Abstract
Ecotypic specialization among populations within plant species can result in adaptational lag when the climate changes directionally. However, disturbances, whether caused by direct effects of human activities or indirect effects such as climate change, may represent zones within which natural selection is relaxed. We compared the genetically based variation in leaf morphology in Dryas octopetala within three natural populations arrayed along a snowbank gradient, to that found in a recently colonized gravel pad less than 100 m away (1600 total leaf lengths measured; 4 sites × 10 transects/site × 4 plants/transect × 10 leaves/plant). Elevated among-clone leaf length variation within the disturbed site supported the idea that disturbances may represent “hotspots” of evolutionarily significant genetic variation. In the Arctic, where colonization of disturbances is primarily by native species, adaptive evolution may be more rapid than previously thought due to relaxation of selection and subsequent mixing of previously isolated gene pools in such areas.
Introduction
In organisms such as plants that are stationary for much of their life cycle, limited gene flow and sharp spatial environmental gradients have often led to genetically distinct populations, or ecotypes, over remarkably short distances (CitationLinhart and Grant, 1996; CitationJoshi et al., 2001; CitationSavolainen et al., 2007). In the Arctic, such ecotypes have allowed species to occupy a broader niche space than would be possible in the absence of such differentiation (CitationMcGraw, 1995). One well-documented example of such differentiation across a gradient from moist snowbed to rocky fellfield is that of Dryas octopetala ecotypes (CitationMcGraw and Antonovics, 1983a, Citation1983b; CitationMcGraw, 1985a, Citation1985b, Citation1987a, Citation1987b; CitationMax et al., 1999; CitationBennington et al., 2012). Reciprocal transplants of pollen, seeds, seedlings, and adults revealed multiple stages of selection favoring local populations at both ends of the gradient, with intermediates (presumed hybrids) occupying a narrow band of vegetation between the two. After thirty years, selection was so effective within reciprocal transplant gardens that alien ecotypes were completely absent from the fellfield and snowbed gardens (CitationBennington et al., 2012).
Dryas octopetala ecotypes are recognized taxonomically as subspecies (ssp. alaskensis and ssp. octopetala, for snowbed and fellfield ecotypes, respectively; CitationHultén, 1959, Citation1968; CitationMax et al., 1999). The presence of “octopetala scales” on the underside of the fellfield ecotype's pubescent leaves, and distinct glands with largely glabrous leaf surfaces in the snowbed ecotype, distinguish the two subspecies. In addition, mature leaves senesce on fellfield plants prior to winter, whereas snowbed plants retain a cohort of leaves through winter. In their home sites, leaf sizes are dramatically different, with snowbed leaves typically 2–10 times longer than fellfield leaves (CitationMcGraw and Antonovics, 1983a). Reciprocal transplanting revealed that leaf size responded differentially to the snowbed-fellfield gradient; the snowbed ecotype had more plastic leaf morphology, while the fellfield ecotype was unable to grow large leaves in response to the snowbed environment. Two independent common environment studies showed that leaf size differences were maintained within environments, implying a genetic basis to those differences (CitationMcGraw and Antonovics, 1983a; CitationMax et al., 1999).
Local genetic specialization confers an advantage to a population in stable environments, but in directionally changing environments, such specialization may rapidly become a disadvantage (CitationHolt, 1990; CitationDavis and Shaw, 2001; CitationJump and Peñuelas, 2005). Climate change, and warming in particular, represents a dramatic directional environmental shift with important expected consequences for ecological and evolutionary processes (CitationDavis and Shaw, 2001; CitationParmesan, 2006). Constraints on migration and the rate of adaptive change within populations increase the probability that a species will go extinct in a rapidly changing environment (CitationDavis and Shaw, 2001). As directional climate change occurs, a mismatch between ecotypes and the environment will emerge, resulting in “adaptational lag” (CitationJump et al., 2006; CitationAitken et al., 2008). Thus, even though range shifts have already been observed for some plant species (CitationKelly and Goulden, 2008; CitationLenoir et al., 2008) and dispersal itself may not be limiting in the long-term in the Arctic (CitationAlsos et al., 2007), establishment may be limiting, and migration of populations is therefore unlikely to be fast enough to keep up with their optimum environments (CitationDavis and Shaw, 2001).
