greater than sites around Kasatochi Island (B). Furthermore, these sites were mostly dominated by polychaetes, gastropods, and sand dollars, rather than amphipods. These two sites also had evidence of a well-established macrobenthic community, with biotic mounds and holes, and numerous macrofaunal components, like burrowing anemones, polychaetes, hermit crabs, sea urchins, sand dollars, sea stars, and adult and juvenile flatfishes.
Discussion
The rocky nearshore community in the Aleutian archipelago from intertidal to ~20 m subtidal depths has been in transition for the past two decades. Following nearly a century of sea otter range expansion and population growth, sea otter numbers began to decline about 1990 across the Aleutian Islands (CitationDoroff et al., 2003), generally causing an ecosystem phase shift from a kelp-dominated to an urchin-dominated subtidal community (CitationEstes et al., 2004). While today the Aleutians are generally in an urchin-dominated state (i.e., urchin barren) (CitationEstes et al., 2010), canopy kelp beds, primarily the annuals Eualaria fistulosa and Nereocystis luetkeana, as well as numerous species of understory algal species, e.g., the annual Cymathaere triplicate, and the perennials Saccharina subsimplex, Laminaria spp., Agarum spp., and Thalasiophyllum clathrus, still occur in some areas of the archipelago (CitationKonar and Estes, 2003; Bodkin, Chenelot, Hoberg, Jewett, personal observations).
The only previous information on the subtidal community of Kasatochi Island prior to the eruption was obtained from surface observations by Alaska Maritime National Wildlife Refuge personnel J. Williams and W. Pepper (personal communication, 2009) while aboard the M/V Tiglax. They maintained that in years leading up to the 2008 eruption beds of canopy kelp, Eualaria fistulosa, were prevalent around Kasatochi Island, except along the northern shore. The M/V Tiglax makes annual sojourns to the island, as well as elsewhere in the Aleutians.
Also, two dives from the M/V Tiglax were made on 3 September 2002 along the east side of Kasatochi (R. Lauth, NOAA, personal communication, 2009). The first dive (52°10.250′N, 175°29.395′W) was approximately 200 m north of our 2009 10 m site along Transect 2 (). On the first dive, at a depth of ~9 m, video recording revealed a dense canopy of Eualaria fistulosa at the surface. At this depth, where their temperature data logger was retrieved, the hard substrate was covered by Eualaria fistulosa and dense understory of the brown alga Cymathaere triplicata. On the second dive, ~200 m WNW of the data logger position, divers descended a wall to 25 m. Brown algae Agarum turneri and Saccharina subsimplex were present along the upper portion of the wall. Algae deeper than ~20 m were the green algae Codium ritteri and encrusting red coralline algae, Clathromorphum nereostratum and Lithothamnion spp. The wall and the floor at the base of the wall were completely covered with sessile organisms such as sponges, sea anemones, gorgonian corals (e.g., Paragorgia spp.), hydroids, bryozoans, rock jingles (Pododesmus macrochisma), barnacles, and tunicates. Mobile epifauna consisted of a scale crab (Placetron wosnessenskii), sea stars (Henricia spp. and Solaster sp.), brittle stars, and sea urchins. Numerous black (Sebastes melanops) or dusky rockfish (Sebastes ciliatus) were noted along the dive, especially below 20 m. Based on widespread changes in benthic community structure in recent years (CitationEstes et al. 2010), it is difficult to predict what the community at Kasatochi looked like prior to the eruption. Nevertheless, whatever dominated the nearshore community around Kasatochi prior to the eruption was mostly destroyed by the 2008 eruption.
