Abstract
Rapid climate change in arctic environments is leading to a widespread expansion in woody deciduous shrub populations. However, little is known about the reproductive, dispersal, and establishment mechanisms associated with shrub expansion. It is assumed that harsh environmental conditions impose limitations on plant sexual reproduction in the Arctic, such that population survival and expansion is predominately a function of clonal recruitment. We present contrary evidence from microsatellite genetic data suggesting the prevalence of recruitment by seed. Further, we present a conceptual model describing modes of recruitment in relation to the abiotic environment. Climate change may be alleviating abiotic stress so that resources are available for more frequent recruitment by seed. Such changes have widespread implications for ecosystem structure and functioning, including species composition, wildlife habitat, biogeochemical cycling, and surface energy balance.
Introduction
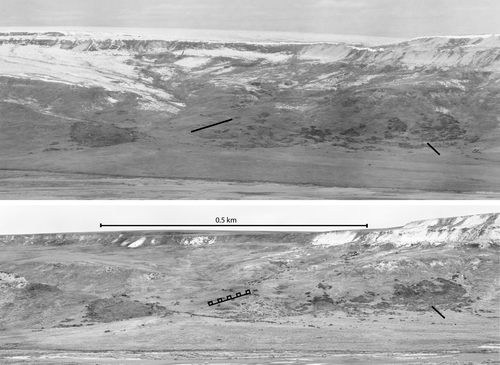
Warming is occurring at an unprecedented pace in the Arctic, leading to changes in terrestrial ecosystem structure and function (CitationHinzman et al., 2005). Among these changes is a widespread increase in the area occupied by woody deciduous shrubs in arctic tundra, concurrent with an increase in land surface temperatures (CitationSturm et al., 2001; CitationStow et al., 2004; CitationTape et al., 2006). Increases in shrub biomass have been observed directly from ground studies (CitationChapin et al., 1995), repeat aerial photography (CitationTape et al., 2006) (), remote sensing imagery (CitationSilapaswan et al., 2001), and in experimentally warmed tundra (CitationBret-Harte et al., 2001). Evidence for previous shrub expansion has also been observed in the paleoecological record, suggesting that expansion in response to a warmer climate may be a recurrent phenomenon (CitationAnderson and Brubaker, 1994).
FIGURE 1 Shrub expansion along the Sagavanirktok River on North Slope, Alaska. The old photo (top panel) was taken during the growing season of 1948 and has evidence of a recent snowfall (U.S. Geological Survey/U.S. Navy). The new photo (bottom panel) was taken during the growing season of 2001 (Ken Tape). Transects are indicated on both old and new photos.
Little research has focused on the reproductive, dispersal, and establishment mechanisms (hereafter referred to as recruitment) associated with shrub expansion. Clonal growth is assumed to be the predominant mode of recruitment in the Arctic, where harsh abiotic conditions limit sexual recruitment (CitationBliss, 1959; CitationJónsdóttir et al., 1996). Clonal growth refers to a plant's expansion and maintenance via growth of new, potentially independent shoots (ramets). Many arctic plants are long-lived and demonstrate growth patterns consistent with clonal recruitment, thus promoting a widespread belief in the prevalence of this strategy (CitationBliss, 1971; CitationBillings, 1974; CitationMadan et al., 2007).
The relative contribution of clonal recruitment to arctic shrub expansion remains unknown. Here, we tested for evidence of clonal identity in a population of Salix spp. shrubs at an arctic site with a known history of woody shrub expansion. Specifically, we sought to (1) test the extent of clonal recruitment in an expanding community of arctic woody shrubs, and (2) propose a theoretical, conceptual model depicting the balance of clonal and sexual recruitment in the Arctic that may be helpful in framing future work.
Methods
SITE DESCRIPTION
This study was conducted at Sagwon Bluffs (69°24′N, 148°38′W; elevation 262 m) adjacent to the Sagavanirktok River on the North Slope, Alaska. Vegetation at the site is composed of a high percent cover of deciduous woody shrubs (~38%) and mosses (~38%), with a smaller percent cover of graminoids (~10%), evergreen woody shrubs (~4%) and lichens (~4%) (Goldsmith and Tape, unpublished data). Average percent cover estimates indicate that Salix spp. accounts for approximately 16% of the total cover, Alnus crispa accounts for 6%, and the remaining shrub cover is comprised of Betula nana and Vaccinium uliginosum. Salix spp. in the arctic region are considered facultative clonal, capable of a range of growth forms including lateral roots with adventitious buds, as well as creeping stems. Previously established microsatellite primers designed and tested for willow species make Salix spp. an ideal genus in which to test clonal diversity of arctic expanding shrub populations (CitationBarker et al., 2003; CitationStamati et al., 2003).
