Abstract
The alpine páramo of Chingaza National Park, Colombia, has a highly variable cloud regime typical of many tropical alpine areas. Yet, little information is available regarding the effects of such dynamic sunlight regimes on alpine temperatures. A close association between changes in incident sunlight and corresponding air (T a) and leaf (T l) temperatures occurred in two dominant species with strongly contrasting leaf form and whole-plant architecture. Spikes in sunlight incidence of >3000 μmol m−2 s−1 occurred during cloud cover and corresponded to increases in T l of 4–5 °C in a 1-min-interval in both species. Although T l was predominately above T a, during the day, depressions below T a of over 6 °C occurred during cloudy conditions when photosynthetic photon flux density (PFDs) was <400 μmol m−2 s−1. The greatest frequency (69%) of changes in incident sunlight (PFD s; over 2-min intervals) was less than 100 μmol m−2 s−1, although changes >1000 μmol m−2 s−1 occurred for 2.4% of the day, including a maximum change of 1512 μmol m−2 s−1. These data may be valuable for predicting the ecophysiological impact of climate warming and associated changes in future cloud regimes experienced by tropical alpine species.
Introduction
Alpine plants have often been characterized adaptively as being short in stature so that leaf temperatures can be more tightly coupled to the protective boundary layer microclimate of the soil surface. This coupling results in lower wind speeds and greater departures from air temperature due to heating by solar radiation during the daytime or cooling due to long-wave radiation exchange with clear skies at night (CitationGrace, 1977; CitationSmith and Young, 1987; CitationJordan and Smith, 1994; CitationKörner, 2007, Citation2012). However, considerable variation in plant temperatures can occur due to microsite differences at ground level (CitationSalisbury and Spomer, 1964; CitationCernusca, 1976; CitationMoser et al., 1977; CitationFetene et al., 1997; CitationScherrer and Körner, 2010; CitationLarcher, 2012), as well as plant stature regardless of microsite (CitationKörner et al., 1983; CitationGauslaa, 1984; CitationDiemer, 1996; CitationTaschler and Neuner, 2004) and leaf size (CitationGrace, 1977). In general, plants with smaller leaves and taller plants exposed to higher wind speeds are more coupled to air temperature and less to radiational heat exchange due to enhanced convective heat exchange.
Although clouds can be a frequent occurrence in mountain ecosystems (CitationMoser et al., 1977; CitationJohnson and Smith, 2008; CitationBerry and Smith, 2012), few comparisons of their specific influence on air and leaf temperatures have been reported. One study measured sunlight levels and leaf temperatures at a lower and higher elevation site in New Guinea (CitationKörner et al., 1983). Several other studies have reported the influence of plant stature and form on temperatures of various plant parts at higher elevations, although only under sunny conditions (e.g., CitationSalisbury and Spomer, 1964; CitationKörner and DeMoraes, 1979; CitationKörner and Cochrane, 1983, Citation1985; CitationFetene et al., 1997; CitationKörner, 2003; CitationCui et al., 2004; CitationLarcher, 2012). Similar to the present study, one study reported temperature differences between two herbaceous perennial species of the Ecuadorian Andes, one a flat rosette habit with dark leaves and the other an erect-leaved species with pubescent leaves (CitationDiemer, 1996). Another similar study in the Qinghai-Tibetal plateau reported differences in leaf temperatures based on leaf and whole-plant structure and reported significant photoinhibition of photosynthesis beginning at a sunlight incidence about 1000 μmol m-2 s-1 in both species, which appeared exacerbated by higher leaf temperatures (CitationCui et al., 2004). It has also been recognized that large-leaved herbs of mountain areas around the world, including the equatorial areas of the Andes, can be substantially warmer than air temperature (CitationGrace, 1977). The more compact leaf crown closer to the ground (e.g., cushion plants) appeared to generate the greatest departures from air temperature during both day and night due to lower wind speeds and less convective heat exchange (CitationKleier and Rundel, 2009).
The aim of our study was to evaluate the influence of a highly variable cloud regime and corresponding effects on leaf and air temperatures in two common páramo species of this northern Andean region. Diurnal air and leaf temperatures, plus incident sunlight levels, were compared under highly dynamic cloud conditions that are characteristic of this alpine páramo region, as well as other tropical alpine systems.
These data will serve to initiate the development of an empirical model for evaluating the influence of such a variable cloud regime on photosynthesis and water relations, as well as potential effects of altered cloud regimes due to continued global warming (CitationEastman and Warren, 2013).