Extension of the shoreline to 400 m offshore to a depth of ~20 m buried the algal community with mainly medium and coarse sands. In all likelihood, the three small patches of kelp observed in August 2009 were the only remnants of the once robust Eualaria fistulosa community that surrounded most of the island. This kelp can attain lengths up to 25 m (CitationO'Clair and Lindstrom, 2000), and it is likely that some portions of the kelp community were not completely buried, especially along the outer margin of the Eualaria zone. While video recordings approximately a year after the eruption further underscored the near complete elimination of kelps, it did reveal isolated kelp remnants. Recordings at 12 locations along transects revealed relatively small (<2 m) algal plants at a few locations where the top of boulders were protruding through the soft substrate (Appendix). The kelp Eualaria fistulosa mainly dominated at 14 m on Transect 3 and Saccharina subsimplex dominated at 10–12 m on Transect 4. That some fertile Saccharina subsimplex were observed indicates some understory kelps survived the eruption. The absence of hard substrate is apparently the major factor limiting kelp recolonization around Kasatochi Island. Studies in temperate systems have shown that algal communities can recover to previous densities within one year of denuding (CitationFoster, 1975; CitationBertness et al., 2004). In situations where algal communities are buried, recovery is entirely dependent upon the rate of sediment erosion. There is no information on the depth of the overlying subtidal fines, the erosion rate of subtidal fines by ocean currents, and the erosion rate of terrestrial fines on Kasatochi (from land to sea). Little information exists on currents around Kasatochi or through the closest passes, Fenimore and Atka, but net northerly current speeds through Seguam Pass (east of Kasatochi) and Tanaga Pass (west of Kasatochi) are moderate (20–25 cm s−1) (CitationStabeno et al., 2005). Current velocities necessary to erode medium and coarse sands (0.25–1.0 mm) are about 20 cm s−1 and greater (CitationHjulström, 1939). Thus, if currents, especially the tidally-driven ones, are >20 cm s−1 around Kasatochi, it may not be long before they erode the subtidal fines down to hard substratum. This would result in increased hard substrate in the nearshore environment that would enable a diverse algal community to become established again.
Another important component of the subtidal community that presumably was buried by the eruption was the long-lived and slow-growing red crustose coralline algae (CitationFrantz et al., 2005; CitationHalfar et al., 2007). In the Aleutian Islands, red crustose coralline algae (Clathromorphum nereostratum and Lithothamnion spp.) are widespread in the low intertidal and shallow subtidal regions, and cover most available hard substrates (Jewett, personal observation). Crustose coralline algae were observed at one location near Kasatochi in 2002, as noted above, and it is thought to have been pervasive in the nearshore zone based on observations made during surveys in 2006–2007. Subtidal habitats dominated by crustose coralline algae are often associated with sea urchin-barren grounds and regarded as supporting limited invertebrate communities, especially compared to the adjacent kelp forests. Despite the desolate appearance of crustose habitats, a recent study in the eastern Aleutians revealed the interstitial spaces of the crustose environments support faunal communities as diverse and abundant as those found in rich macroalgal habitats (CitationChenelot et al., 2008). Thus, the loss of this habitat is viewed as significant as the loss of the algal community. It is unknown if this crustose community will recover when suitable substrate is re-exposed by erosion.
Around Kasatochi, the newly deposited sand substrate was largely devoid of life, since video recordings revealed only barnacles (Transect 6 at 10 m) and no mounds or holes formed by infauna. The dominant macrofaunal species present were motile crustaceans, such as amphipods, that presumably moved into the devastated area from outer unaffected regions. This is in contrast to the Atka and Amlia sites with similar depth and substrate (Jewett, unpublished). Sand communities in the latter region were well-established and showed no effects of physical or biological disturbance. They were characterized by high macrobenthic diversity and abundance with dominant faunal components (i.e., polychaetes, gastropods, sand dollars) less motile or sessile than the amphipods found at Kasatochi. Numerous investigators have found amphipods among the first opportunistic species on soft substrates following disturbances (e.g., CitationOliver et al., 1980; CitationVanBlaricom, 1982; CitationOliver and Slattery, 1985; CitationJewett et al., 1999). CitationVanBlaricom (1982) discovered that shallow sand sites disturbed by digging rays were characterized by low macroinvertebrate abundance, but four families of tube-dwelling and burrowing amphipods dominated. Benthic amphipods (13 genera including tube-dwelling corophiids and burrowing oedicerotids) were among the dominant early colonizing taxa at sandy locations subjected to placer gold mining in Norton Sound, Alaska (CitationJewett et al., 1999). Little is known about the life history of the amphipods in the family Pontogeneiidae that dominated near Kasatochi, but this family is reported to be a nestler (CitationYu et al., 2002) and a free surface dweller that feeds as an herbivore, surface detritivore, and carnivore (CitationBiernbaum, 1979). Diatom films or mats were pervasive in the nearshore zone around Kasatochi, and the pontogeneiid amphipods appear to have been the first and only grazer to utilize this algal resource.
