Abstract
Global temperature increase would seem likely to result in general upwards shifts of altitudinal margins of tree stands. However, range expansion of trees could be significantly affected by both negative and positive interactions with alpine shrubs in existing treeline ecotones. We examined the effects of dwarf pine (Pinus mugo) shrubs on the vegetative propagation and height growth of Norway spruce (Picea abies) trees in the treeline ecotone of the Hrubý Jeseník Mountains, Czech Republic. Here, the non-native dwarf pine was planted above timberline during the 19th and 20th centuries. In the treeline ecotone, vegetative propagation is important both for generation of clonal groups from seed-originated individuals and for persistence of such stands. We found that increasing density of dwarf pine stands strongly reduced vegetative propagation of spruce, as shown by the spruce clonal groups surrounded by dense pine having fewer layering branches and ramets than such groups outside pine stands. This has likely resulted from competitive pressure of pine causing decreased spruce layering mainly through mechanical damage and shading. In contrast, dense pine stands increased spruce height growth, presumably by providing shelter against wind and/or browsing. Our results indicate that interactions of prostrate dwarf pine and Norway spruce clonal groups include both competitive and facilitative components, which probably change in importance along climatic stress gradients.
Introduction
The alpine treeline ecotone represents a prominent temperature-driven altitudinal boundary of forests in mountain regions (CitationHoltmeier, 2009). Along the gradient of increasing stress due to temperature, trees lose their competitive advantage over low, prostrate shrubs (CitationKörner, 2012). Whereas tall trees are thermally coupled with the ambient atmosphere, prostrate shrubs profit from near-ground heating of the surface air layer (CitationKörner, 1998; CitationGeiger et al., 2003). Therefore, direct temperature limitation of tree growth is not the only major driver of treeline ecotone dynamics, as the interaction between upright tree species and prostrate shrubs can also have a particularly strong influence (CitationDullinger et al., 2004; CitationDufour-Tremblay et al., 2012). The stress-gradient hypothesis (CitationCallaway et al., 2002; CitationHe et al., 2013) predicts that prostrate shrubs should have prevalently competitive effects on trees in the lower part of treeline ecotone, with facilitation more common towards the tree species limit (e.g. CitationMaestre et al., 2009).
Indeed, a range of positive and negative effects of prostrate shrubs on treeline trees has been documented. Positive effects can include protection against herbivorous insects and mammals (CitationDullinger et al., 2005; CitationGrau et al., 2010), frost (CitationMichiels, 1993), and strong winds (CitationVacek et al., 2012). In particular, these positive effects facilitate tree seedling survival and performance (CitationCamarero and Gutiérrez, 1999; CitationGómez-Aparicio et al., 2005; CitationGrau et al., 2010). On the other hand, prostrate shrubs compete with trees for light (CitationJeník and Lokvenc, 1962), nutrients, and water (CitationSchönenberger, 1975; CitationWeih and Karlsson, 2002; CitationGrau et al., 2012) and can reduce tree germination success (CitationDullinger et al., 2005) and cool surface microclimates during the growing season (CitationSvoboda, 2001; CitationKöck et al., 2003). Trees can have negative effects on prostrate shrubs, with their overstory overgrowing them, thereby likely limiting the responses of shrub species to increasing temperatures (Švajda et al., 2011; CitationBoudreau and Villeneuve-Simard, 2012).
In the treeline ecotone, trees often occur in clonal groups (CitationBliss, 1971; CitationTranquillini, 1979; CitationLaberge et al., 2000). Indeed, vegetative reproduction is an important strategy enabling trees to form and maintain stands in environments in which seedling growth and survival are limited by cold (CitationHoltmeier, 2009). Thus, alpine and northern treeline ecotones often contain clonally reproduced tree groups surrounded by prostrate shrubs (CitationHarsch and Bader, 2011; CitationGrau et al., 2012). For example, treeline ecotones in western North American mountains contain Abies lasiocarpa, Picea engelmannii, and shrubby Chamaecyparis nootkatensis (CitationBrooke et al., 1970; CitationArno and Hammerly, 1984); those in the Carpathians include Picea abies and Juniperus communis ssp. alpina (CitationMihai et al., 2007); and those in central Kamchatka have Larix gmelinii and Pinus pumila (CitationOkitsu, 1998).
Norway spruce (Picea abies L., Karst) and dwarf pine (Pinus mugo, Turra) play the roles, respectively, of clonal group-forming tree and prostrate shrub species in treeline ecotones of Central Europe. There, Norway spruce is the most abundant treeline-forming species (CitationScotti et al., 2008; CitationTreml and Banaš, 2008; CitationHertel and Schöling, 2011). In the treeline ecotone, the reduced sexual reproduction of Norway spruce is replaced by layering, in which new ramets are generated by the rooting of plagiotropic branches of the parent tree (CitationKuoch and Amiet, 1970; CitationTranquillini, 1979; CitationKozlowski, 2002; CitationŠenfeldr and Maděra, 2011; CitationVacek et al., 2012). Prostrate dwarf pine occurs in alpine areas of Europe from the Pyrenees to the Balkan peninsula, and is a widespread species in the altitudinal belt above the upper limit of closed forest in the eastern Alps, Sudetes, and Carpathians (CitationNagy et al., 2003; CitationÚradníček et al., 2010).
The distribution and altitudinal limits of Norway spruce and dwarf pine have been strongly influenced by past agricultural activities such as grazing, grass mowing, burning, and logging (CitationDirnböck and Grabherr, 2000; CitationSitko and Troll, 2008). Thus, treeline ecotones were shifted downwards and dwarf pine stand distribution became more scattered between the 11th and 18th centuries (CitationTreml et al., 2008). Later, from the second half of the 19th century through the mid-20th century, dwarf pine was frequently planted in deforested or steeply sloping mountain areas to protect soil against mass movement and surface erosion (CitationBukovčan, 1960; CitationHošek, 1964; CitationSouček and Špulák, 2011; CitationRoštínský et al., 2013). This occurred in the Hrubý Jeseník Mountains (eastern part of the Sudetes Mountains) where the pine was planted as a non-indigenous species on summit forest-free areas. There, some Norway spruce clonal groups are surrounded by dwarf pine stands of known planting date, whereas others are not. This provides a special opportunity to examine the effects of prostrate dwarf pine on dynamics of Norway spruce clonal groups by comparing the spruce groups inside and outside dwarf pine stands.