Materials and Methods
SITES AND SPECIES
Chingaza National Park (CNP) is located on the Eastern Cordillera in Colombia, less than 70 km east of Bogotá, and páramo ecosystems in CNP have been classified as humid (CitationCleef, 1981; CitationSarmiento, 1986). The park comprises more than 50,000 hectares ranging in altitude from ∼800 to ∼4000 m and contains an estimated plant biodiversity of over 2000 species (CitationParque Nacional Natural Chingaza, 2005–2009). Above ∼3200 m, the vegetation of the park changes from high-elevation mountain forests to the alpine páramos where more than 350 plant species occur within a mosaic of natural glacial lakes.
Chingaza has a unimodal monthly precipitation regime, with a wet season lasting from April through November and a short dry season from December to late February or early March (CitationSarmiento, 1986). However, El Niño (ENSO) years can lead to extreme drought seasons such as occurred in late 2009 and early 2010, in contrast to 2012 when the dry season was less than two months and rainfall was frequent by early February (A. Sanchez, J. M. Posada, and W. K. Smith, unpublished data; CitationNOAA, 2013). Mean annual air temperatures at CNP range between ∼6 and 10.5 °C but are most variable during the dry season, with maximums greater than 20 °C and nighttime minimums several degrees below freezing; during the wet season nighttime temperatures rarely go below freezing (CitationTol and Cleef, 1994; Citationde los Ángeles et al., 2002). The annual maximum and minimum precipitation are highly variable, ranging between 1690 and 3320 mm, with an annual mean of around 2000 mm (CitationTol and Cleef, 1994; Citationde los Ángeles et al., 2002).
We chose three páramo sites located around Laguna Seca, at 3557, 3568, and 3696 m of altitude; the sites were located at 4°41′7″N, 73°45′59″W; 4°41′12″N, 73°46′21″W; 4°41′27″N, 73°48′6″W, respectively. We considered these sites representative of the páramo of this region in terms of topography, species composition, and climate (CitationParque Nacional Natural Chingaza, 2005–2009). Two species of this páramo were chosen for study: Espeletia grandiflora Bonpl. (Asteraceae) and Chusquea tessellata Munro (Poaceae). Both species are dominant members of the CNP páramo community and grow sympatrically. Espeletia grandiflora (Asteraceae) is a caulescent rosette endemic of the Eastern Andes of Colombia that can grow to 4–5 m in height with thick leaves that are covered with a dense, white pubescence on both adaxial (top) and abaxial (bottom) leaf surfaces (; CitationSmith and Koch, 1935; CitationFagua and Gonzalez, 2007). Chusquea tessellata (Poaceae) is a dwarf bamboo with a broad distribution that extends from Venezuela to northwestern Bolivia; it can grow to more than 3 m in height and has glabrous leaves on both adaxial and abaxial sides (CitationTol and Cleef, 1994; CitationClark, 2003). Both species had relatively laminar and upright leaf orientations—in E. grandiflora, the third and fourth whorls have angles of ∼65°–75° (leaf angle varies depending on the specific location of individual leaves within the rosette) and Chusquea tessellata of ∼0°–80° from horizontal (). Thus, these species had contrasting leaf form and whole-plant architecture.
INCIDENT SUNLIGHT, AIR AND LEAF TEMPERATURES
To associate the high variability in sunlight at CNP, photosynthetic photon flux density (PFD, 0.4–0.7 μm) incident on adaxial leaf surfaces and corresponding abaxial leaf temperatures were measured for both species. In addition, air temperature (T a) and PFD incident on a horizontal plane measured at 1.5 m above ground at the site (PFD s) were collected only meters away from measurement plants. Incident PFD s was measured with a quantum sensor (model LI-190s, LiCor, Lincoln, Nebraska) placed in an open area with an unobstructed view of the sky. Measurements were continuous beginning ∼0.5 hr after sunrise and ∼0.5 hr prior to sunset. Three individuals per species were measured simultaneously at each of the three sites and three leaves per individual were recorded continuously for a total of 11 days (N = 9 total individuals and 27 total leaves per species). Individual plants were approximately 0.5–1.0 m tall and selected leaves appeared healthy and fully mature. Leaf temperatures (T l) were measured using fine-wire (36 ASU gauge), copper-constantan thermocouples, while sunlight incident on individual leaves (PFD l) was measured using GaAsP light sensors (G1118 Hamamatsu). The PFD leaf sensors and thermocouples were connected to a Campbell multiplexer (AM16/32B and AMT25, respectively, Campbell Scientific, Utah) and recorded by a Campbell datalogger (model CR-1000, Campbell Scientific, Utah). All leaf sensors were logged every minute. All PFD1 sensors and thermocouples were placed at the same locations on sampled leaves, approximately in the center of the leaf, midway between the tip and base. Thermocouples were placed on the abaxial (bottom) side, while PFD sensors were placed on the adaxial (top) side of the leaf. Air temperatures were recorded using three Thermochron ibuttons (model DS1921G) located 1.5 m aboveground, shielded from direct sunlight and the cold night sky using a 20-cm-diameter circular shield painted with 3M reflective white paint and positioned 5 cm above the sensors. These sensors were located within 3 m of the study plants.