COMPARISONS WITH SURTSEY ERUPTION
Since no post-eruption information on eruption effects or recolonization of nearshore communities on other Aleutian volcanic islands is available, comparisons are made between this study and the volcanic island of Surtsey, off the coast of Iceland. During the ~3.5 year volcanic eruption of Surtsey, which began 14 November 1963, the cinder cone built up from a water depth of 120 m and eventually covered an area of 6.5 km2, of which 2.8 km2 were above water to a height of 172 m (CitationFridriksson, 1975). Smaller satellite volcanoes were added to the submarine base of the Surtsey structure. During the later period of volcanic activity ash flowed down and destroyed all benthic organisms on the sea floor. Ash reached as far out as 1.8 km off the island, where a thin layer of ash was deposited over a layer of mud. The immediate effects of the eruption on the temperature and nutrient composition of sea water were negligible and there was no effect on salinity.
One element of the algal community around Kasatochi bears resemblance to the first stages of algal settlement around Surtsey. Pioneers of the marine benthic algae that colonized Surtsey 21 months after the island emerged were composed only of diatoms (Navicula mollis and Nitzschia bilobata) and one species of filamentous green algae, Urospora penicilliformis (CitationFridriksson, 1975). We also found the first algae colonizers off Kasatochi 10–12 months after the eruption to be unidentified diatoms, mixed with Urospora spp., young brown alga (Alariaceae: Eualaria fistulosa and Saccharina spp.), and red alga (Porphyra spp.). It was not until nearly three years after Surtsey emerged did any brown (Alariaceae) and red algae (Porphyra spp.) appear (CitationFridriksson, 1975). The number of benthic algae discovered growing on Surtsey 3 and 4 years later were 17 and 27 species, respectively.
As for benthic marine fauna around Surtsey, in November 1964, one year after the island formed, coarse substrate prevented sampling at shallow depths. However, at a depth of 70 m 0.4 km off the island a few invertebrates were found on a rough scoria (fragments of burnt, crustlike lava) bottom, e.g., the tube-dwelling polychaete Pectinaria koreni. The scoria substrate reached out to 0.7 km offshore, except to the north side where it was mostly gravel. Between 0.7 and 1.8 km offshore the ash did not seem to affect the fauna. Three years after the island formed there were 17 species of polychaete worms at depths greater than 100 m. At this time several benthic species were found on the slope in shallow water (undetermined, but shallower than 70 m), such as the polychaetes Pectinaria koreni, Scoloplos armiger, and Capitella capitata, six bivalve species of which the most common was Abra nitida, and the brittle star, Ophiura affinis (CitationFridriksson, 1975). Fine sediments with particulate organics must have been prevalent for these opportunistic species to become established. It is of interest that highly mobile amphipods were not among the early colonizers at Surtsey as we found at Kasatochi. Presumably amphipods at Kasatochi were opportunists derived from the remnants of the kelp bed immediately outside the devastated area. No such kelp was available at the newly formed Surtsey.
In summary, the 2008 eruption of Kasatochi Island resulted in catastrophic change to marine habitats and the near complete loss of biological communities surrounding the island. The rocky reef habitats that presumably were dominated by sea urchins and remnant canopy kelp prior to the eruption were replaced by a new substrate of coarse sand dominated by benthic amphipods. There is some evidence that erosion processes may eventually reveal larger sediment sizes or bedrock and algal colonization may occur as a precursor to the recovery of the system.
Acknowledgments
We thank the North Pacific Research Board (Project #923) and the U.S. Geological Survey (USGS) Alaska Science Center for funding this study. We also thank the crew of the U.S. Fish and Wildlife Service (USFWS) M/V Tiglax and USGS Research Diver Sandra Baldwin for her support in field operations. We thank Tony DeGange, Alaska Science Center, and Vernon Byrd, USFWS, for organizing the Kasatochi eruption science program; Jeff Williams, Alaska Maritime National Wildlife Refuge, for providing the water temperature data; Robert L. Lauth, Alaska Fisheries Science Center, National Oceanic and Atmospheric Administration (NOAA), for providing a video on the pre-eruption benthic community; Gary S. Drew, USGS, for providing help with the figures; and Mandy Lindeberg, Alaska Fisheries Science Center, NOAA, for providing help with the algal identifications. Finally, we thank Dr. James A. Estes, Dr. Howard M. Feder, and two anonymous reviewers for their helpful comments. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. This is contribution 258 of the North Pacific Research Board.