To date, the literature on interactions between Norway spruce and dwarf pine only reflects examination of the effects of the pine on spruce sexual reproduction and height growth (CitationDullinger et al., 2005). Studies of similar tree—shrub systems from alpine treeline ecotones indicate both competition among species of different sizes and different competition strategies and facilitation through protective effects of shrubs at early life stages of tree seedlings on extreme sites (CitationAnthelme et al., 2003, CitationTakahashi, 2003, CitationGrau et al., 2012). However, no published study has investigated the effect of prostrate shrubs on vegetative reproduction of treeline trees. Since vegetative reproduction is important for the persistence and spread of spruce at its upper distributional limit, in the present study we examine (1) the influence of dwarf pine stand density on indicators of spruce clonal group vegetative reproduction ability, and (2) the effect of distance from dwarf pine shrubs on actual spruce vegetative reproduction.
Material and Methods
STUDY SITES
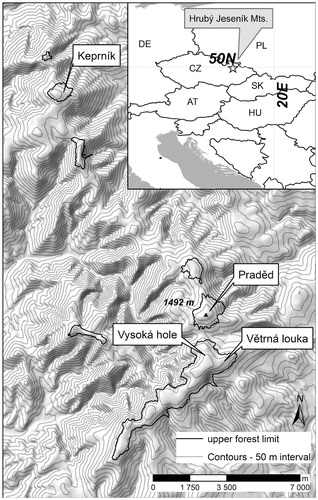
Our study sites (Praděd, Keprník, Větrná Louka, and Vysoká Hole) are situated on the highest peaks of the Hrubý Jeseník Mountains, and comprise all the locations on these mountains in which spruce groups and planted dwarf pine co-occur (see ). The Hrubý Jeseník Mountains reach their maximum elevation at Mount Praděd, at 1492 m a.s.l. (). The climate is relatively cold and humid, with the summit areas characterized by annual precipitation of around 1400 mm and average temperature around 1.1 °C (CitationTolasz et al., 2007). The mountain tops of the Hrubý Jeseník Mountains are among the windiest locations in Europe (CitationMigala, 2005).
The highest elevations are thought to have been naturally forest-free (CitationJeník, 1961), but the extent of alpine meadows was significantly enlarged by human activities (CitationNovák et al., 2010), e.g. grass mowing, cattle grazing, and woodland burning and logging. The average altitude of the upper limit of closed forest is 1300 m a.s.l. Above this, scattered Norway spruce groups occur. Spruce groups typically consist of one seed-originated parent tree accompanied by variable numbers of its ramets. Indeed, within these groups, trees of clonal origin clearly dominate, accounting for 90–95% of the trees (CitationŠenfeldr and Maděra, 2011). Therefore, we term the spruce groups “clonal groups.” Nevertheless, sparse sexual reproduction is present at treeline, with seedlings of height 10–80 cm at a density of 25–39 specimens per hectare found at the study sites (CitationŠenfeldr and Maděra, 2011).
FIGURE 1. Topographic map showing location of study sites and elevation of the upper forest limit in the Hrubý Jeseník Mountains; inset shows position of the Hrubý Jeseník Mountains in Europe.
Dwarf pine was planted on these mountains during the 19th and 20th centuries, mostly between 1874 and 1928, at spacings of 1.25 × 1.25 m to 2 × 2 m square (CitationHošek, 1964). This species now covers 179.2 ha (6.8%) of the area above the upper limit of closed forest (CitationTreml et al., 2010). Since designation of the Šerák-Keprník and Praděd nature reserves in 1955, both Norway spruce populations and dwarf pine stands have developed spontaneously, without any direct human intervention. Of particular conservation concern, dwarf pine stands rapidly expanded into surrounding alpine grasslands, leading to a loss of rare alpine plants and insects (CitationKuras et al., 2001; CitationTreml et al., 2010; CitationZeidler et al., 2012).
FIELD DATA COLLECTION
To examine the effects of dwarf pine density on spruce clonal growth characteristics, we distinguished three types of pine stands (see ) based on pine canopy cover: no pine presence (type “no-pine”), sparse pine (pine cover 20–70%; type “sparse”), and dense pine (pine cover 70–100%; type “dense”). Precise information about dwarf pine cover was obtained from orthorectified images with 0.25 m resolution, using supervised classification followed by manual error correction. Although we searched for all types of stands at each site, two sites lacked dense pine stands (Praděd and Větrná Louka) and one lacked sparse pine stands (Vysoká Hole) (). Cover of dwarf pine stands was usually related to their age, with some deviations caused by site factors and rates of seedling establishment (CitationTreml et al., 2010).
FIGURE 2. Photographs showing (A) extensive clonal spruce groups in a no-pine plot; (B) detail of a clonal group with layering branches growing in a no-pine plot; (C) spruce groups growing in a dense pine stand; and (D), close mechanical contact of pine with spruce resulting in absent layering branches in a dense pine stand.
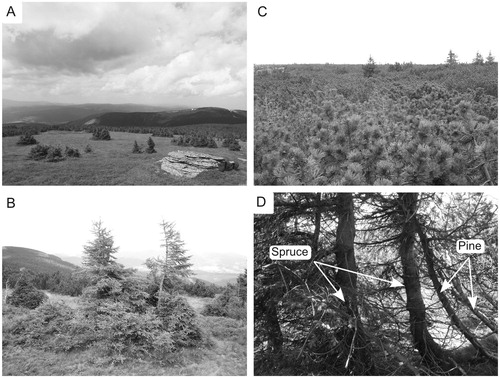
In each study site, at similar altitudes, we randomly placed 4 to 9 circular 30-m-diameter sample plots in each pine stand type (no-pine, sparse, dense). In each of these plots, data were recorded from all clonal spruce groups and solitary spruce trees. For study design simplicity, each solitary tree was treated as a clonal group. For each clonal group, the number of layering branches, the total number of trees, and the height of the highest tree in the group (maximum tree height) were recorded. Additionally, to detect possible effects of dwarf pine on juvenile spruce stem-breaks, for each tree growing in the dense and no-pine plots, we recorded the presence/absence of multiple stems, up to the height of 130 cm (roughly corresponding to the average height of dwarf pine stands).
Next, we randomly chose two spruce clonal groups on each plot for age structure analysis. Two groups per plot were sufficiently representative because across sites, the average number of clonal groups per plot was 3.8 ± 0.8 SD; (n = 60). Within each of these groups, all trees were cored using a Pressler borer. Cores were taken from the stem base (5 to 20 cm above ground). Tree rings were measured on a positioned table. If off-center cores were collected, we used the age correction method employing the mean annual width of the five rings nearest the pith (CitationBatllori and Gutiérrez, 2008). Coring height correction was based on mean height growth rate of seedlings and juvenile ramets (height 10–200 cm; Šenfeldr, Treml—unpublished data). We considered the following three variables derived from age structure analysis: mean age, age of the oldest tree, and number of juvenile ramets (i.e. 20 years old or younger).