The fragmentation of landscapes by human activities could provide significant barriers to migration of ecotypes that are responding to climate change (CitationPitelka, 1997), reinforcing adaptational lag. Alternatively, an increased frequency of disturbances across the landscape, due to direct human causes, or indirectly caused by climate change, might allow movement of species and ecotypes at greater than historical rates. Disturbances allow recruitment of new individuals dispersed from nearby sites. Indeed, within such disturbances, increased levels of resources associated with low levels of competition (CitationWilson and Tilman, 1993) may result in relaxation of natural selection, allowing a mixing of ecotypes, along with formation of new adaptive gene complexes. Incorporating genetic variation and rates of adaptation into species distribution models used to predict responses to climate change is acknowledged as a major challenge (CitationThuiller et al., 2008).
In the present study, we observed invasion of a disturbed area adjacent to a snowbed-fellfield gradient containing spatially sorted ecotypes of Dryas octopetala. We assessed the genetically based phenotypic variation within this disturbed area relative to that observed in the adjacent tundra communities to test the hypothesis that such disturbed zones would allow a relaxation of selection and, potentially, more rapid evolution in response to environmental change.
Methods
STUDY SITE
The study took place near Mile 106 on the Steese Highway in central Alaska northeast of Fairbanks. The study site consisted of Arctic alpine tundra above timberline at an elevation of 1035 m. The fellfield community was characterized by 10%–50% vegetative cover, presence of frostboils, and dominance by Dryas octopetala ssp. octopetala (CitationMiller, 1982). The snowbed community was dominated by Dryas octopetala ssp. alaskensis and had 80%–100% vegetative cover. The intermediate zone was a narrow band of vegetation located between the snowbed and the fellfield, and contained a mix of Dryas morphs ranging from ssp. octopetala morphs to alaskensis morphs, but also intermediate morphs that exhibited attributes of both subspecies; most notably, they were intermediate in leaf length. The site was the same one studied by McGraw and Antonovics (Citation1983a) and re-examined more recently to characterize D. octopetala ecotypes (CitationBennington et al., 2012).
The Gravel Pad was adjacent to the rocky fellfield that had high cover of D. octopetala ssp. octopetala, but was also within 20 m of intermediate environments, and within 50 m of snowbed environments containing intermediate and snowbed ecotypes, respectively. The Gravel Pad was left behind following highway construction activities in the mid-1980s. The mineral soil there originated on the formerly fellfield site, but the organic matter had been bulldozed off. Over the ensuing 30 yr, the area was gradually reinvaded by colonizing tundra species (McGraw, personal observation, 1977–2012). Mineral soil contains few viable seeds (CitationMcGraw and Vavrek, 1989); therefore, it is likely that most colonization was from establishment of new individuals. The center of the pad has remained disturbed by vehicle traffic and is vegetation-free. Qualitatively, the Gravel Pad resembled long stretches of the Steese Highway road shoulder and extensive nearby gold mine tailings; bare mineral soil on these sites is slowly recolonized by willow shrubs and other wind-dispersed native species. Although microenvironmental gradients existed within the Gravel Pad, the site was relatively flat and uniform, so within the transects used for sampling, variation among phenotypes was considered primarily genetically based, consistent with prior common garden studies (CitationMcGraw and Antonovics, 1983a; CitationMax et al., 1999).
SAMPLING
On 15–17 July 2012, ten 10 m transects were haphazardly laid out within each of three “undisturbed” community zones delineated as fellfield, intermediate, and snowbed zones. The short length of the transects and the criteria for designating the zones ensured that each transect occurred in a relatively uniform environment, such that phenotypic variation among individuals represented primarily genetically based variation. Ten additional transects were haphazardly located within the revegetating perimeter of a disturbed Gravel Pad.
LEAF PHENOTYPE MEASUREMENTS
On each transect, the nearest D. octopetala individuals to four random points on the transect were chosen and the length of the longest leaf was measured on ten haphazardly chosen shoots within each clone. Along a shoot, D. octopetala leaves are exserted sequentially, reaching a maximum size; within a particular environment, this maximum size characterizes the individual phenotype in that environment. Mean maximum leaf length was calculated for each individual. In total, we measured 1600 leaf lengths (4 sites × 10 transects/site × 4 plants/transect × 10 leaves/plant).