Repeat aerial photography identified the site as having expanded in deciduous woody shrubs over the past 50 years, increasing from 8 to 13% shrub cover (CitationTape et al., 2006).
LEAF GENETICS SAMPLING AND ANALYSIS
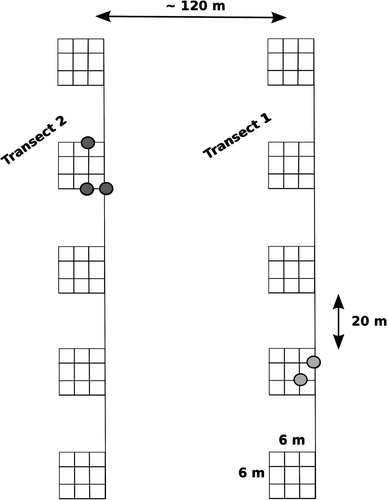
In August 2006, we placed two random transects on a toe slope (<10°) located between the bluffs and the river (). Along each transect, 6 × 6 m grid plots were established at 20 m intervals and a leaf sample of Salix spp. was collected at 2 m grid intervals in each plot (n = 160 total samples). This spacing was considered adequate to capture clonal growth commensurate with the observed shrub expansion. Leaf samples were identified in the field per CitationHultén (1968) and frozen within 6 hours of collection until DNA extraction. Salix collections were found to consist of five species, including S. pulchra Cham., S. glauca L., S. lanata ssp. richardsonii (Hook.) A. Skvortz, S. arbusculoides Anderss., and S. barclayi Anderss.
FIGURE 2 Sampling design and results of survey exploring the recruitment mode of arctic Salix spp. We established 10 plots at 20 m intervals on two random transects ~120 m apart. Sixteen leaf samples of Salix spp. were collected in each plot at 2 m grid intervals (n = 160 total samples). Filled circles represent detected ramets of a multi-ramet clone.
Genomic DNA was extracted from leaves per CitationCullings (1992). Microsatellite markers (SSR) developed for a diverse group of willows by CitationBarker et al., (2003) were used to genotype all samples and determine the extent of clonality (SB85, SB100, SB194). PCR included 5 ng DNA, 25 ng forward primer, 25 ng reverse primer, 200 µm of each dNTP (Promega), 0.5 U Taq DNA polymerase (GibcoBRL), 20 mm Tris–HCl pH 8.4, 50 mm KCl, and 1.5 mm MgCl 2. Cycling conditions were 94°C/2 min; 35 cycles of 94°C/40 s, 54°C/1 min, 72°C/2 min; 72°C/20 min. Amplified DNA was analyzed according to PE Applied Biosystems protocols on an ABI Prism 3100 DNA Sequencing System using Genemapper software. Using PAUP 4.0 (Sinauer Associates) and GenoDive (CitationMeirmans and Van Tienderen, 2004), genetic fingerprints were compared and clonal groups assigned per CitationDouhovnikoff and Dodd (2003). In a preliminary test, a small number of markers were found to have adequate resolving power for disproving clonality, which can be shown once genetic fingerprints do not match. Due to limitations in willow species' identification by morphometric traits, as well as wide phenotypic variation and hybridization (CitationHultén, 1968), all willows were treated as a group for testing of clonality. This should not impact results because we are only considering two conditions: (1) two samples have identical genetic fingerprints and are thus clonal, or (2) two samples have different genetic fingerprints and thus vary at the genotype, species, or higher level.
In a broad survey of references pertaining to arctic plant ecology, we also reviewed literature that offered a model for plant recruitment. For each reference, we identified the stated dominant mode of recruitment (clonal vs. sexual), and explored the empirical basis for the stated model.