References Cited
- Bertness, M. D. , G. C. Trussell , P. J. Ewanchuk , B. R. Silliman , and C. M. Crain . 2004. Consumer-controlled community states on Gulf of Maine rocky shores. Ecology 85:1321–1331.
- Biernbaum, C. K. 1979. Influence of sedimentary factors on the distribution of benthic amphipods of Fishers Island Sound, Connecticut. Journal of Experimental Marine Biology and Ecology 38:201–223.
- Chenelot, H. , S. Jewett , and M. Hoberg . 2008. Invertebrate communities associated with various substrates in the nearshore eastern Aleutian Islands, with emphasis on thick crustose coralline algae. In Bruggeman, P. and N. W. Pollock . (eds.). Diving for Science 2008 Proceedings of the 27th American Academy of Underwater Sciences Symposium, Dauphin Island, AL. 13–36.
- Dayton, P. K. 1971. Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecological Monographs 41:351–389.
- Dayton, P. K. and M. J. Tegner . 1984. Catastrophic storms, El Niño, and patch stability in a southern California kelp community. Science 224 4646:283–285.
- Dayton, P. K. , M. J. Tegner , P. E. Parnell , and P. B. Edwards . 1992. Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecological Monographs 62:421–445.
- Doroff, A. M. , J. A. Estes , M. T. Tinker , D. M. Burn , and T. J. Evans . 2003. Sea otter population declines in the Aleutian archipelago. Journal of Mammalogy 84:55–64.
- Estes, J. A. and D. O. Duggins . 1995. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecological Monographs 65:75–100.
- Estes, J. A. and J. F. Palmisano . 1974. Sea otters: their role in structuring nearshore communities. Science 185:1058–1060.
- Estes, J. A. , D. O. Duggins , and G. B. Rathbun . 1989. The ecology of extinctions in kelp forest communities. Conservation Biology 3 3:252–264.
- Estes, J. A. , E. M. Danner , D. F. Doak , B. Konar , A. M. Springer , P. D. Steinberg , M. T. Tinker , and T. M. Williams . 2004. Complex trophic interactions in kelp forest ecosystems. Bulletin of Marine Science 74:621–638.
- Estes, J. A. , M. T. Tinker , and J. L. Bodkin . 2010. Using ecological function to develop recovery criteria for depleted species: sea otters and kelp forests in the Aleutian archipelago. Conservation Biology 24 3:852–860.
- Folk, R. L. 1980. Petrology of Sedimentary Rocks. Austin, Texas Hemphil Publishing Co. 182 pp.
- Foster, M. S. 1975. Algae succession in a Macrocystis pyrifera forest. Marine Biology 32:313–329.
- Frantz, B. R. , M. S. Foster , and R. Riosmena-Rodriguez . 2005. Clathromorphum nereostratum (Corallinales, Rhodophyta): the oldest alga? Journal of Phycology 41:770–773.
- Fridriksson, S. 1975. Surtsey. New York Halsted Press, John Wiley & Sons. 198 pp.
- Gulliksen, B. , T. Haug , and O. K. Sandnes . 1980. Benthic macrofauna on new and old lava grounds at Jan Mayen. Sarsia 65:137–148.
- Halfar, J. , R. Steneck , B. R. Schöne , G. W. K. Moore , M. Joachimski , A. Konz , J. Fietzke , and J. Estes . 2007. Coralline alga reveals first marine record of subarctic North Pacific climate change. Geophysical Research Letters 34:article L07702.
- Hjulström, F. 1939. Transportation of detritus by moving water. (Part 1. Transportation). In Trask, P. D. (ed.). Recent Marine Sediments. New York Dover Publications, Inc. 5–31.
- Jewett, S. C. , H. M. Feder , and A. Blanchard . 1999. Assessment of the benthic environment following offshore placer mining in northeastern Bering Sea. Marine Environmental Research 48:1–32.