PHENOLOGICAL SEPARATION IN THE GRAVEL PAD
Even if ecotypes were found growing together on the Gravel Pad, gene flow may not be enhanced if flowering phenology remained separate, as it is along the snowbank gradient (CitationMcGraw and Antonovics, 1983a). As a test for this, we analyzed 50 images containing 32 separate seed-producing D. octopetala individuals on the Gravel Pad, classifying them as snowbed, intermediate, or fellfield phenotypes, then classifying the dispersal stage of their infructescences as predispersal (achenes green and twisted together) or postdispersal (achenes dried and at least some missing). The dispersal stage was taken as an indicator of phenological stage of reproduction.
STATISTICAL ANALYSIS
Leaf length data were first analyzed with a three-level nested ANOVA (Site, with random effects of Transect[Site] and Clone[Transect(Site)]) to compare sites in terms of mean leaf length and to verify that the snowbank gradient showed the expected pattern documented three decades previously.
To test the hypothesis that the Gravel Pad site contained higher levels of phenotypic variation among clones than the undisturbed sites, we performed two analyses. First, we estimated the coefficient of variation (CV) among clones within transects. The within-transect measure of the CV estimated the among plant leaf size variation. Using transect as the sampling unit within sites, we performed a one-way ANOVA to test for differences among sites in the CV. Although the first analysis examined variation at the 10 m scale, a second analysis was performed for the whole site. In this second analysis, we ignored transect and calculated 1000 bootstrapped estimates of the CV at each site as a whole, and estimated sitewide mean and standard deviation of the CV.
To test for differences in phenological stage among ecotypes in the Gravel Pad, we performed a G-test.
Results and Discussion
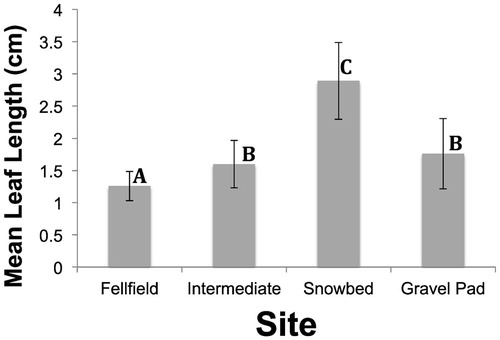
The phenotypic expression of maximum leaf length data in undisturbed communities reflected the previously documented differences in leaf length (CitationMcGraw and Antonovics, 1983a; ), with fellfield plants having the smallest and snowbed plants having the largest leaves (Fsite = 106.3, p < 0.0001; Fsite = 83.9, p < 0.0001 for log transformed data to correct for heterogeneity of variances). Only plants in Intermediate and Gravel Pad environments were not significantly different in mean leaf length (Tukey-Kramer HSD). The standard deviations of leaf lengths were positively related to overall leaf length (), so it was appropriate to examine the CVs as a way to standardize the comparison of variation among individuals for the four sites (CitationSokal and Rohlf, 2012).
FIGURE 1. Mean leaf length (±1 s.d.) across the snowbank gradient from fellfield to snowbed, and in the adjacent gravel pad disturbed site for Dryas octopetala. A total of 1600 leaves were measured (4 sites × 10 transects/site × 4 plants/transect × 10 leaves/plant). Standard deviations around the mean are shown to illustrate the correlation between mean and variance. Means with the same letters are not significantly different according to the Tukey-Kramer HSD a posteriori test.
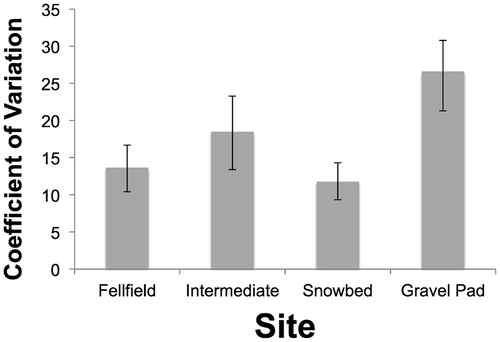
The CV of maximum leaf length among individuals within transects differed significantly among sites (Fsite = 4.6343, p = 0.0077). Both fellfield and snowbed sites had significantly lower CVs than the Gravel Pad site. These differences in level of variation were confirmed by the bootstrapped estimates of mean CV and standard errors of CV, by site ().
