Abstract
Aim & methods: To assess the impact of pretreatment serum levels of IL-18 and soluble IL-2 receptor (sIL-2R) on the clinical outcome of patients with diffuse large B-cell lymphoma treated with an R-CHOP protocol. Total 73 patients were included. Results: Elevated serum IL-18 (using mean as cutoff) was associated with numerically lower complete remission, and 3-year disease-free survival rates; however, the difference was not statistically significant. Nevertheless, the 3-year overall survival rates were significantly more favorable for the lower serum level group. Correspondingly, the complete remission, 3-year disease-free survival and overall survival rates for patients with low pretreatment sIL-2R levels were significantly better than individuals with higher levels. Conclusion: There is a growing body of evidence supporting the utility of pretreatment serum levels of sIL-2R and IL-18 as prognostic factors in diffuse large B-cell lymphoma patients.
Lay abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphomas in Egypt. Since the introduction of rituximab, the utility of International Prognostic Index in DLBCL patients treated in the rituximab era has been questioned. Instead, biologic prognostic factors including cytokines are increasingly being investigated to stratify DLBCL patients. In this prospective single arm Phase II study, there is evidence supporting the utility of pretreatment serum levels of IL-18 and sIL-2R, which can be easily measured in clinical practice, as potential prognostic factors that may add additional information regarding response to treatment and outcome in DLBCL patients and could help stratify poor risk patients for more aggressive treatment.
Diffuse large B-cell lymphomas (DLBCLs) are the most common non-Hodgkin lymphomas (NHLs) in adults [Citation1]. In Egypt, DLBCL represents 55% of NHLs [Citation2]. Before rituximab, the International Prognostic Index (IPI) was considered the most reliable prognostic index for NHL patients [Citation3]. However, in the rituximab era, IPI failed to distinguish the four risk groups previously identified in chemotherapy-alone based treatment [Citation4]. Therefore, concerns regarding the utility of IPI were raised [Citation5].
IL-18 is a cytokine that has been shown to affect the immune defense against tumor cells [Citation6]. It has been reported that serum IL-18 may be a useful marker for monitoring outcome in some malignancies including gastric carcinoma [Citation7], colon carcinoma [Citation8] and NHL [Citation9].
Soluble IL-2 receptor (sIL-2R) is the soluble form of IL-2 receptor that plays important roles in lymphocytes activation [Citation10]. Some lymphoid malignancies including lymphoblastic leukemia [Citation11], Hodgkin’s disease [Citation12], B-cell chronic lymphocytic leukemia [Citation13], adult T cell leukemia [Citation14] and peripheral T cell lymphoma unspecified [Citation15] displayed elevated levels of sIL-2R. Serum levels of IL-18 and sIL-2R were found to be prognostic factors for DLBCL patients [Citation5,Citation16–20].
This study aimed to assess the impact of serum levels of IL-18 and soluble IL-2 receptor on the clinical course and in determination of the treatment outcome in DLBCL patients treated with R-CHOP, considering disease-free survival (DFS) and overall survival as primary end points, and complete remission (CR) rate and treatment related toxicity as secondary end points.
Patients & methods
Patients
This study included 73 patients with CD20-positive DLBCL who presented to the Medical Oncology Department, Zagazig University Hospital, between November 2014 and November 2018. Patients were eligible when their age was 18 years or older with no prior radiotherapy or chemotherapy in the absence of any medical contraindications for receiving R-CHOP. Patients were excluded if they were pregnant, lactating or positive for HIV. Patients with 1ry central nervous system (CNS) lymphoma or Composite lymphoma were also excluded.
Pretreatment evaluation
Pretreatment evaluation included history; physical examination; laboratory studies (blood counts, LDH and liver and kidney functions), and virology serology (HCV Ab, HBsAg, HBcAb, PCR for serologically positive patients); and bone marrow biopsy when there was unexplained cytopenia. Staging radiology comprised CT scans of chest, abdomen and pelvis; and PET-CT scan for all patients. Patients were staged according to Lugano modification of Ann Arbor staging system. Performance status was reported according to Eastern Cooperative Oncology Group performance scale, and we used standard IPI to stratify our patients into different prognostic groups.