Results
Molecular analysis of 160 leaf samples at a 2 m grid scale on 10 sites identified only 2 clonal genets (). On transect 1, only one out of 5 sites was identified as containing a clone, which was made up of two sample points (). On transect 2, only one out of 5 sites was identified as containing a clone, this time made up of three sample points. The molecular markers showed that all other samples were genetically distinct and thus not clonal.
TABLE 1 Clonal diversity results for two transects with five sampling sites each. Genetic fingerprints were used to distinguish genotypes. Percent distinguishable, a standard measure of clonal diversity (CitationEllstrand and Roose, 1987), is calculated by dividing the number of genotypes detected by the number of samples taken.
Percent distinguishable is a standard measure of clonal diversity (CitationEllstrand and Roose, 1987) calculated by dividing the number of genotypes detected by the number of samples taken. The lowest site percent distinguishable values were very high (0.88 and 0.94), and all others were entirely distinguishable (1.00).
In the field, we were unable to observationally distinguish individual seedlings. The average height of the shrub canopy across all plots was 32.2 cm ± 28.9 S.D. The average density of shrubs was 1.7 individuals ± 0.7 S.D. per m2. The average volume of a shrub (calculated as a cube using one height and two width measurements) was 0.143 m3 ± 0.099 S.D..
In a review of over 175 references on arctic plant ecology, 61 specifically referred to the modes of plant recruitment in arctic communities. Of those references, all described clonal growth as being important or predominant and only 22 considered the role of sexual reproduction as a mode of recruitment. Eleven studies were identified wherein clonal growth was cited as a mode of recruitment based on empirical in situ data generated by the author ().
TABLE 2 Eleven arctic studies identified where clonal growth is cited as a mode of recruitment based on empirical in situ data generated by the author.
Discussion
Contrary to expectations, we found little evidence for clonality at a site with a known history of shrub expansion. Although our results are limited in scope, it appears that sexual recruitment by seed is the predominant form of Salix spp. recruitment at this site and scale. This study represents one of the first to use molecular genetic markers to examine clonal diversity in arctic plant species. In a similar study, CitationSteltzer et al. (2008) found equivalent results in Salix arctica plants sampled across multiple arctic sites. Such results demonstrate the need to reexamine the prevailing view of arctic plant recruitment, its emphasis on clonal growth, and associated climate change implications.
Important early work on arctic plants by authors such as CitationBillings and Mooney (1968), CitationBliss (1971), CitationCallaghan and Collins (1976), and CitationBell and Bliss (1980) established a general consensus that sexual reproduction is rare in the Arctic relative to clonal reproduction, but this assumption has seldom been tested. In our survey of citations addressing arctic plant recruitment, all described clonal growth as being important, and only 36% considered a role for sexual reproduction in recruitment. Yet, we found only 11 references where clonal growth is cited as a mode of recruitment based on empirical in situ data generated by the corresponding author (). These studies were limited to 9 plant species, of which 5 were in the same genus (Carex spp.). Furthermore, almost all of these studies used allozyme markers known to underestimate clonal diversity and thus overemphasize clonality (CitationHonnay and Jacquemyn, 2008). Conversely, a growing body of literature demonstrates higher-than-expected levels of genetic diversity in many arctic plant populations presumed to be facultatively clonal, illustrating the potential importance of sexual recruitment (CitationGabrielsen and Brochmann, 1998, CitationSchonswetter et al., 2008). While clonal growth plays a role in arctic plant ecology, further research is necessary to best clarify its extent and associated dynamics.
While environmental conditions of arctic and alpine zones are similar enough to support the same species, the body of work that permits direct comparisons of clonal plant reproductive dynamics is extremely limited. Based on cpDNA haplotypes of Salix arctica, CitationSteltzer et al. (2008) proposed the possibility that sexual reproduction occurs more frequently in the Arctic than in alpine zones, but are more inclined to the argument that population isolation explains differences in genetic architecture. In contrast, CitationBauert (1996), describing high arctic, subarctic, and alpine Polygonum viviparum populations, found very similar genetic structure across zones. Studies on clonal diversity in solely alpine and subarctic species are available and similarly describe a range of clonal diversity levels (CitationMcClintock and Waterway, 1993; CitationLiu et al., 2009). For instance, CitationForbis (2003) described “evidence for the presence of a continuum from complete reliance on clonal reproduction to complete reliance on sexual reproduction.” Yet, it is questionable as to how germane these results are to arctic clonal plant reproductive dynamics due to differences such as the isolated nature of alpine zones.