Results
SITE PFDS AND AIR TEMPERATURE (TA)
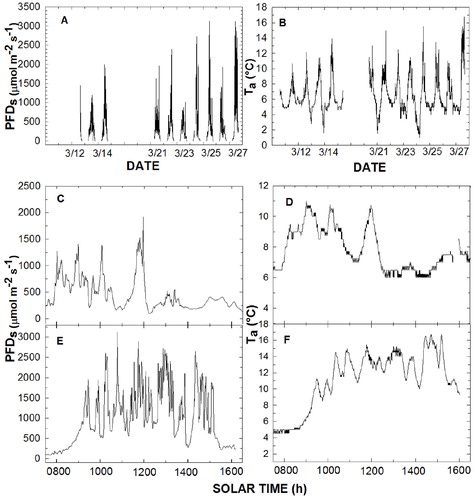
There was considerable variation in measured PFD s between all measurement days, ranging from a maximum instantaneous PFD s value of over 3000 μmol m-2 s-1 and a lowest maximum of ∼1000 μmol m-2 s-1 (, part A). Greater variability in PFD occurred on the warmest day with the greatest PFD s values occurring as single, higher-intensity episodes that lasted only minutes or less (, part E). For example, in a one-minute interval, PFDs changed from 2200 to 3200 μmol m-2 s-1 and from near 3200 to 500 μmol m-2 s-1, then back to 2500 μmol m-2 s-1 over a 5-minute interval. On a cooler day, PFD s declined from near 1900 to 50 μmol m-2 s-1, also over a 5-minute interval (, part C). Thus, differences between the coldest and warmest days during the measurement days showed distinct shifts in maximum values, but a prolonged, low morning PFD on the warm day (due to cloud immersion) (, part E).
For daily comparisons of both PFD s and T a, times and days with high PFD values also corresponded to days with warmer T (). A maximum daily T a of between 11 and 12 °C occurred on cooler days compared to maximums of ca. 17–18 °C on warmer days, and both types of days occurred throughout the study period. Most days had multiple temperature peaks throughout both morning and afternoon, especially for the warm days (, part F). In general, there was an increase in T a during early morning on the cooler days that led to higher air temperatures in the morning than afternoon (, part D), although warmer days showed a somewhat opposite trend (e.g., , part F).
During an average day, a bell-shaped curve of hourly PFD s measurements occurred, with maximum mean PFD s near 800 μmol m-2 s-1 at midday, increasing and decreasing almost linearly from 0600 h and 1800 h, respectively (, part A). A near linear increase in PFD s occurred between 0600 and 1200 h compared to a more delayed decline from 1200 to 1800 h. Thus, afternoon PFD s values tended to be higher overall than morning values (, part A). The greatest duration (percentage of daytime) of specific PFD s values was for values <100 μmol m-2 s-1 (20.4%) followed by the total duration of all flux densities of between 100 and 350 μmol m-2 s-1 (37.6%, , part B). A precipitous decrease in the intensity and daily duration of incident PFD began at about 450 μmol m-2 s-1. Also, PFD < 400 μmol m-2 s-1 occurred 67.3% of the time during the day while PFD > 500 μmol m-2 s-1 and PFD > 1000 μmol m-2 s-1 occurred for 28.6% and 9.4% of the time, respectively (, part B).
FIGURE 3. (A). Mean hourly PFD measured at the central location (PFDs) for all sampling days and (B) mean percent of daylight hours when indicated values (intervals) of PFD occurred. PFD Intervals were 50 to 1500 μmol m-2 s-1 and then 100 μmol m-2 s-1. Greatest values at 2800–2900 and at more than 3000 μmol m-2 s-1 had a frequency of 0.02% and 0.03%, respectively.