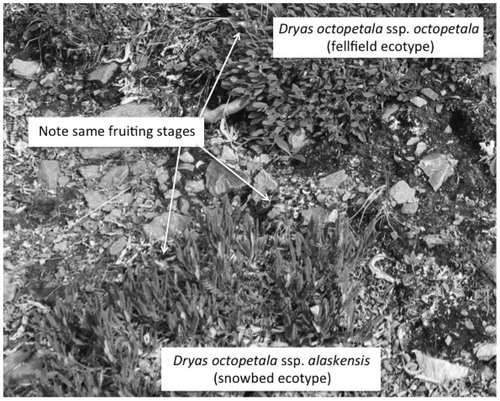
Indeed, other than the extensive patches of bare ground in the Gravel Pad, the diverse assemblage of phenotypes found there resembled that in the narrow Intermediate environment. Fellfield, intermediate, and snowbed ecotypes could be found growing in close proximity within the Gravel Pad, often within centimeters of each other ().
Although much less is known about ecotypic differentiation of other species found on the disturbed Gravel Pad, at least twenty other species were noted. Many of these were also found in more than one adjacent natural community type. Therefore, although our measures focused on Dryas octopetala because of its well-understood pattern of ecotypic variation, the opportunity for ecotypes of other species to intermix in the disturbance existed as well.
FIGURE 2. Coefficient of variation (CV) of maximum leaf length of Dryas octopetala in four sites; mean and standard error of the CV determined from 1000 bootstrapped samples drawn with replacement.
In Arctic tundra, two classes of disturbance are increasing globally in spatial extent and temporal frequency. Anthropogenic disturbances have spread due to expanding human populations and resource exploitation (CitationForbes et al., 2001). Accompanying these increasing activities are more road corridors, such as the north-south—running Dalton highway paralleling the Alaska oil pipeline (CitationAlexander and Van Cleve, 1983), as well as increased off-road vehicle traffic (CitationSlaughter et al., 1990). Resource exploitation such as oil field development (CitationMaki, 1992) and mining (CitationMcLeay et al., 1987) directly disturbs tundra soils and downstream waters. The second class of disturbance is “natural” and encompasses a wide range of factors including forest fires, landslides, glacial retreat, thermokarst erosion, erosion by streams and rivers, animal activity, and a wide variety of soil-ice related disruptions. In a warming climate, many such natural disturbances are occurring at accelerated rates. General circulation models of the atmosphere predict that climate change, and warming in particular, is predicted to be greater in Arctic regions than elsewhere (CitationHolland and Bitz, 2003; CitationIPCC, 2007), a prediction that has been borne out by observed warming over the past two decades (CitationSerreze et al., 2000). For example, the unprecedented 2007 Anaktuvuk fire on the north slope of Alaska was set by lightning and burned 1039 km2 (CitationJones et al., 2009; CitationMack et al., 2011). Lightning is likely to be an increasingly common weather phenomenon in northern climates (CitationHu et al., 2010). Retreating glaciers worldwide are exposing weathered mineral soils (CitationJones and Henry, 2003; CitationPaul et al., 2004). Permafrost thaw is followed by thermokarst erosion, resulting in increased N availability and enhanced plant growth (CitationSchuur et al., 2007).
Disturbances in the Arctic are colonized primarily by native plant species with effective dispersal mechanisms (CitationForbes et al., 2001; CitationJones and Henry, 2003). If such disturbances generally represent zones where strong natural selection is relaxed, as appears to be the case with Dryas octopetala in the disturbance adjacent to the snowbank gradient in Alaska, several population genetic consequences may follow. First, disturbances may represent corridors for rapid migration (CitationNeilson et al., 2005). Recruitment from seed in “closed” tundra communities occurs at low rates, while seed germination and establishment are enhanced in disturbances (CitationForbes et al., 2001). Once established, recruited seedlings will likely grow to maturity much more rapidly than in closed communities for two reasons: (a) competition is reduced, and (b) growth-limiting nutrients are enhanced (CitationMoulton and Gough, 2011). In the Gravel Pad site, diameter growth of clones was much greater than that observed in adjacent tundra (McGraw, personal observation, 2012). In a directionally changing climate, rapid migration along disturbance corridors, particularly those traversing latitudinal or elevational gradients, may allow re-equilibration of ecotype-environment matching.