Building on recent literature that suggests an important role for sexual reproduction (CitationGabrielsen and Brochmann, 1998; CitationAlsos et al., 2007), we present a theoretical conceptual working model that can help direct future work. The model depicts the relationship between abiotic stress and arctic plant sexual recruitment over time (). The model assumes that sexual recruitment in a given plant species is possible only when stress drops below some critical threshold. Even if these events may be extremely rare in the harsh abiotic environment of the Arctic, high observed genetic diversity can partially be explained by the longevity of clonal plants. In addition to testing the general dynamics of the model, future work should also provide specific data on factors such as reproductive event duration and frequency, as well as the character of the environmental conditions that facilitate these events.
FIGURE 3 A proposed model representing the relationship between abiotic stress and plant recruitment over time. Abiotic stress is represented by the (theoretical) dashed line. The solid horizontal line represents a theoretical threshold where abiotic stress is sufficiently low to permit sexual recruitment (>0%). (A) Gray areas correspond to periods of sexual recruitment and represent all possible values above zero. (B) Under current global climate change, abiotic stress will likely be reduced by increasing temperature and nutrient availability. Two of many hypothetical scenarios include (a) continued increase in successful recruitment in synchrony with diminishing stress and (b) saturation and stabilization of the extent of sexual reproduction. Each species will have its own threshold and response to reduced stress.
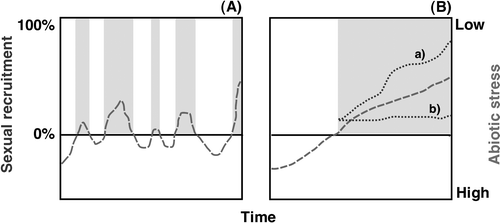
Many references in our literature survey cite a short growing season with cold temperatures and nutrient limitation—both abiotic stresses that generally increase with latitude—as the primary barriers to sexual recruitment by seed. In general, rising terrestrial surface temperatures and increased nutrient availability associated with global climate change are reducing arctic abiotic stress. Our model predicts that if this trend continues, arctic plant species will transition to a mode of recruitment by seed. An observed expansion and shift in plant boundaries, along with the limited data presented here, may be evidence that some species have already crossed this critical threshold. In order to test this, more extensive investigations of clonal diversity using molecular genetic markers need to be completed on more species. Assuming a threshold has been passed, the future trajectory and duration of this state is unknown (). Plant recruitment models, such as the one proposed here, can play an important role in larger-scale efforts to understand the dynamics of global climate change.
Whether sexual recruitment in arctic plants is more prevalent than previously considered, or a transition to reproduction by seed is presently being mediated by climate change, the implications include greater gene flow, genetic crossing, and long-distance dispersal. Increased genetic diversity and mobility improves species ability to adapt to changing environmental conditions and to colonize new areas. While responses are likely species-specific, there may be a cumulative range of critical recruitment thresholds for arctic plant species. A drop in abiotic stress levels below this threshold range could result in a broad shift in plant community recruitment modes. The cumulative effect of such a shift would have widespread effects for community, ecosystem, and landscape level processes. As such, we stress the importance of additional work to elucidate the generality of our finding and its implication in the context of global change.
Acknowledgments
This work was funded by an Arctic Institute of North America Grant-In-Aid and an NSF Graduate Research Fellowship to G. R. Goldsmith and by NSF DEB 0516509. We thank L. Brosius and P. M. Ray for assistance in the field. L. Gough, M. Power, and M. Hutchings provided constructive feedback.
References Cited
- Alsos, I. G. , P. B. Eidesen , D. Ehrich , I. Skrede , K. Westergarde , G. H. Jacobsen , J. Y. Landvik , P. Taberlet , and C. Brochmann . 2007. Frequent long-distance plant colonization in the changing Arctic. Science 316:1606–1609.
- Anderson, P. M. and L. B. Brubaker . 1994. Vegetation history of northcentral Alaska: a mapped summary of late-Quaternary pollen data. Quaternary Science Review 13:71–92.
- Barker, J. H. , A. Pahlich , S. K. Trybush , J. Edwards , and A. Karps . 2003. Microsatellite markers for diverse Salix species. Molecular Ecology Notes 3:4–6.