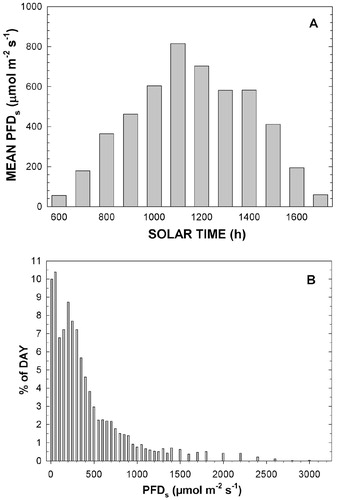
FIGURE 4. Nighttime air temperatures for the coldest (light line; 3/24/12) and warmest night (bold line; 3/23/12) during the study period.
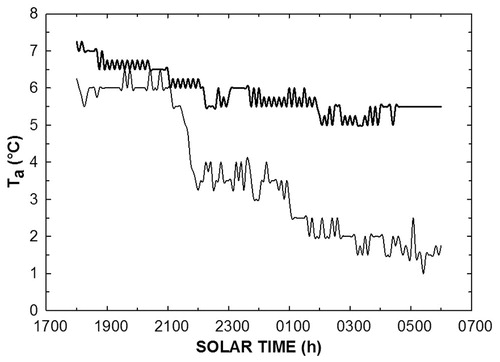
Minimum nighttime T a also varied, with the coldest night (mostly clear night skies) T a of about 1 °C and the warmest night (overcast night) near 5 °C (). During the coldest night, temperatures decreased gradually, reaching minimums at near 0530 h and also decreased gradually during the warmest night where the lowest T a recorded occurred between 0200 and 0400 h. There was >3 °C difference between daily maxima and minima (T a) during the warmest night compared to a 5 °C difference during the coldest night ().
PFDL AND LEAF TEMPERATURE
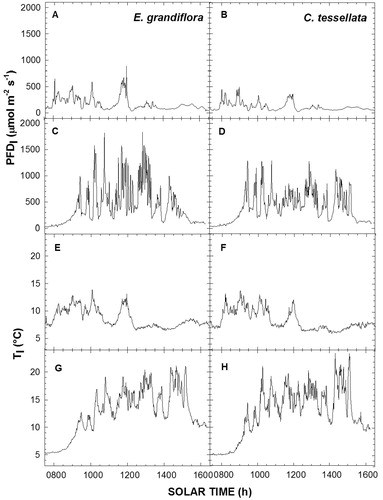
The inclined leaf orientation in both species substantially reduced incident PFD 1 compared to the centrally located, horizontal PFDs sensor (PFD s). This was especially true for C. tessellata where PFD s was ∼70%–80% greater than PFD incident on leaves (PDF l), while for E. grandiflora this value was mostly between 50% and 70% ().
Leaf temperature (T l,) and PDF l for E. grandiflora and C. tessellata paralleled one another, with low T l values corresponding to a low PFD l (). Both species had similar PFD l and T l patterns, although the incident PFD values were higher in E. grandiflora, with a difference with C. tessellata of up to 500 μmol m-2 s-1 (, parts A and C); Chusquea never experienced values over 1300 μmol m-2 s-1 (, parts B and D). Leaf temperatures on both species were similar on cold days, with both species reaching maximums of near 14 °C (, parts E and F), while on warmer days C. tessellata had higher T l by about 3 °C than E. grandiflora during the afternoon (, parts G and H).
FIGURE 5. Estimating leaf orientation effects on PFDl using comparisons with the horizontal PFD sensor located at the central site location. The number of percent reductions in PFD due to a nonhorizontal leaf orientation were calculated as the percent difference between PFDs and PFDl, i.e. [(PFDs - PFDl)/(PFD ) × 100] for Espeletia grandiflora (black) and Chusquea tessellata (gray) for all corresponding PFD measurements. Number of occurrences were pooled from data on nine leaves from three individuals per species measured over 11 days.