The numbers of layering branches and juvenile ramets can serve as indicators of spruce vegetative reproduction intensity, as groups with relatively high vegetative reproduction are characterized by numerous juvenile ramets and layering branches and by low mean age. Additionally, the overall number of trees within a group provides an indicator of long-term vegetative reproduction ability (CitationKuoch and Amiet, 1970). The maximum tree height within each clonal group was recorded to evaluate effects of pine presence on spruce height growth.
TABLE 1 Basic characteristics of sample plots in the four study sites above upper forest limit in the Hrubý Jeseník Mountains.
Further, we assessed the effect of the distance between dwarf pine margins and spruce group crown margins (hereinafter referred as “distance to pine”) on the presence/absence of spruce layering branches. Spruce groups were placed in four distance classes: 0–1 m, 1–3 m, 3–5 m, and 5–7 m, with the corresponding mid-range values of 0.5 m, 2 m, 4 m, and 6 m used in the analysis. The distances to the nearest dwarf pine margin were evaluated in the four cardinal directions, and only those spruce groups having distance values within these classes in all four directions were regarded as suitable for analysis. At each site (excluding Keprník), 10 such clonal groups in each distance class were chosen, and the presence or absence of layering branches in each of the clonal groups was recorded.
DATA ANALYSIS
The data from our sample plots had a hierarchical structure (sample plot/clonal group/tree). To avoid pseudoreplication and simplify analyses (CitationMurtaugh, 2007), we generally used within-plot average values for analyses at the plot level. The exception was the probability analysis of the relationship between distance to pine and layering branch occurrence, due to its different sampling design.
In analyzing our data, we first performed principal component analysis (PCA) to evaluate the relationships among studied spruce clonal group characteristics and also the effect of pine stand type (no-pine/sparse/dense) on the whole set of spruce clonal group characteristics. Data were scaled to unit variance before using PCA. To assess the effect of pine stand type, we fitted this factor onto the first two main components of the ordination. Its significance was tested using a permutation test.
To test differences within studied clonal spruce group characteristics (mean age, age of the oldest tree, maximum height, number of layering branches, and number of juvenile ramets), we used linear mixed effect (LME) models with restricted maximum likelihood (REML), treating pine stand type as a fixed effect and site as a random effect. To evaluate the significance of site effects, we also fitted a simpler model with only pine stand type (i.e., with no random effect) using generalized least squares (GLS) REML estimation. We used likelihood ratio tests and Akaike´s Information Criterion (AIC) to compare this GLS model with the more complex LME model (see CitationZuur et al., 2009). To account for heteroscedasticity of our dependent variables, we did square-root transformation of all dependent variables except age of the oldest tree. Because maximum height was correlated with mean age, a separate analysis of a subset of trees of similar age (60–80 years) was conducted. This allowed us to evaluate the effect of pine stand type on maximum height independent of mean age.
The effect of distance to pine on the probability of spruce layering was analyzed using generalized linear models (binomial family and probit link function) with distance to pine as the explanatory variable and probability of layering as the dependent variable. WALD Z was used to evaluate the significance of distance to pine.
All statistical analyses were carried out using R statistical environment version 2.14 (CitationR Development Core Team, 2011). The ‘vegan’ package (CitationOksanen et al., 2012) was used for multivariate analysis, the ‘nlme’ package (CitationPinheiro et al., 2013) for LME and GLS, and the ‘lattice’ package (CitationSarkar, 2008) for boxplot construction.
Results
PRINCIPAL COMPONENT ANALYSIS
Data on 380 clonal spruce tree groups comprising 1508 trees were collected from a total of 60 plots at the four sites (). The PCA ordination diagram for the six clonal spruce group characteristics showed a clear trend from the dense pine stand type to the no-pine stand type along the first principal component (). The effect of pine stand type on the studied characteristics was significant (p < 0.001). The numbers of juvenile ramets, layering branches, and trees in a group were positively correlated with the first principal component and tended to be higher in no-pine stands. In contrast, maximum height and mean age were negatively correlated with the first principal component and tended to be higher in dense pine stands. The ages of the oldest trees tended to be greater in dense stands than in sparse stands, with this variable distinct in that it was correlated with the second principal component, standing apart from the other variables. The ordination plot showed also strong positive correlations between maximum height and mean age and between number of trees in a group and number of layering branches.
FIGURE 3. Principal component analysis (PCA) ordination plot showing projections of sample plot and clonal spruce groups characteristics. Sample plots in different pine stand types (dense, sparse, no-pine) are distinguished using a spider plot. Dispersion ellipses for pine stand types are plotted using standard deviations of point scores. The first PCA axis explains 52% of variance, and the second explains 20% of variance.
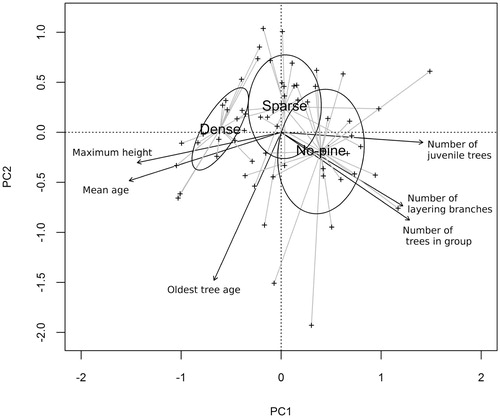
TABLE 2 Evaluation of site effects on clonal spruce group chracteristics; likelihood ratio test and Akaike's Information Criterion (AIC) were used to compare simpler model without site effect (fitted using generalized least squares [GLS]) and model with site linear mixed effect (LME). Results show site effects that were not significant in any case.
DIFFERENCES IN AGE-RELATED VARIABLES AND TREE HEIGHT
At all sites and in all pine stand types, the spruce populations were younger than the surrounding pine stands (compare pine stand age in and age of oldest spruce tree in ). The age differences between the spruce (oldest tree in group, indicating establishment date) and surrounding pine stands ranged from 10 (dense pine, Keprník sites) to 60 years (sparse pine, Praděd site). Thus, most of the spruces have been growing, since their early ontogenetic stages, inside gradually closing pine stands.
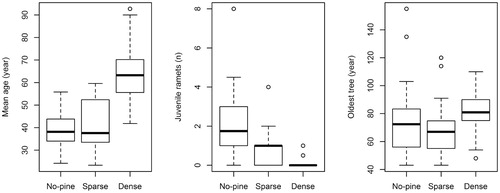
The effect of pine stand type was significant for all variables except the age of the oldest spruce (). In contrast, a site did not have a significant effect on any studied variable (). Mean spruce age was greater in dense pine stands than in no-pine stands and sparse pine stands (). The number of juvenile ramets showed the opposite trend. It was lower in dense pine stands (with almost no juveniles) and higher in no-pine stands (on average, two juvenile ramets per spruce clonal group) (). The age of the oldest tree tended to be higher in dense pine stands, but this trend was not significant (). Overall, the oldest tree was 155 years old, and it was found in a no-pine stand at Keprník (). Stem breaks were more frequent in no-pine plots (55%) than dense pine plots (20%; p < 0.001, t-test). The maximum height of spruce trees in dense pine stands was significantly greater than those of no-pine and sparse pine plots (, ). The height findings were similar to the trend for mean age, which is not surprising, as maximum tree height was correlated with mean age (r = 0.75). However, this trend was also found in the subset of spruce trees of similar age (60–80 years, n = 28). In this subset, trees in dense pine stands were significantly taller (mean height [cm]: 441 ± 72 SD) than trees in no-pine stands (mean height [cm]: 367 ± 56 SD; F = 7.4, p < 0.01).