The precise cause of the phenotypic differences in the “common environment” of the disturbed Gravel Pad is not known, although previous growth chamber and experimental work suggest they are genetically based. Epigenetic differences could also be partly responsible (CitationBräutigam et al., 2013), though virtually nothing is presently known about the level or functional significance of epigenetics in Dryas octopetala.
Regardless of the cause of phenotypic differences among clones, the enhanced phenotypic variation present in the Gravel Pad demonstrates that progeny of ecotypes that were formerly separated, both spatially and temporally, are now growing together. This new juxtaposition will likely promote formation of hybrid swarms, including novel combinations of genes that would be rare in undisturbed communities (CitationRieseberg et al., 2003). Although some crossing among D. octopetala ecotypes can occur along natural gradients, isolation in the closed community was shown to be reinforced by sharp phenological separation of flower times (CitationMcGraw and Antonovics, 1983a) as well as by distance. In the disturbed zone, both distance and phenological isolation were much reduced, allowing high levels of gene exchange among previously isolated populations. We found no difference in phenological stage among phenotype classes (G = 0.877, p > 0.05) and inferred that flowering time overlap would have been great as well, allowing for much greater cross-pollination than previously observed for ecotypes in the undisturbed communities (CitationMcGraw and Antonovics, 1983a). This pattern differed from the adjacent fellfield and snowbed communities, in which plants were predominantly in postdispersal and predispersal phases, respectively, indicating largely separate flowering times. A similar promotion of crossing between previously isolated gene pools by anthropogenic disturbance has been observed before in Banksia species, where a breakdown of phenological isolation appeared to be the root cause (CitationLamont et al., 2003).
Conclusion
A full account of the potential evolutionary consequences of enhanced hybridization is beyond the scope of this study (see CitationArnold, 1997; CitationAbbott et al., 2013). However, one salient aspect is that the enhanced genetic variance associated with mixing of previously isolated ecotypes is likely to result in new combinations of traits that could be crucial for adaptation to a changing climate (CitationPease et al., 1989). Offspring with unique combinations of characters, or pollen of progeny produced in the disturbance, could disperse back into adjacent undisturbed tundra, thereby ultimately increasing genetic variance within established communities as well. This could be particularly important as climate change produces “no analogue” communities in the future (CitationWilliams and Jackson, 2007). A theoretical example in D. octopetala ecotypes would be production of a long-leaved, but deciduous phenotype through hybridization that would be both more competitive against neighbors, yet avoid the destructive effects of winter snowblast in the fellfield environment; such a phenotype does not currently exist, but might confer selective advantages in a future fellfield environment with more competition between neighbors. A second example would be the transmission of genes enhancing growth at warm temperatures from snowbed to fellfield forms. With mechanisms such as these, directional climate change accompanied by increasing levels of disturbance in the Arctic may stimulate the rate of evolution in these hotspots of genetic diversity beyond what would be predicted by current levels of genetic variation and selection within extant communities.
While an account of enhanced inter-ecotype mixing at one site in one species cannot definitively demonstrate that disturbances will generally act as hotspots of genetic variation, Dryas octopetala ecotypes and, indeed, species (Dryas drummondii and Dryas integrifolia) have been observed to be growing in close proximity in other disturbed sites (McGraw, personal observation, 2010–2012). If the phenomenon is widespread both spatially and taxonomically, this could have important evolutionary implications for the tundra flora as climate continues to change over the next century and beyond.
Acknowledgments
The authors thank Ned Fetcher and Cynthia Bennington for discussions and comments on this manuscript. We are grateful for financial support from National Science Foundation grant ARC-0908936 to McGraw.