![FIGURE 5. Estimating leaf orientation effects on PFDl using comparisons with the horizontal PFD sensor located at the central site location. The number of percent reductions in PFD due to a nonhorizontal leaf orientation were calculated as the percent difference between PFDs and PFDl, i.e. [(PFDs - PFDl)/(PFD ) × 100] for Espeletia grandiflora (black) and Chusquea tessellata (gray) for all corresponding PFD measurements. Number of occurrences were pooled from data on nine leaves from three individuals per species measured over 11 days.](/cms/asset/94caa3ab-fc1c-49c8-93ed-aec75f31a95c/uaar_a_11957740_f0005.jpg)
FIGURE 6. Hourly variation in PFDl and Tl in E. grandiflora and C. tessellata leaves on the coldest (A and B; E and F; 3/26/12) and warmest day (C and D; G and H; 3/27/12).
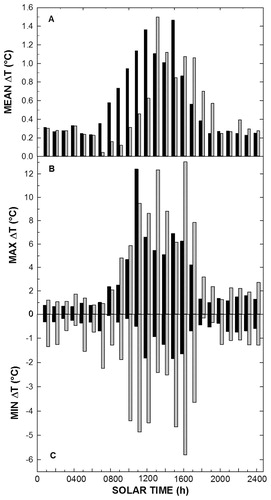
Considering differences between T l and T a, both E. grandiflora and C. tessellata maintained average T l values above T a, especially during the day (Fig. 7). However, maximum T l T a differences (ΔT) were over 12 °C (Fig. 7, part B), while T l < T a was more frequent in C. tessellata and up to -5.7 °C for both species, and for all T a (Fig. 7, part C). Temporally, morning AT in both species showed steep linear increases, although C. tessellata warmed more slowly (Fig. 7, part A). In contrast, C. tessellata cooled more slowly in the afternoon, and both species showed less linear decreases during the afternoon compared to morning increases.
Discussion
SITE PFD AND AIR TEMPERATURES
Data describing the influence of cloud dynamics on air and leaf temperatures in high elevation alpine zones are rare in the literature, especially for equatorial alpine areas (see review by CitationKörner, 2002; CitationPellicciotti et al., 2011). Our páramo study site, northern Andes, was characterized by high levels of incident sunlight, along with high temporal variability due to cloud patterns that ranged from high cirrus clouds to full cloud immersion (A. Sanchez, J. M. Posada, and W. K. Smith, unpublished data). At high elevations, increased levels of insolation during clear skies are well known (e.g., CitationSmith and Geller, 1980; CitationBader et al., 2007; CitationKörner, 2007). However, as reported for other tropical alpine areas (CitationDiemer, 1996), lower levels of incident sunlight (<400 μmol m-2 s-1) may dominate the frequency distribution of sunlight levels due to clouds and fog, although maximum, much higher values may occur intermittently. Specifically, Diemer (Citation1996) reported that over 50% of incident sunlight values were below about 400 μmol m-2 s-1 with maximum values of >2400 μmol m-2 s-1 occurring for less that 2% of the total day in an Ecuadorian Andes site, similar to our data (). PFD values of over 3000 μmol m-2 s-l (), well above the Solar Constant (an average of 1361 W m-2 or approximately 2790 μmol m-2 s-1; CitationPearcy, 1989), have been reported elsewhere and are associated with specific cloud patterns (CitationGu et al., 2001; CitationDye et al., 2009).
FIGURE 6. (A) Hourly mean, (B) maximum, and (C) minimum temperature differences between the leaf (Tl) and air temperature (Ta) for E. grandiflora (black) and C. tessellata (gray) measured over the entire study period, i.e. ΔT = Tl - Ta.
Although rapid changes in PFD levels () have been associated with both photosynthetic induction (CitationPearcy and Way, 2012) and photoinhibitory effects (e.g., CitationGermino and Smith, 1999; CitationCui et al., 2004), these associations have yet to be studied comprehensively (see CitationSmith and Berry, 2013, for recent review). In both species studied here, inclined leaf orientations reduced incident PFD compared to the horizontal-sensor PFD s by more than 50%–60%. This is a potentially important architectural trait that avoids high PFD, as well as the potentially stressful, short transition times from low to high PFD, e.g., from <400 to over 3000 μmol m-2 s-1. The lower values of PFD l relative to the horizontal sensor (PFD s) in C. tessellata indicate a greater decline in PFD interception due to a more inclined leaf orientation, approximately 70%–80% lower compared to ∼50% in E. grandiflora ().