FIGURE 4. Boxplots of mean age, number of juvenile ramets (20 years old or younger), and age of the oldest tree for spruce groups in each pine stand type (dense, sparse, no-pine). The horizontal line in each box represents the median; the hinges represent the 25th and 75th percentiles; the whiskers represent 1.5 times the interquartile range; open circles represent values outside this interval.
NUMBER OF TREES AND NUMBER OF LAYERING BRANCHES
The numbers of trees and layering branches were both significantly affected by pine stand type (), showing similar trends to the number of juvenile ramets: numbers low in dense pine, higher in sparse pine, and even higher in no-pine stands (). At the site level, the average number of trees per group ranged from 3 at Keprník (dense pine stand) to 11 at Praděd (no-pine stands). Layering branches occurred in 41–80% of the clonal spruce groups in no-pine stands, in 20–39% in sparse pine stands, and in 6–14% in dense pine stands. The highest number of layering branches for a single clonal spruce group was 20 in a no-pine stand at Praděd ().
TABLE 3 Effects of pine stand type (referred to as “pine” in table) on clonal spruce group characteristics. Because site effect was not significant, F and p-values from only GLS models are shown. Pine stand type had significant effects on all studied characteristic except the age of the oldest tree.
EFFECT OF DISTANCE TO PINE ON LAYERING PROBABILITY
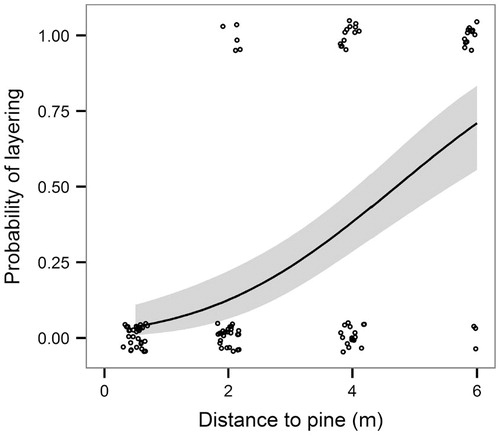
The probability of layering, calculated as the number of spruce clonal groups having layering branches divided by total number of spruce groups in the distance class, was significantly affected by the distance between the spruce group crown and dwarf pine crown margin (p <0.001; Wald Z value = -5.603). None of the spruce groups in the distance class 0–1 m had any layering branches, resulting in zero layering probability in this distance class. The probability of layering increased with increasing distance to pine: from 17% in the 1–3 m distance class, to 43% in the 3–5 m class, and finally to 67% in the 5–7 m class ().
FIGURE 5. Boxplots of number of trees in a clonal group, number of layering branches, and maximum height for spruce groups in each pine stand type (dense, sparse, no-pine). The horizontal line in each box represents the median; the hinges represent the 25th and 75th percentiles; the whiskers represent 1.5 times the interquartile range; open circles represent values outside this interval.
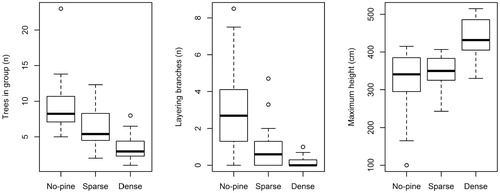
FIGURE 6. Spruce branches layering probability in relation to distance between spruce tree crown and pine shrub crown margins. Layering probability is gradually increasing from zero to 67% with increasing distance to pine. Gray area is within 95% confidence intervals. Small circles (slightly jittered to avoid overplotting) represent the observed values.
Discussion
Our results clearly show that vegetative propagation of spruce was strongly affected by the density of surrounding dwarf pine stands. In particular, this was demonstrated by the spruce populations in pine stands being older, with fewer juveniles, than the spruce groups surrounded by alpine meadows. Furthermore, in dense pine stands the reduced occurrence of layering branches indicated the lower potential for vegetative propagation (see CitationKuoch and Amiet, 1970; CitationSchönenberger, 1981; CitationTranquillini, 1979).
We suggest that the scarcity of layering branches probably resulted from a combination of the competitive pressure of closely occurring pine (causing light deficiency, CitationSoukupová et al., 2001; CitationWild and Wildová, 2002; CitationDullinger et al., 2005) and increased branch mortality from prolonged snow cover and wetter microclimate in pine stands (CitationCulek, 2012). Indeed, both the snow cover prolongation and increased moisture can lead to fungal infection (CitationVacek et al., 2012). The mortality of actual or potential layering branches could also be increased by mechanical damage from prostrate branches of dwarf pine (see , part D). In fact, we have observed high dieback of spruce branches in dense pine stands in the study area. The relative importance of the various explanatory phenomena is likely to differ among the distance classes, as they would operate over different distances. Thus, mechanical damage would likely have been especially important in the class of shortest distances to pine margins (0–1 m), thereby at least partly explaining the zero layering probability found for that class. However, at this distance, shading (CitationWild and Wildová, 2002; CitationDullinger et al., 2005) and snow cover prolongation (CitationCulek, 2012) also likely contributed to the extremely low layering probability. The reduced layering probability of spruces even at relatively large distances from pine (3–5 m), about 25% less than for those in meadows, can probably be ascribed largely to snow prolongation and to a lesser degree to shading. Apparently, none of these mechanisms would operate at distances greater than 6 m, as no effect on spruce layering was observed.
Layering success is also known to be affected by ground vegetation (CitationArno and Hammerly, 1984; CitationVacek et al., 2012), waterlogging (CitationVacek et al., 2012), and soil moisture scarcity, but none of these is likely to have substantially influenced our observed outcomes. In particular, increasing dwarf pine cover is characterized by increasing dominance of ground vegetation cover by Avenella flexuosa (CitationZeidler et al., 2012). However, ground vegetation dominated by this species is associated with very low mortality of spruce layering branches (CitationVacek et al., 2012). Therefore, such a change in vegetation cover could not underlie the inhibitory effect we found of dwarf pine on spruce layering (if anything, it would have reduced the strength of this effect). Similarly, mortality of juvenile ramets is higher in waterlogged areas (CitationVacek et al., 2012), but our sites were not waterlogged (as shown by the absence of hygrophilous vegetation). Too little soil moisture can also limit vegetative reproduction of some woody species in alpine areas (e.g., Salix and Rhododendron at sites in the Alps having substrate that does not retain water), with adventitious root development strongly dependent on available soil moisture (CitationKörner, 2003). However, in our study system, there is unlimited water availability in the root zone soil substrate during the entire growing season (CitationŠenfeldr et al., 2013).