References Cited
- Abbott, R. , Albach, D. , Ansell, S. , Arntzen, J. W. , Baird, S. J. E. , Bierne, N. , Boughman, J. , Brelsford, A. , Buerkle, C. A. , Buggs, R. , Butlin, R. K. , Dieckmann, U. , Eroukhmanoff, F. , Grill, A. , Cahan, S. H. , Hermansen, J. S. , Hewitt, G. , Hudson, A. G. , Jiggins, C. , Jones, J. , Keller, B. , Marczewski, T. , Mallet, J. , Martinez-Rodriguez, P. , Most, M. , Mullen, S. , Nichols, R. , Nolte, A. W. , Parisod, C. , Pfennig, K. , Rice, A. M. , Ritchie, M. G. , Seifert, B. , Smadja, C. M. , Stelkens, R. , Szymura, J. M. , Vainola, R. , Wolf, J. B. W. , and Zinner, D. , 2013: Hybridization and speciation. Journal of Evolutionary Biology , 26: 229–246.
- Aitken, S. N. , Yeaman, S. , Holliday, J. A. , Wang, T. , and Curtis-McLane, S. , 2008: Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications , 1: 95–111.
- Alexander, V. , and Van Cleve, K. , 1983: The Alaska pipeline: a success story. Annual Review of Ecology and Systematics , 14: 443–463.
- Alsos, I. G. , Eidesen, P. B. , Ehrich, D. , Skrede, I. , Westergaard, K. , Jacobsen, G. H. , Landvik, J. Y. , Taberlet, P. , and Brochmann, C. , 2007: Frequent long-distance plant colonization in the changing Arctic. Science , 316(5831): 1606–1609.
- Arnold, M. L. , 1997: Natural Hybridization and Evolution. Oxford: Oxford University Press.
- Bennington, C. C. , Fetcher, N. , Vavrek, M. C. , Shaver, G. R. , Cummings, K. J. , and McGraw J. B. , 2012: Home site advantage in two long-lived Arctic plant species: results from two 30-year reciprocal transplant studies. Journal of Ecology , 100: 841–851.
- Bräutigam, K. , Vining, K. J. , Lafon-Placeette, C. , Fossdal, C. G. , Mirouze, M. , Marcos, J. G. , Fluch, S. , Fraga, M. F. , Guevara, M. A. , Abarca, D. , Johnsen, Ø. , Maury, S. , Strauss, S. H. , Campbell, M. M. , Rohde, A. , Diaz-Sala, C. , and Cervera, M.-T. , 2013: Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecology and Evolution , 3(2): 399–415.
- Davis, M. B. , and Shaw, R. G. , 2001: Range shifts and adaptive responses to Quaternary climate change. Science , 292: 673–679.
- Forbes, B. C. , Ebersole, J. J. , and Strandberg, B. , 2001: Anthropogenic disturbance and patch dynamics in circumpolar Arctic ecosystems. Conservation Biology , 15: 954–969.
- Holland, M. M. , and Bitz, C. M. , 2003: Polar amplification of climate change in coupled models. Climate Dynamics , 21: 221–232.
- Holt, R. D. , 1990: The microevolutionary consequences of climate change. Trends in Ecology and Evolution , 5: 311–315.
- Hu, F. S. , Higuera, P. E. , Walsh, J. E. , Chapman, W. L. , Duffy, P. A. , Brubaker, L. B. , and Chipman, M. L. , 2010: Tundra burning in Alaska: linkages to climatic change and sea ice retreat. Journal of Geophysical Research—Biogeography , 115: G04002, doi: http://dx.doi.org/10.1029/2009JG001270.
- Hultén, E. , 1959: Studies in the genus Dryas. Svensk Botanisk Tidskrift , 53: 507–542.
- Hultén, E. , 1968. Flora of Alaska and Neighboring Territories. Stanford, California: Stanford University Press.
- IPCC , 2007: Climate Change 2007: the Physical Science Basis. Summary for Policy Makers—A Report of Working Group II of the Intergovernmental Panel on Climate Change. Fourth Assessment Report. Paris: IPCC.
- Jones, B. M. , Kolden, C. A. , Jandt, R. , Abatzoglou, J. T. , Urban, F. , and Arp, C. D. , 2009: Fire behavior, weather, and burn severity of the 2007 Anaktuvuk River tundra fire, North Slope, Alaska. Arctic, Antarctic, and Alpine Research , 41: 309–316.