Comparisons of the warmest and coldest days during the sampling period showed considerably greater T a values along with a greater number of PDF s values above approximately 1500 μmol m-2 s-1 on the warmest day of the sampling period. The warmest day also had a shift to higher T a, in correspondence with PFD s above about 400–500 μmol m-2 s-1. Thus, solar heating appeared to have had a significant impact on T a during both the afternoon and morning ().
LEAF PFD AND TEMPERATURES
Leaf temperature patterns relative to PFD l and T a were similar between the two species, despite contrasting differences in whole-plant architecture and individual leaf form. Thus, in terms of PFD effects on T l, leaf orientation similarities seemed to override differences in leaf morphology such as the dense pubescence found in E. grandiflora. Based upon differences in leaf form and plant architecture, the slightly more horizontal leaf inclination in E. grandiflora should have also led to greater nighttime sky exposure and, thus, colder minimum temperatures. However, thicker leaves and a denser leaf pubescence could lead to less Tl variability (CitationJordan and Smith, 1994; CitationMeinzer et al., 1994).
Some studies have pointed to the importance of nighttime minimum air temperatures in the alpine as dictating the functional beginning and end of the growth season, plus the frequent occurrence of low-temperature photoinhibition of photosynthesis following near-freezing nights even during summer (CitationHamerlynck and Smith, 1994; CitationGermino and Smith, 1999, Citation2000; CitationJohnson and Smith, 2005). Because minimum air temperatures often occur at or near sunrise (), minimum leaf temperatures could occur almost simultaneously with relatively high sunlight exposure (, part C). In addition, the accumulation of cold air settling next to the ground, enhanced by wind-sheltered, yet sky-exposed microsites, can also contribute to minimum leaf temperatures at night that are well below air temperature (e.g., Fig. 7; CitationJordan and Smith, 1995; CitationGermino and Smith, 1999). Moreover, cloud cover and leaf orientation away from the sky can act to curtail both leaf warming during the day and cooling during the night by minimizing the radiational heat exchange that uncouples leaves from air temperature.
Conclusions
The results presented here show a tight coupling between a highly variable PFD regime and corresponding air and leaf temperatures for two dominant species of this northern Andes páramo, despite having contrasting leaf morphology, leaf form, and whole-plant architecture. Rapid changes in PFD levels of over 2000 μmol m-2 s-1 occurred over time intervals of minutes or less, especially on warmer days. Leaf orientation reduced both PFD l and leaf temperature, and seemed to outweigh contrasting differences in leaf morphology (heavy pubescence in E. grandiflora) and whole-plant architecture (upright rosette versus multiple erect stems, ) between the two species. This information could be critical for anticipating functional responses of native species to potential changes in future cloud regimes under current scenarios of climate warming.
Acknowledgments
The authors would like to thank Camilo Rey for his help in the field and calibration of all instruments, and Chingaza National Park (CNP) for providing logistical support and permits for this research. This project was funded partially by the Center for Energy, Environment, and Sustainability (CEES) at Wake Forest University and the National Science Foundation, U.S.A. (IOS-1122092).
References Cited
- Bader, M. Y. , van Geloof, I. , and Rietkerk, M. , 2007: High solar radiation hinders tree regeneration above the alpine treeline in northern Ecuador. Plant Ecology , 191: 33–45.
- Berry, Z. C. , and Smith, W. K. , 2012: Cloud pattern and water relations in Picea rubens and Abies fraseri, southern Appalachian Mountains, USA. Agricultural and Forest Meteorology , 162–163: 27–34.
- Cernusca, A. , 1976: Bestandesstruktur, Bioklima, und Energiehaushalt von alpinen Zwergstrauchbeständen. Oecologia Plantarum , 11 : 71–102.
- Clark, L. G. , 2003: A new species of Chusquea Sect. Swallenochloa (Poaceae: Bambusoideae) from Bolivia. The Journal of the American Bamboo Society , 17: 55–58.
- Cleef, A. M. , 1981: The vegetation of the páramos of the Colombian Cordillera Oriental. Dissertation Botany , 61: 1–321.
- Cui, X. , Tang, Y. , Gu, S. , Shi, S. , Zhao, X. , and Seiichi, N. , 2004: Leaf orientation, incident sunlight, and photosynthesis in the alpine species Suassurea superba and Gentiana straminea on the Qinghai-Tibet Plateau. Arctic, Antarctic, and Alpine Research , 36: 219–227.
- De los Angeles, C. , Posada Vergara, C. , and Vargas, O. , 2002: Banco de semillas germinable de una comunidad vegetal de páramo húmedo sometida a quema y pastoreo (Parque Nacional Natural Chingaza, Colombia). Ecotropicos , 15: 51–60.