In contrast to hampering the vegetative reproduction of spruce, dense pine stands positively affected spruce height growth. As shown by the lower ages of spruce groups compared to their surrounding pine stands, many of these spruces have been growing in gradually increasing pine cover. The improved height growth in the dense pine stands might be related to decreased browsing pressure from herbivores (CitationRao et al., 2003; CitationRussell and Fowler, 2004, CitationDullinger et al., 2005) and/or lower wind abrasion of aboveground biomass in the juvenile ontogenetic phases. The latter explanation in particular is supported by our finding of lower numbers of broken stems in dense pine plots in comparison to no-pine plots. Protection against frost (CitationMichiels, 1993) might also have played a role. We suggest that these benefits accelerated height growth at least until the shrub layer was overtopped, with the added growth retained such that trees that lacked this protection as juveniles would not catch up. We do not believe that the higher spruce growth was the result of competition for light and growing space with pine, because we find no differences in spruce tree slenderness coefficients between dense pine and no-pine plots (not shown). In most cases, gaps in pine stands were probably large enough for the juvenile phase of the oldest trees in a group not to experience light deficiencies. According to Wild and Wildová (Citation2002), the negative effect of pine on low-stature plants is manifested only within a ca. 0.4- to 0.6-m-wide buffer zone along the pine margin. In our study, it appears that the benefit from sheltering by dwarf pine outweighed possible suppression due to competition for light and nutrients. These findings are in contrast to results from the eastern Alps, where poorer height growth was found in pine stands than in meadows. This dissimilarity is probably related to stronger effects (abrasion, breaks) of wind on alpine/subalpine ecosystems in the Sudetes than in the Alps (see CitationTreml et al., 2012). Besides strong winds, high population densities of browsers (red deer [Cervus elaphus]) in the treeline areas of the Sudetes might be key height growth-limiting factors for Norway spruce in its early ontogenetic stages. Our finding of a positive effect of dwarf pines on spruce height growth along with a negative effect on vegetative propagation is consistent with the overall view that interactions between treeline species can have both positive and negative components (CitationCallaway and Walker, 1997; CitationSong et al., 2010).
At the upper forest limit in the study area, the spruce trees occur as “competitive stress tolerators” (C-S strategists, sensu CitationBrzeziecki and Kienast, 1994) in dense sexual populations, reaching heights of about 10 m (CitationTreml, 2007). There, the pine-spruce interaction is mainly competitive, and, as documented from many areas, spruce is gradually overgrowing pine stands (CitationJeník and Lokvenc, 1962; CitationDullinger et al., 2005; Švajda et al., 2011; CitationŠenfeldr et al. 2012). In the upper part of the treeline ecotone, this interaction switches to at least partly facilitative, with pine, representing strong “stress tolerators” (S — strategist, sensu CitationBrzeziecki and Kienast, 1994) benefitting the height growth of spruce at its range limit (CitationKörner, 2012). However, this facilitative role is ambiguous, since spruce layering is suppressed. This pattern is consistent with special cases of the stress-gradient hypothesis (CitationMaestre et al., 2009) posed by competition between C-S and S strategists (CitationWang et al., 2008). In our study system, different spruce morphotypes are represented by high stature sexual populations and low stature clonal populations (CitationSchöb et al., 2013). Moreover, changes between competition and facilitation can also occur during the ontogenies of interacting species (CitationMiriti, 2006). Taking these considerations into account, the pine-spruce interactions along the alpine treeline ecotone can be understood within the framework of the stress-gradient hypothesis (CitationCallaway et al., 2002).
Most treelines in Europe have recently been subjected to forest and shrub invasion following the cessation of mountain agriculture, along with climate amelioration (CitationAnthelme et al., 2003; CitationGehrig-Fasel et al., 2007). Generally, for upward shifts of island-form, wind-affected treelines, as found in the study area, the combination of both sexual and vegetative reproduction is needed (CitationHoltmeier, 2009; CitationŠenfeldr and Maděra, 2011). Our findings suggest that potentially quick upward expansion of forest within the zone adjacent to the current upper forest limit is likely to be slowed by closed dwarf pine stands, since they hamper both seedling recruitment of spruce (CitationDullinger et al., 2005) and spruce layering. In the upper part of the treeline ecotone at climatically extreme sites, the interactions are complex as shown by our documentation of both facilitative and competitive effects of dwarf pine on spruce establishment and growth. Future scenarios of spruce-dwarf pine interactions will strongly depend on the texture of dwarf pine stands and the availability of space for spruce germination (CitationWild and Winkler, 2008). Moreover, although interactions of spruce and dwarf pine within the treeline ecotone may follow their current patterns, they will probably also depend on differing individual responses of both species to climate change (CitationWalther et al., 2002).
Conclusions
Our results show that tree-shrub interactions at wind-affected treelines significantly determine dynamics of clonal tree groups. We found that the distance between spruce trees and surrounding dwarf pine proved to be a key limiting factor of the spruce's vegetative reproduction. As a result of strong competitive pressure of dwarf pine, the numbers of layering branches and juvenile spruce ramets decreased with increasing pine stand density. On the other hand, spruce height growth was facilitated in dense pine stands. This study indicates that both competition and facilitation between shrubs and trees will influence dynamics of the alpine treeline ecotone. The expansion of spruce coverage will probably be slowed significantly at sites with dense dwarf pine stands adjacent to the current upper forest limit. At climatically more extreme sites, facilitative interactions should also be considered. Similar patterns of interactions between shrubs and trees are likely to occur at treelines involving other tree and shrub species. Thus, the results of this study can contribute to the understanding of processes driving treeline dynamics, not only in the particular study system, but also more generally, especially in the context of treeline responses to climatic change.
Acknowledgments
This paper was supported by the Internal Grant Agency of the Faculty of Forestry and Wood Technology at Mendel University in Brno (projects 38/2010) and by the project for creation and development of a multidisciplinary landscape team (no. CZ.1.07/2.3.00/20.0004), with financial contribution from the European Union and the state budget of the Czech Republic. V. Treml was supported by project GA ČR P504/11/P557. The authors wish to thank J. Rosenthal for improving the English. Additionally, we are grateful to T. Kyncl for initial suggestions and help in the field.
References Cited
- Anthelme, F. , Michalet, R. , Barbaro, L. , and Brun, J. J. , 2003: Environmental and spatial influences of shrub cover (Alnus viridis DC.) on vegetation diversity at the upper treeline in the inner western Alps. Arctic, Antarctic, and Alpine Research , 35: 48–55.