Pretreatment serum levels of IL-18 were assessed using Human IL-18, IL-18 ELISA Kit (E0064h) from EIAAB science (Wuhan, China). For assessment of serum levels of sIL-2R, we used humans IL-2-receptor, sIL-2R ELISA Kit (BE51121) from IBL international (Hamburg, Germany).
Treatment plan
Patients meeting eligibility criteria were scheduled to receive R-CHOP therapy (rituximab 375 mg/m2 day 1, cyclophosphamide 750 mg/m2 day 1, doxorubicin 50 mg/m2 day 1 vincristine 1.4 mg/m2 day 1, prednisone 100 mg PO daily for 5 days) repeated at 21-day intervals. Six cycles of R-CHOP-21 were planned for patients with stage I or II disease. Patients with advanced stage disease (III, IV) were scheduled to receive six to eight cycles of R-CHOP-21. Locoregional RT (36 Gy) was prescribed for patients with bulky disease (≥7.5 cm).
Cut-off level definition
We evaluated the optimal cut-off values for serum IL-18 and sIL2R levels to predict CR using the area under the receiver operating characteristic (ROC) curve. In the current study, the ROC curve generated cut-off value for serum IL-18 was 11.3 pg/ml. Corresponding value for sIL-2R was 592.4 U/ml. We also tested the median (10.8 pg/ml) and mean serum IL-18 level (22.2 pg/ml) to determine the most appropriate cut-off value for serum IL-18.
Statistical analysis
SPSS, version 25 (SPSS Inc., IL, USA) was used for data management and analysis. Chi-square or Fisher exact tested proportion independence. Correlation analysis was used to show strength and significance of association between quantitative variables. Kaplan–Meier method estimated overall survival (OS) and DFS and log rank test compared survival curves. Multivariate analysis was done by Cox regression model. P value was considered significant at ≤0.05 levels. Overall survival was calculated from the date of diagnosis to date of death or last follow-up. Disease-free survival was calculated as the period the patient lived without evidence of disease relapse or death.
Ethical conduct
The authors state that they have obtained appropriate institutional review board approval from the Egyptian National Cancer Institute, Cairo University and have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, informed consent has been obtained from the participants involved.
Results
Baseline characteristics
Clinical characteristics of the 73 patients included are listed in . The mean ± standard deviation (SD) of the serum IL-18 level was 22.2 ± 36.3 pg/ml (range = 3.8–231.7) with a median of 10.8 pg/ml. On the other hand, the mean ± SD of the serum sIL-2R level was 732.3 ± 645.6 U/ml (range = 67.5–2362.6) with a median of 468.8 U/ml.
Table 1. Patients characteristics.
Response to chemoimmunotherapy & survival
Complete response was achieved in 76% (54/72), while no CR (PR, SD and PD) was reported in 34%. After a median follow-up time of 15 months (range = 1–44 months), the 3-year OS rate was 72.9%. The median OS was not reached. The 3-year DFS rate for the 54 patients who achieved CR was 60.5%. At the end of follow-up, 20% of patients suffered relapse.
Association of serum cytokines with clinical features
Serum IL-18 levels were significantly associated with elevated LDH (p = 0.017), but not with other poor prognostic indicators or HCV seropositivity. On the other hand, various poor prognostic indicators, such as being elderly, increased LDH, advanced disease, existence of B symptoms and unfavorable IPI were associated with high serum sIL-2R levels; but not with poor PS, multiple extranodal sites, bulky disease or HCV seropositivity ().
Table 2. Serum IL-18 level according to conventional prognostic factors.