- Bauert, M. 1996. Genetic diversity and ecotypic differentiation in arctic and alpine populations of Polygonum viviparum . Arctic and Alpine Research 28:190–195.
- Bell, K. L. and L. C. Bliss . 1980. Plant reproduction in high arctic environments. Arctic and Alpine Research 12:1–10.
- Billings, W. D. 1974. Arctic and alpine vegetation: plant adaptations to cold summer climates. In Ives, J. D. and R. D. Barry . (eds.). Arctic and Alpine Environments. London, England Methuen. 403–443.
- Billings, W. D. and H. A. Mooney . 1968. Ecology of arctic plants. Biological Reviews of the Cambridge Philosophical Society 43:481–529.
- Bliss, L. C. 1959. Seed germination in arctic and alpine species. Arctic 11:180–188.
- Bliss, L. C. 1971. Arctic and alpine plant life cycles. Annual Review of Ecology, Evolution, and Systematics 2:405–438.
- Bret-Harte, M. S. , G. R. Shaver , J. P. Zoerner , J. F. Johnstone , J. L. Wagner , A. S. Chavez , R. F. Gunkelman IV , S. C. Lippert , and J. A. Laundre . 2001. Developmental plasticity allows Betula nana to dominate tundra subjected to an altered environment. Ecology 82:18–32.
- Callaghan, T. V. and N. J. Collins . 1976. Strategies of growth and population dynamics of tundra plants. Oikos 27:383–388.
- Chapin III, F. S. , G. R. Shaver , A. E. Giblin , K. J. Nadelhoffer , and J. A. Laundre . 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76:694–711.
- Cullings, K. 1992. Design and testing of a plant-specific PCR primer for ecological and evolutionary studies. Molecular Ecology 1:233–244.
- Douhovnikoff, V. and R. S. Dodd . 2003. Intra-clonal variation and a similarity threshold for identification of clones: application to Salix exigua using AFLP molecular markers. Theoretical and Applied Genetics 106:1307–1315.
- Ellstrand, N. C. and M. L. Roose . 1987. Patterns of genotypic diversity in clonal plant species. American Journal of Botany 74:123–131.
- Forbis, T. A. 2003. Seedling demography in an alpine ecosystem. American Journal of Botany 90:1197–1206.
- Gabrielsen, T. M. and C. Brochmann . 1998. Sex after all: high levels of diversity detected in the arctic clonal plant Saxifraga cernua using RAPD markers. Molecular Ecology 7:1701–1708.
- Hermanutz, L. A. , D. Innes , and I. Weis . 1989. Clonal structure of arctic dwarf birch ( Betula glandulosa ) at its northern limit. American Journal of Botany 76:755–761.
- Hinzman, L. D. , N. D. Bettez , W. R. Bolton , F. S. Chapin , M. B. Dyurgerov , C. L. Fastie , B. Griffith , R. D. Hollister , A. Hope , H. P. Huntington , A. M. Jensen , G. J. Jia , T. Jorgenson , D. L. Kane , D. R. Klein , G. Kofinas , A. H. Lynch , A. H. Lloyd , A. D. McGuire , F. E. Nelson , W. C. Oechel , T. E. Osterkamp , C. H. Racine , V. E. Romanovsky , R. S. Stone , D. A. Stow , M. Sturm , C. E. Tweedie , G. L. Vourlitis , M. D. Walker , D. A. Walker , P. J. Webber , J. M. Welker , K. S. Winker , and K. Yoshikawa . 2005. Evidence and implications of recent climate change in northern Alaska and other arctic regions. Climatic Change 72:251–298.
- Honnay, O. and H. Jacquemyn . 2008. A meta-analysis of the relation between mating system, growth form and genotypic diversity in clonal plant species. Evolutionary Ecology 22:299–312.
- Hultén, E. 1968. Flora of Alaska and Neighboring Territories. Stanford, California Stanford University Press.
- Jónsdóttir, I. , T. Callaghan , and A. Headley . 1996. Resource dynamics within arctic clonal plants. Ecological Bulletin 45:53–64.
- Jónsdóttir, I. , R. Virtanen , and I. Kärnefelt . 1999. Large-scale differentiation and dynamics in tundra plant populations and vegetation. Ambio 28:230–238.