- Diemer, M. , 1996: Microclimatic convergence of high-elevation tropical páramo and temperate-zone alpine environments. Journal of Vegetation Science , 7: 821–830.
- Dye, D. G. , Kobayashi, H. , Wu, P. , Sulistowati, R. , Sarodja, D. , and Syamsudin, F. , 2009: Cloud-induced variability of photosynthetically active radiation, tropical forest PAR absorption and photosynthesis in central Borneo. American Geophysical Union, Spring Meeting 2008, Abstract #B33A-05. <http://adsabs.harvard.edu/abs/2008AGUSM.B33A..05D>.
- Eastman, R. , and Warren, S. G. , 2013: A 39-yr survey of cloud changes from land stations worldwide 1971–2009: long-term trends, relation to aerosols, and expansion of the tropical belt. Journal of Climate , 26: 1286–1303.
- Fagua, J. C. , and Gonzalez, V. H. , 2007: Growth rates, reproductive phenology, and pollination ecology of Espeletia grandiflora (Asteraceae), a giant Andean caulescent rosette. Plant Biology , 9: 127–135.
- Fetene, M. , Nauke, P. , Luttge, U. , and Beck, E. , 1997: Photosynthesis and photoinhibition in a tropical alpine giant rosette plant, Lobelia rhynchopetalum. New Phytologist , 137: 453–461.
- Gauslaa, Y. , 1984: Heat resistance and energy budget in different Scandinavian plants. Ecography , 7: 5–6.
- Germino, M. J. , and Smith, W. K. , 1999: Sky exposure, crown architecture, and low-temperature photoinhibition in conifer seedlings at alpine treeline. Plant, Cell and Environment , 22: 407–415.
- Germino, M. J. , and Smith, W. K. , 2000: High resistance to low-temperature photoinhibition in two alpine snowbank species. Physiologia Plantarum , 110: 89–95.
- Grace, J. , 1977: Plant Responses to Wind. London: Academic Press, 204 pp.
- Gu, L. , Fuentes, J. D. , Garstang, M. , Tota da Silva, J. , Heitz, R. , Sigler, J. , and Shugart, H. H. , 2001: Cloud modulation of surface solar irradiance at a pasture site in southern Brazil. Agricultural and Forest Meteorology , 106: 117–129.
- Hamerlynck, E. P. , and Smith, W. K. , 1994: Subnivean and emergent micro-climate, photosynthesis, and growth in Erythronium grandiflorum Pursh, a snowbank geophyte. Arctic, Antarctic, and Alpine Research , 26: 21–28.
- Johnson, D. M. , and Smith, W. K. , 2005: Refugial forests of the southern Appalachians: photosynthesis and survival in high altitude, current-year Abies fraseri seedlings. Tree Physiology , 25: 1379– 1387.
- Johnson, D. M. , and Smith, W. K. , 2008: Cloud immersion alters microclimate, photosynthesis and water relations in Rhododendron and Abies seedlings, southern Appalachian Mountains, USA. Tree Physiology , 28: 385–392.
- Jordan, D. , and Smith, W. , 1994: Energy balance analysis of nighttime leaf temperatures and frost formation in a subalpine environment. Agricultural and Forest Meteorology , 71: 359–372.
- Jordan, D. N. , and Smith, W. K. , 1995: Radiation frost susceptibility and the association between sky exposure and leaf size. Oecologia , 103:43–48.
- Kleier, C. , and Rundel, P. , 2009: Energy balance and temperature relations of Azorella compacta, a high-elevation cushion plant of the Central Andes. Plant Biology , 11: 351–358.
- Körner, C. , 2002: Mountain biodiversity, its causes and function: an overview. In Körner, C. , and Spehn, E. M. (eds.), Mountain Biodiversity: a Global Assessment. New York: Parthenon Publishing, 3–20.
- Körner, C. , 2003: Alpine Plant Life. Berlin: Springer, 362 pp.
- Körner, C. , 2007: The use of ‘altitude’ in ecological research. Trends in Ecology and Evolution , 22: 569–574.
- Körner, C. , 2012: Alpine Treelines. Berlin: Springer, 220 pp.
- Körner, C. , and Cochrane, P. M. , 1983: Influence of plant physiognomy on leaf temperatures on clear midsummer days in the Snowy Mountains, south-eastern Australia. Acta Oecologia Plantarum , 4: 117–124.