- Arno, S. F. , and Hammerly, R. P. , 1984: Timberline. Mountain and Arctic Forest Frontiers. Seattle: The Mountaineers, 304 pp.
- Batllori, E. , and Gutiérrez, E. , 2008: Regional tree line dynamics in response to global change in the Pyrenees. Journal of Ecology, 96: 1275–1288, doi: <http://dx.doi.org/10.1111/j.1365-2745.2008.01429.x>.
- Bliss, L. C. , 1971: Artic and alpine plant life cycles. Annual Review of Ecology, Evolution, and Systematics , 2: 405–438.
- Boudreau, S. , and Villeneuve-Simard, M. P. , 2012: Dendrochronological evidence of shrub growth suppression by trees in a subarctic lichen woodland. Botany , 90: 151–156, doi: <http://dx.doi.org/10.1111/j.1365-2486.2012.02708.x>.
- Brooke, R. C. , Peterson, E. B. , and Krajina, V. J. , 1970: The Subalpine Mountain Hemlock Zone: Subalpine Vegetation in Southwestern British Columbia, its Climatic Characteristics, Soils, Ecosystems and Environmental Relationships. Volume 2. Department of Botany, University of British Columbia, 147–349.
- Brzeziecki, B. , and Kienast, F. , 1994: Classifying the life-history strategies of trees on the basis of the Grimian model. Forest Ecology and Management , 69: 167–187.
- Bukovčan, V. , 1960: Laviny a lesy [Avalanches and Forest]. Bratislava, Slovakia: Slovenské vydavateľstvo pôdohospodárskej literatúry, 196 pp.
- Callaway, R. M. , and Walker, L. R. , 1997: Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology , 78: 1958–1965.
- Callaway, R. M. , Brooker, R. W. , Choler, P. , Kikvidze, Z. , Lortie, C. J. , Michalet, R. , Paolini, L. , Pugnaireq, F. I. , Newingham, B. , Aschehoug, E. T. , Armasq, C. , Kikodze, D. , and Cook, B. J. , 2002: Positive interactions among alpine plants increase with stress. Nature , 417: 844–848.
- Camarero, J. J. , and Gutiérrez, E. , 1999: Structure and recent recruitment at alpine forest-pasture ecotones in the Spanish central Pyrenees. Ecoscience , 6: 451–464.
- Culek, M. , 2012: Vliv borovice kleče na klima, hydrické a nivální procesy [Effect of dwarf pine on climate and hydrological processes]. In Šenfeldr, M. , Maděra, P. , and Buček, A. (eds.), Kleč v horské krajině Hrubého Jeseníku [Dwarf pine in the mountain landscape of the Hrubý Jeseník Mts.]. Brno, Czech Republic: CERM, Geobiocoenological papers 16, 69–138.
- Dirnböck, T. , and Grabherr, G. , 2000: GIS assessment of vegetation and hydrological change in a high mountain catchment of the Northern Limestone Alps. Mountain Research and Development , 20: 172–179.
- Dufour-Tremblay, G. , De Vriendt, L. , Lévesque, E. , and Boudreau, S. , 2012: The importance of ecological constraints on the control of multi-species treeline dynamics in eastern Nunavik, Québec. American Journal of Botany , 99: 1638–1646.
- Dullinger, S. , Dirnböck, T. , and Grabherr, G. , 2004: Modelling climate change-driven treeline shifts: relative effects of temperature increase, dispersal and invasibility. Journal of Ecology , 92: 241–252.
- Dullinger, S. , Dirnbock, T. , Kock, R. , Hochbichler, E. , Englisch, T. , Sauberer, N. , and Grabherr, G. , 2005: Interactions among tree-line conifers: differential effects of pine on spruce and larch. Journal of Ecology , 93: 948–957.
- Gehrig-Fasel, J. , Guisan, A. , and Zimmerman, N. E. , 2007: Tree line shifts in the Swiss Alps: climate change or land abandonment? Journal of Vegetation Science , 18: 571–582.
- Geiger, R. , Aron, R. H. , Todhunter, P. , 2003: The Climate near the Ground. Lanham: Rowman and Littlefield Publishers, 584 pp.
- Gómez-Aparicio, L. , Valladares, F. , Zamora, R. , and Luis Quero, J. , 2005: Response of tree seedlings to the abiotic heterogeneity generated by nurse shrubs: an experimental approach at different scales. Ecography , 28: 757–768.
- Grau, O. , Ninot, J. M. , Blanco-Moreno, J. M. , Logtestijn, R. S. P. , Cornelissen, J. H. C. , and Callaghan, T. V. , 2010: An ericoid shrub plays a dual role in recruiting both pines and their fungal symbionts along primary succession gradients. Oikos , 119: 1727–1734.
- Grau, O. , Ninot, J. M. , Blanco-Moreno, J. M. , van Logtestijn R. S. P. , Cornelissen, J. H. C. , and Callaghan, T. V. , 2012: Shrub-tree interactions and environmental changes drive treeline dynamics in the subarctic. Oikos , 121: 1680–1690.
- Harsch, M. A. , and Bader, M. Y. , 2011: Treeline form-A potential key to understanding treeline dynamics. Global Ecology and Biogeography , 20: 582–596, doi: ttp://dx.doi.org/10.1111/j.1466-8238.2010.00622.x.
- He, Q. , Bertness, M. D. , and Altieri, A. H. , 2013: Global shifts towards positive species interactions with increasing environmental stress. Ecology Letters , 16: 695–706.
- Hertel, D. , and Schöling, D. , 2011: Below-ground response of Norway spruce to climate conditions at Mt. Brocken (Germany)—A re-assessment of Central Europe's northernmost treeline. Flora — Morphology, Distribution, Functional Ecology of Plants , 206: 127–135.
- Holtmeier, F. K. 2009: Mountain Timberlines. Ecology, Patchiness, and Dynamics. Advances in Global Change Research 36. Berlin: Springer Science, 437 pp.
- Hošek, E. , 1964: Zalesňování horských holí na Králickém Sněžníku a Keprníku kolem r. 1900 [Afforestation of the areas above timberline in the Kralický Sněžník and Keprník around 1990]. Časopis Slezského Muzea (C) , 3: 65–73.
- Jeník, J. , 1961: Alpinská vegetace Krkonoš, Králického Sněžniku a Hrubého Jeseníku. Teorie anemo-orografických systémů [Alpine vegetation of Giant Mts., Hrubý Jeseník Mts. and Kralický Sněžník Mts. Theory of Anemo-Orographic systems]. Praha, Czech Republic: NČSAV, 407 pp.