- Jewett, S. C. , R. Brewer , H. Chenelot , R. Clark , D. Dasher , S. Harper , and M. Hoberg . 2008. Scuba techniques for the Alaska Monitoring and Assessment Program (AKMAP) of the Aleutian Islands, Alaska. In Bruggeman, P. and N. W. Pollock . (eds.). Diving for Science 2008 Proceedings of the 27th American Academy of Underwater Sciences Symposium, Dauphin Island, AL. 71–89.
- Konar, B. 2000. Limited effects of a keystone species: trends of sea otters and kelp forests at the Semichi Islands, Alaska. Marine Ecology Progress Series 199:271–280.
- Konar, B. and J. A. Estes . 2003. The stability of boundary regions between kelp beds and deforested areas. Ecology 84:174–185.
- Michener, W. K. and R. A. Haeuber . 1998. Flooding: natural and managed disturbances. BioScience 48:677–680.
- O'Clair, R. M. and S. C. Lindstrom . 2000. North Pacific Seaweeds. Auke Bay, Alaska Plant Press. 162 pp.
- Okey, T. A. 1997. Sediment flushing observations, earthquake slumping, and benthic community changes in Monterey Canyon head. Continental Shelf Research 17 8:877–897.
- Oliver, J. S. and P. N. Slattery . 1985. Destruction and opportunity on the sea floor: effects of gray whale feeding. Ecology 66 6:1965–1975.
- Oliver, J. S. , P. N. Slattery , L. W. Hulberg , and J. W. Nybakken . 1980. Relationships between wave disturbance and zonation of benthic invertebrate communities along a subtidal high-energy beach in Monterey Bay, California. Fishery Bulletin 78 2:437–454.
- Paine, R. T. 1974. Intertidal community structure: experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 14:93–120.
- Paine, R. T. and S. A. Levin . 1981. Intertidal landscapes: Disturbance and the dynamics of disturbance. Ecological Monographs 51:145–178.
- Reisewitz, S. E. , J. A. Estes , and C. A. Simenstad . 2006. Indirect food web interactions: sea otters and kelp forest fishes in the Aleutian archipelago. Oecologia 146:623–631.
- Scott, W. E. , C. J. Nye , C. F. Waythomas , and C. A. Neal . 2010. August 2008 eruption of Kasatochi Volcano, Aleutian Islands, Alaska—Resetting an island landscape. Arctic, Antarctic, and Alpine Research 42:250–259.
- Sousa, W. P. 2001. Natural disturbance and the dynamics of marine benthic communities. In Bertness, M. D. , S. D. Gaines , and M. E. Hay . (eds.). Marine Community Ecology. Sunderland, Massachusetts Sinauer Associates. 85–130.
- Stabeno, P. J. , D. G. Kachel , N. B. Kachel , and M. E. Sullivan . 2005. Observations from moorings in the Aleutian passes: temperature, salinity and transport. Fisheries Oceanography 14 Suppl. 1:39–54.
- Townsley, S. J. , L. Trott , and E. Trott . 1962. A preliminary report of the rehabilitation of the littoral marine community on a new lava flow at Kapoho, Hawaii. Ecology 43:728–730.
- VanBlaricom, G. R. 1982. Experimental analyses of structural regulation in a marine sand community exposed to oceanic swell. Ecological Monographs 52 3:283–305.
- Vicknair, K. E. 1997. Interactions between the sea otter ( Enhydra lutris ) and a subtidal assemblage of sea stars in the western Aleutian Islands. MS thesis. University of California, Santa Cruz. 36 pp. + appendices.
- White, P. S. and S. T. A. Pickett . 1985. Natural disturbance and patch dynamics: an introduction. In Pickett, S. T. A. and P. S. White . (eds.). The Ecology of Natural Disturbance and Patch Dynamics. New York Academic Press. 3–13.
- Yu, O. H. , H. Y. Soh , and H-L. Suh . 2002. Seasonal zonation patterns of benthic amphipods in a sandy surf zone of Korea. Journal of Crustacean Biology 22 2:459–466.
APPENDIX
APPENDIX
Summary of video observations and samples collected from six nearshore (<20 m depths) transects off Kasatochi Island, 12–15 June and 10–12 August 2009 from the R/V Tiglax.