- Jones, G. A. , and Henry, G. H. R. , 2003: Primary plant succession on recently deglaciated terrain in the Canadian High Arctic. Journal of Biogeography , 30: 277–296.
- Joshi, J. , Schmid, B. , Caldeira, M. C. , Dimitrakopoulos, P. G. , Good, J. , Harris, R. , Hector, A. , Huss-Danell, K. , Jumpponen, A. , Minns, A. , Mulder, C. P. H. , Pereira, J. S. , Prinz, A. , Scherer-Lorenzen, M. , Siamantziouras, A.-S. D. , Terry, A. C. , Troumbis, A. Y. , and Lawton, J. H. , 2001: Local adaptation enhances performance of common plant species. Ecology Letters , 4: 536–544.
- Jump, A. S. , and Peñuelas, J. , 2005: Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters , 8: 1010–1020.
- Jump, A. S. , Hunt, J. M. , Martinez-Izquierdo, J. A. , and Penuelas, J. , 2006: Natural selection and climate change: temperature-linked spatial and temporal trends in gene frequency in Fagus sylvatica. Molecular Ecology , 15: 3469–3480.
- Kelly, A. E. , and Goulden, M. L. , 2008: Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences of the USA. 105: 11823–11826.
- Lamont, B. B. , He, T. , Enright, N. J. , Krauss, S. L. , and Miller, B. P. , 2003: Anthropogenic disturbance promotes hybridization between Banksia species by altering their biology. Journal of Evolutionary Biology , 16: 551–557.
- Lenoir, L. , Gégout, J. S. , Marquet, P. A. , Reffray, P. D. , and Brisse, H. , 2008: A significant upward shift in plant species optimum elevation during the 20th century. Science , 320: 1768–1771.
- Linhart, Y. C. , and Grant, M. C. , 1996: Evolutionary significance of local differentiation in plants. Annual Review of Ecology and Systematics , 27: 237–277.
- Mack, M. C. , Bret-Harte, S. , Hollingsworth, T. N. , Jandt, R. R. , Schuur, E. A. G. , Shaver, G. R. , and Verbyla, D. L. , 2011: Carbon loss from an unprecedented Arctic tundra wildfire. Nature , 475: 489–492.
- Maki, A. W. , 1992: Of measured risks: the environmental impacts of the Prudhoe Bay, Alaska, oil field. Environmental Toxicology and Chemistry , 11: 1691–1707.
- Max, K. N. , Mouchaty, S. K. , and Schwaegerle, K. E. , 1999: Allozyme and morphological variation in two subspecies of Dryas octopetala (Rosaceae) in Alaska. American Journal of Botany , 86: 1637–1644.
- McGraw, J. B. , 1985a: Experimental ecology of Dryas octopetala ecotypes: relative response to competitors. New Phytologist , 100: 233–241.
- McGraw, J. B. , 1985b: Experimental ecology of Dryas octopetala ecotypes. III. Environmental factors and plant growth. Arctic and Alpine Research , 17: 229–239.
- McGraw, J. B. 1987a: Experimental ecology of Dryas octopetala ecotypes. IV. Fitness response to transplanting in ecotypes with differing plasticity. Oecologia , 73: 465–468.
- McGraw, J. B. , 1987b: Experimental ecology of Dryas octopetala ecotypes. V. Field photosynthesis of reciprocal transplants. Holarctic Ecology , 10: 308–311.
- McGraw, J. B. , 1995: Patterns and causes of genetic diversity in Arctic plants. In Chapin, F. S., III , and Körner, C. (eds.), Arctic and Alpine Biodiversity , Berlin: Springer-Verlag, 33–43.
- McGraw, J. B. , and Antonovics, J. , 1983a: Experimental ecology of Dryas octopetala ecotypes. I. Ecotypic differentiation and life cycle stages of selection. Journal of Ecology , 71: 879–897.
- McGraw, J. B. , and Antonovics, J. , 1983b: Experimental ecology of Dryas octopetala ecotypes. II. A demographic model of growth, branching, and fecundity. Journal of Ecology , 71: 899–912.