- Jeník, J. , and Lokvenc, T. , 1962: Die alpine Waldgrenze im Krkonoše Gebirge [Alpine timberline in the Giant Mts.]. Rozpravy Československé akademie věd. Řada matematických a přírodních věd, 72: 1–65.
- Köck, R. , Härtel, E. , Holtermann, C. , Hochbichler, E. , Hager, H. , and Schönthaler, K. , 2003: Monitoring hydrological processes in montane and subalpine regions: comparison between different types of vegetation. Experimental design, techniques and first results. Paris: UNESCO, Technical Documents in Hydrology, 67: 73–78.
- Körner, Ch. , 1998: A re-assessment of high elevation treeline positions and their explanation. Oecologia , 115: 445–459.
- Körner, Ch. , 2003: Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Berlin: Springer, 344 pp.
- Körner, Ch. , 2012: Treelines will be understood once the functional difference between a tree and a shrub is. Ambio , 41: 197–206.
- Kozlowski, T. T. , 2002: Physiological ecology of natural regeneration of harvested and disturbed forest stands: implications for forest management. Forest Ecology and Management , 158: 195–221.
- Kuoch, R. , and Amiet, R. , 1970: Die Verjüngung im Bereich der oberen Waldgrenze der Alpen mit Berücksichtigung von Vegetation und Ablegerbildung [The treeline regeneration with consideration of layering]. Eidgenössischen Anstalt für das Forstliche Versuchswes , 46: 159–328.
- Kuras, T. , Beneš, J. , and Konvička, M. , 2001: Behaviour and within-habitat distribution of adult Erebia sudetica sudetica, endemic of the Hruby Jesenik Mts., Czech Republic (Nymphalidae, Satyrinae). Nota Lepidopterologica , 24: 69–83.
- Laberge, M. J. , Payette, S. , and Bousquet, J. , 2000: Life span and biomass allocation of stunted black spruce clones in the subarctic environment. Journal of Ecology , 88: 584–593.
- Maestre, F. T. , Callaway, R. M. , Valladares, F. , and Lortie, C. J. , 2009: Refining the stress-gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology , 97: 199–205.
- Michiels, H. G. , 1993: Die Stellung einiger Baum und Straucharten in der Struktur und Dynamik der Vegetation im Bereich der hochmontanen und subalpinen Waldstufe der Bayrischen Kalkalpen. Forstliche Forschungsberichte Münche , 135: 1–300.
- Migala, K. , 2005: Piętra klimatyczne w górach Europy a problem zmian globalnych [Climate levels in the European Mountains and the problem of global changes]. Wrocław: Wydawnictwo Uniwersytetu Wrocławskiego, Studia Geograficzne, 144 pp.
- Mihai, B. , Savulescu, I. , and Sandric, I. , 2007: Change detection analysis (1986-2002) of vegetation cover in Romania. Mountain Research and Development , 27: 250–258.
- Miriti, M. N. , 2006: Ontogenetic shift from facilitation to competition in a desert shrub. Journal of Ecology, 94: 973–979.
- Murtaugh, P. , 2007: Simplicity and complexity in ecological data analysis. Ecology , 88: 56–62.
- Nagy, L. , Grabherr, G. , Körner, Ch. , and Thompson, D. B. A. , 2003: Alpine Biodiversity in Europe. Ecological Studies, vol. 167. Berlin: Springer, 477 pp.
- Novák, J. , Petr, L. , and Treml, V. , 2010: Late-Holocene human-induced changes to the extent of alpine areas in the East Sudetes, Central Europe. Holocene , 20: 895–905, doi: <http://dx.doi.org/10.1177/0959683610365938>.
- Okitsu, S. , 1998: Distribution and growth of Pinus pumila Regel along the Larix gmelinii (Rupr.) Rupr. timberline ecotone of Mt. Dal'Nyaya Ploskaya, central Kamchatka. Proceedings NIPR Symposium of Polar Biology , 11: 159–168.
- Oksanen, J. , Blanchet, G. F. , Kindt, R. , Legendre, P. , Minchin, P. R. , O'Hara, R. B. , Simpson, G. L. , Solymos, P. , Stevens, M. H. H. , and Wagner, H. , 2012: Vegan: Community Ecology Package. R package version 2.0-4. <http://CRAN.R-project.org/package=vegan>.
- Pinheiro, J. , Bates, D. , DebRoy, S. , Sarkar, D. , and the R Development Core Team, 2013: Nlme: linear and nonlinear. Vienna , Austria: R Foundation for Statistical Computing.
- R Development Core Team , 2011: R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
- Rao, S. J. , Lason, G. R. , Hulbert, A. R. , Elston, D. A. , and Racey, P. A. , 2003: The effect of sapling density, heather height and season on browsing by mountain hares on birch. Journal of Applied Ecology , 40: 626–638.
- Roštínský, P. , Šenfeldr, M. , and Maděra, P. , 2013: Effects of dwarf pine stands on slope deformation processes, as a basis for their management in the Hrubý Jeseník Mountains. Journal of Landscape Ecology , 6: 63–83.
- Russell, F. L. , and Fowler, N. L. , 2004: Effects of white-tailed deer on the population dynamics of acorns, seedlings and small saplings of Quercus buckleyi. Plant Ecology , 173: 59–72.
- Sarkar, D. , 2008: Lattice: Multivariate Data Visualization with R. New York: Springer, 265 pp.
- Schöb, C. , Armas, C. , Guler, M. , Prieto, I. , and Pugnaire, F. I. , 2013: Variability in functional traits mediates plant interactions along stress gradients. Journal of Ecology , 101: 753–762.
- Schönenberger, W. , 1975: Standortseinflüsse auf Versuchsaufforstungen an der alpinen Wald-grenze [The local effects on planting at the treeline] (Stillberg, Davos). Mitteilungen-Eidgenössische Anstalt für das Forstliche Versuchswesen, 51: 357–428.
- Schönenberger, W. , 1981: Die Wuchsformen der Bäume an der alpinen Waldgrenze [The growth form of the trees at the treeline]. Schweiz. Z. Forstwesen , 132: 149–162.
- Scotti, I. , Gugerli, F. , Pastorelli, R. , Sebastiani, F. , and Vendramin, G. G. , 2008: Maternally and paternally inherited molecular markers elucidate population patterns and inferred dispersal processes on a small scale within a subalpine stand of Norway spruce (Picea abies [L.] Karst.). Forest Ecology and Management , 255: 3806–3812.
- Sitko, I. , and Troll, M. , 2008: Timberline changes in relation to summer farming in the Western Chornohora (Ukrainian Carpathians). Mountain Research and Development , 28: 263–271.
- Song, M. , Hu, Q. , Tian, Y. , and Ouyang, H. , 2010: Seasonal patterns of root and shoot interactions in an alpine meadow on the Tibetan Plateau. Journal of Plant Ecology , 5: 182–190.