- Körner, C. , and Cochrane, P. M. , 1985: Stomatal responses and water relations of Eucalyptus pauciflora in summer along an elevational gradient. Oecologia , 66: 443–455.
- Körner, C. , and Demoraes, J. , 1979: Water potential and diffusion resistance in alpine cushion plants on clear summer days. Oecologia Plantarum , 14: 109–120.
- Körner, C. , Allison, A. , and Hilscher, H. , 1983: Altitudinal variation in stomatal conductance, nitrogen content and leaf anatomy in heliophytes of montane New Guinea and their interrelation with microclimate. Flora , 174: 91–135.
- Larcher, W. , 2012: Bioclimatic temperatures in the high Alps. In Lütz, C. (ed.), Plants in Alpine Regions: Cell Physiology of Adaptation and Survival Strategies. New York: Springer-Verlag, 21–27.
- Meinzer, F. C. , Goldstein, G. , and Rundel, P. W. , 1994: Comparative water relations of tropical alpine plants. In Rundel, P. W. , Smith, A. P. , and Meinzer, F. C. (eds.), Tropical Alpine Environments. Cambridge: Cambridge University Press, 61–76.
- Moser, W. , Brzoska, W. , Zachhuber, K. , and Larcher, W. , 1977: Ergebnisse des IBP-Projekts “Hoher Nebelkogel 3184m. ” Sitzungsberichte/Österreichische Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftliche Klasse Abteilung I, Biologische Wissenschaften und Erdwissenschaften, 186: 387–419.
- NOAA , 2013: Cold and warm episodes by season. National Oceanic and Atmospheric Administration, <http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ensoyears.shtml>, accessed 20 September, 2013.
- Parque Nacional Natural Chingaza , 2005–2009: Plan estratégico y de manejo del Parque Nacional Natural Chingaza. <http://www.parquesnacionales.gov.co/PNN/portel/libreria/pdf/ParqueCHINGAZA.pdf>, accessed 15 February 2013.
- Pearcy, R. W. , 1989: Radiation and light measurements. In Pearcy, R. W. , Ehleringer, J. R. , Mooney, H. A. , and Rundel, P. W. (eds.), Plant Physiological Ecology: Field Methods and Instrumentation. London: Chapman and Hall, 97–116.
- Pearcy, R. W. , and Way, D. A. , 2012: Two decades of sunfleck research: looking back to move forward. Tree Physiology , 32: 1059–1061.
- Pellicciotti, F. , Raschle, T. , Huerlimann, T. , Carenzo, M. , and Burlando, P. , 2011: Transmission of solar radiation through clouds on melting glaciers: a comparison of parameterizations and their impact on melt modeling. Journal of Glaciology , 57: 367–381.
- Salisbury, F. B. , and Spomer, G. G. , 1964: Leaf temperatures of alpine plants in the field. Planta , 60: 497–505.
- Sarmiento, G. , 1986: Ecologically crucial features of climate in high tropical mountains. In Vuilleumier, F. , and Monasterio, M. (eds.), High Altitude Tropical Biogeography. Oxford: Oxford University Press, 11–45.
- Scherrer, D. , and Körner, C. , 2010: Infra-red thermometry of alpine landscapes challenges climatic warming projections. Global Change Biology , 16: 2602–2613.
- Smith, A. C. , and Koch, M. F. , 1935: The genus Espeletia. Brittonia , 1: 479–530.
- Smith, A. P. , and Young, T. P. , 1987: Tropical alpine plant ecology. Annual Review of Ecology and Systematics , 18: 137–158.
- Smith, W. K. , and Berry, Z. C. , 2013: Sunflecks? Tree Physiology , 33: 233–237.
- Smith, W. K. , and Geller, G. N. , 1980: Leaf and environmental parameters influencing transpiration at high elevation: theory and field measurement. Oecologia , 46: 308–314.
- Taschler, D. , and Neuner, G. , 2004: Summer frost resistance and freezing patterns measured in situ in leaves of major alpine plant growth forms in relation to their upper distribution boundary. Plant Cell and Environment , 27: 737–746.
- Tol, G. J. , and Cleef, A. M. , 1994: Above-ground biomass structure of a Chusquea tessellata bamboo páramo, Chingaza National Park, Cordillera Oriental, Colombia. Vegetatio , 115: 29–39.