- Jónsdóttir, I. , M. Augner , T. Fagerström , H. Persson , and A. Stenström . 2000. Genet age in marginal populations of two clonal Carex species in the Siberian Arctic. Ecography 23:402–412.
- Jonsson, B. , I. Jónsdóttir , and N. Cronberg . 1996. Clonal diversity and allozyme variation in populations of the arctic sedge Carex bigelowii (Cyperaceae). The Journal of Ecology 84:449–459.
- Kjolner, S. , S. M. Sastad , P. Taberlet , and C. Brochmann . 2004. Amplified fragment length polymorphism versus random amplified polymorphic DNA markers: clonal diversity in Saxifraga cernua . Molecular Ecolology 13:81–86.
- Kjolner, S. , S. M. Sastad , and C. Brochmann . 2006. Clonality and recombination in the arctic plant Saxifraga cernua . Botanical Journal of the Linnean Society 152:209–217.
- Liu, W. S. , W. Wei , and M. Dong . 2009. Clonal and genetic diversity of Carex moorcroftii on the Qinghai-Tibet plateau. Biochemical Systematics and Ecology 37:370–377.
- Madan, N. J. , L. J. Deacon , and C. H. Robinson . 2007. Greater nitrogen and/or phosphorous availability increase plant species' cover and diversity at a high arctic polar semidesert. Polar Biology 30:559–570.
- McClintock, K. A. and M. J. Waterway . 1993. Patterns of allozyme variation and clonal diversity in Carex lasiocarpa and C. pellita (Cyperaceae). American Journal of Botany 80:1251–1263.
- Meirmans, P. G. and P. H. Van Tienderen . 2004. Genotype and Genodive: two programs for analysis of genetic diversity of asexual organisms. Molecular Ecology Notes 4:792–794.
- Schonswetter, P. , R. Elven , and C. Brochmann . 2008. Trans-Atlantic dispersal and large-scale lack of genetic structure in the circumpolar, arctic-alpine sedge Carex bigelowii (Cyperaceae). American Journal of Botany 95:1006–1014.
- Silapaswan, C. S. , D. L. Verbyla , and A. D. McGuire . 2001. Land cover change on the Seward Peninsula: the use of remote sensing to evaluate the potential influences of climate warming on historical vegetation dynamics. Canadian Journal of Remote Sensing 27:542–554.
- Stamati, K. , S. Blackie , J. S. W. Brown , and J. Russell . 2003. A set of polymorphic SSR loci for subarctic willow ( Salix lanata , S. lapponum and S. herbacea ). Molecular Ecology Notes 3:280–282.
- Steltzer, H. , R. A. Hufbauer , J. M. Welker , M. Casalis , P. F. Sullivan , and R. Chimner . 2008. Frequent sexual reproduction and high intraspecific variation in Salix arctica: implications for a terrestrial feedback to climate change in the High Arctic. Journal of Geophysical Research 113:article G03S10.
- Stenstrom, A. , B. Jonsson , and I. Jónsdóttir . 2001. Genetic variation and clonal diversity in four clonal sedges ( Carex ) along the arctic coast of Eurasia. Molecular Ecolology 10:497–513.
- Stenstrom, A. , I. Jónsdóttir , and M. Augner . 2002. Genetic and environmental effects on morphology in clonal sedges in the Eurasian Arctic. American Journal of Botany 89:1410–1421.
- Stow, D. A. , A. Hope , D. McGuire , D. Verbyla , J. Gamon , F. Huemmrich , S. Houston , C. Racine , M. Sturm , K. Tape , L. Hinzman , K. Yoshikawa , C. Tweedie , B. Noyle , C. Silapaswan , D. Douglas , B. Griffith , G. Jia , H. Epstein , D. Walker , S. Daeschner , A. Petersen , L. M. Zhou , R. Myneni , et al . 2004. Remote sensing of vegetation and land-cover change in arctic tundra ecosystems. Remote Sensing of Environment 89:281–308.
- Sturm, M. , C. Racine , and K. Tape . 2001. Increasing shrub abundance in the Arctic. Nature 411:546–547.
- Tape, K. D. , M. Sturm , and C. Racine . 2006. The evidence for shrub expansion in northern Alaska and the pan-Arctic. Global Change Biology 12:686–702.