- McGraw, J. B. , and Vavrek, M. C. , 1989: The role of buried viable seeds in Arctic and alpine plant communities. In Leck, M. A. , Parker, V. T. , and Simpson, R. L. (eds.), Ecology of Soil Seed Banks. New York: Academic Press, 91–106.
- McLeay, D. J. , Birtwell, I. K. , Hartman, G. F. , and Ennis, G. L. , 1987: Responses of Arctic grayling (Thymallus arcticus) to acute and prolonged exposure to Yukon placer mining sediment. Canadian Journal of Fisheries and Aquatic Sciences , 44: 658–673.
- Miller, P. C. , 1982: Environmental and vegetational variation across a snow accumulation. Holarctic Ecology , 5: 85–98.
- Moulton, C. A. , and Gough, L. , 2011: Effects of soil nutrient availability on the role of sexual reproduction in an Alaskan tundra plant community. Arctic, Antarctic, and Alpine Research , 43: 612–620.
- Neilson, R. P. , Pitelka, L. F. , Solomon, A. , Nathan, R. , Midgley, G. F. , Fragoso, J. , Lischke, H. , and Thompson, K. , 2005: Forecasting regional to global plant migration in response to climate change: challenges and directions. BioScience , 55: 749–759.
- Parmesan, C. , 2006: Ecological and evolutionary responses to recent climate change. Annual Review of Ecology and Systematics , 37: 637–669.
- Paul, F. , Kaab, A. , Maisch, M. , Kellenberger, T. and Haeberli, W. , 2004: Rapid disintegration of Alpine glaciers observed with satellite data. Geophysical Research Letters , 31: L21402, doi: http://dx.doi.org/10.1029/2004GL020816.
- Pease, C. , Lande, R. , and Bull, J. J. , 1989: A model of population growth, dispersal and evolution in a changing environment. Ecology , 70: 1657–1664.
- Pitelka, L. , 1997: Plant migration and climate change: a more realistic portrait of plant migration is essential to predicting biological responses to global warming in a world drastically altered by human activity. American Scientist , 85: 464–474.
- Rieseberg, L. , Raymond, O. , Rosenthal, D. M. , Lai, Z. , Livingstone, K. , Nakazato, T. , Durphy, J. L. , Schwarzbach, A. E. , Donovan, L. A. , and Lexer, C. , 2003: Major ecological transitions in wild sunflowers facilitated by hybridization. Science , 301: 1211–1216.
- Savolainen, O. , Pyhajarvi, T. , and Knurr, T. , 2007: Gene flow and local adaptation in trees. Annual Review of Ecology and Systematics , 38: 595–619.
- Schuur, E. A. G. , Crummer, K. G. , Vogel, J. G. , and Mack, M. C. , 2007: Plant species composition and productivity following permafrost thaw and thermokarst in Alaskan tundra. Ecosystems , 10: 280–292.
- Serreze, M. C. , Walsh, J. E. , Chapin, F. S. , Osterkamp, T. , Dyurgerov, M. , Romanvsky, V. , Oechel, W. C. , Morison, J. , Zhang, T. , and Barry, R. G. , 2000: Observational evidence of recent change in the northern high-latitude environment. Climatic Change , 46: 159–207.
- Slaughter, C. W. , Racine, C. H. , Walker, D. A. , Johnson, L. A. and Abele, G. , 1990: Use of off-road vehicles and mitigation of effects in Alaska permafrost environments: a review. Environmental Management , 14: 53–72.
- Sokal, R. R. , and Rohlf, F. J. , 2012: Biometry. 4th edition. New York: Freeman.
- Thuiller, W. , Alberta, C. , Araujo, M. B. , Berry, P. M. , Cabezad, M. , Guisane, A. , Hickler, T. , Midgley, G. F. , Paterson, J. , Schurr, F. M. , Sykes, M. T. , and Zimmermann, N. E. , 2008: Predicting global change impacts on plant species distributions: future challenges. Perspectives in Plant Ecology , 9: 137–152.
- Williams, J. W. , and Jackson, S. T. , 2007: Novel climates, no-analog communities, and ecological surprises. Frontiers in Ecology and the Environment , 5: 475–482.
- Wilson, S. D. , and Tilman, D. , 1993: Plant competition and resource availability in response to disturbance and fertilization. Ecology , 74: 599–611.