- Souček, J. , and Špulák, O. , 2011: Cluster reforestation near the timber line. Journal of Forestry Science , 57: 16–23.
- Soukupová, L. , Frantík, T. , and Jeník, J. , 2001: Grasslands versus krummholz in arctic-alpine tundra of the Giant Mountains. Opera Corcontica , 38: 63–76.
- Svoboda, M. , 2001: The effect of Pinus mugo (Turra.) plantations on alpine-tundra microclimate, vegetation distribution, and soils in Krkonoše national park, Czech Republic. Opera Corcontica , 38: 189–206.
- Šenfeldr, M. , and Maděra, P. , 2011: Population structure and reproductive strategy of Norway spruce (Picea abies L . Karst) above the former pastoral timberline in the Hrubý Jeseník Mountains, Czech Republic. Mountain Research and Development , 31: 131–143.
- Šenfeldr, M. , Maděra, P. , Buček, A. , Roštínský, P. , Špinlerová, Z. , Culek, M. , Friedl, M. , Štykar, J. , Vavříček, D. , Pecháček, J. , Tippner, A. , and Sedláček, A. , 2012: Kleč v horské krajině Hrubého Jeseníku [Dwarf pine in the mountain landscape of the Hrubý Jeseník Mts.]. Brno: CERM, Geobiocoenological papers 16, 235 pp.
- Šenfeldr, M. , Urban, J. , Maděra, P. , and Kučera, J. , 2013: Bidirectional flows in the layering branches between parent and daughter tree in a Norway spruce polycormon. Acta Horticulturae, 991: 277–284.
- Švajda, J. , Solár, J. , Janiga, M. , and Buliak, M. , 2011: Dwarf pine (Pinus mugo) and selected abiotic habitat conditions in the western Tatra Mountains. Mountain Research and Development , 31: 220–228.
- Takahashi, K. , 2003: Effects of climatic conditions on shoot elongation of alpine dwarf pine (Pinus pumila) at its upper and lower altitudinal limits in central Japan. Arctic, Antarctic, and Alpine Research , 35: 1–7.
- Tolasz, R. , Brázdil, R. , Bulíř, O. , Dobrovolný, P. , Dubrovský, M. , Hájková, L. , Halásová, O. , Hostýnek, J. , Janouch, M. , Kohut, M. , Krška, K. , Křivancová, S. , Květoň, V. , Lepka, Z. , Lipina, P. , Macková, J. , Metelka, L. , Míková, T. , Mrkvica, Z. , Možný, M. , Nekovář, J. , Němec, L. , Pokorný, J. , Reitschläger, J. D. , Richterová, D. , Rožnovský, J. , Řepka, M. , Semerádová, D. , Sosna, V. , Stříž, M. , Šercl, P. , Škáchová, H. , Štěpánek, P. , Štěpánková, P. , Trnka, M. , Valeriánová, A. , Valter, J. , Vaníček, K. , Vavruška, F. , Voženílek, V. , Vráblík, T. , Vysoudil, M. , Zahradníček, J. , Zusková, I. , Žák, M. , and Žalud, Z. , 2007: Atlas podnebí česka [Climate Atlas of Czechia]. Prague: Czech Hydrometeorological Institute, 255 pp.
- Tranquillini, W. , 1979: Physiological Ecology of the Alpine Timberline. Tree Existence at High Altitudes with Special Reference to the European Alps. Ecological Studies 31. Berlin: Springer, 137 pp.
- Treml, V. , 2007: Dynamic of the Alpine Timberline in the High Sudetes. Ph.D. thesis, Department of Physical Geography and Geoecology, Charles University in Prague, Czech Republic, 198 pp.
- Treml, V. , and Banaš, M. , 2008: The effect of exposure on alpine treeline position: a case study from the High Sudetes, Czech Republic. Arctic, Antarctic, and Alpine Research , 40: 751–760.
- Treml, V. , Jankovská, V. , and Petr, L. , 2008: Holocene dynamics of the alpine timberline in the High Sudetes. Biologia , 63: 73–80.
- Treml, V. , Wild, J. , Chuman, T. , and Potůčková, M. , 2010: Assessing the change in cover of non-indigenous dwarf pine using aerial photographs, a case study from the Hrubý Jeseník Mountains, the Sudetes. Journal of Landscape Ecology , 4: 90–104.
- Treml, V. , Ponocná, T. , and Büntgen, U. , 2012: Growth trends and temperature responses of high-elevation Norway spruce (Picea abies (L.) H.Karst.) in the Czech Sudetes Mountains. Climate Research , 55: 91–103, doi: <http://dx.doi.org/10.3354/cr01122>.
- Úradníček, L. , Maděra, P. , Tichá, S. , and Koblížek, J. 2010: Woody Plants of the Czech Republic. Kostelec nad Černými lesy: Lesnická práce, 368 pp.
- Vacek, S. , Hejcmanová, P. , and Hejcman, M. , 2012: Vegetative reproduction of Picea abies by artificial layering at the ecotone of the alpine timberline in the Giant (Krkonoše) Mountains, Czech Republic. Forest Ecology and Management , 263: 199–207.
- Walther, G. R. , Post, E. , Convey, P. , Menze, A. , Parmesan, C. , Beebee, T. J. C. , Fromentin, J. M. , Hoegh-Guldberg, O. , and Bairlein, F. , 2002: Ecological responses to recent climate change. Nature , 416: 389–395.
- Wang, Y. , Chu, C. , Maestre, F. T. , and Wang, G. , 2008: On the relevance of facilitation in alpine meadow communities: an experimental assessment with multiple species differing in their ecological optimum. Acta Oecologica , 33: 108–113.
- Weih, M. , and Karlsson, P. S. , 2002: Low winter soil temperature affects summertime nutrient uptake capacity and growth rate of mountain birch seedlings in the subarctic, Swedish Lapland. Arctic, Antarctic, and Alpine Research , 34: 434–439.
- Wild, J. , and Wildová, R. , 2002: Interactions between dwarf pine shrubs and grassland vegetation under different management. Opera Corcontica , 39: 17–34.
- Wild, J. , and Winkler, E. , 2008: Krummholz and grassland coexistence above the forest-line in the Krkonoše Mountains: grid-based model of shrub dynamics. Ecological Modelling , 213: 293–307.
- Zeidler, M. , Duchoslav, M. , Banaš, M. , and Leškovám, M. , 2012: Impacts of introduced dwarf pine (Pinus mugo) on the diversity and composition of alpine vegetation. Community Ecology , 12: 213–220.
- Zuur, A. F. , Leno, E. N. , Walker, N. , Saveliev, A. A. , and Smith, G. M. , 2009: Mixed Effects Models and Extensions in Ecology with R. New York: Springer, 574 pp.