Univariate & multivariate analyses for cytokines & conventional prognostic variables on remission rates & survival outcomes
Utilizing ROC curve cut-off value (11.3 pg/ml), high serum levels of IL-18 were associated with numerically lower CR rate compared with lower levels (69.74 vs 79.5% [p = 0.339]). Similar results were obtained when we stratified patients according to the median (10.8 pg/ml) and the mean (22.2 pg/ml) serum IL-18 level. On the other hand, the CR rates for patients with sIL-2R levels ≤592.4 U/ml were significantly better than patients with higher serum levels (90.9 vs 50.0% [p = 0.001]). Additionally, the CR rates were significantly worse in patients with elevated LDH, advanced stage and unfavorable IPI, whereas bulky disease was marginally associated with lower CR rates. Conversely, the CR rates were not significantly associated with other clinical features, such as being elderly, poor PS, multiple extranodal sites, existence of B symptoms or HCV seropositivity ().
Table 3. Remission rate and survival outcome according to serum levels IL-18 and sIL-2R and other prognostic factors.
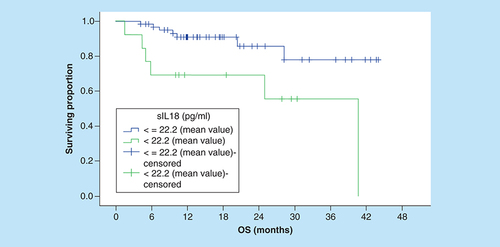
Serum IL-18 level was not predictive of OS using ROC curve value or the median serum IL-18 level as cut-off points (). Using the mean serum IL-18 level as a cutoff, the 3-year OS rates for patients with low and high serum levels were 77.8 and 55.4%, respectively, this difference was statistically significant (p = 0.008; ).
OS: Overall survival.
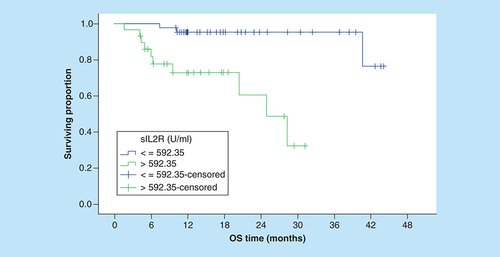
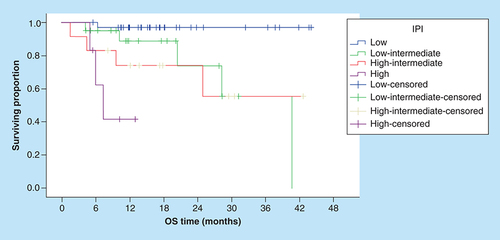
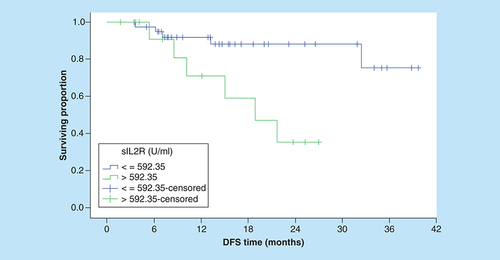
Correspondingly, the 3-year OS rates for patients with sIL-2R levels ≤592.4 U/ml were significantly better compared with patients with higher serum levels (95.4% vs NR, [p = 0.001]; ). In addition, the OS rates were significantly worse in patients with increased LDH, multiple extranodal involvement, advanced stage, presence of B symptoms () and unfavorable IPI ().
IPI: International prognostic index; OS: Overall survival.
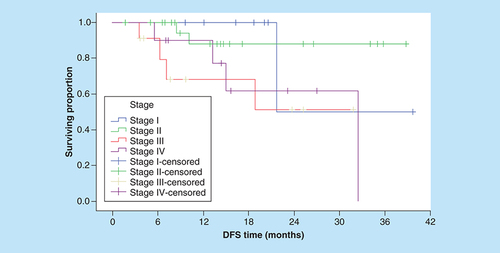
With respect of DFS, serum level of IL-18 was not predictive of 3-year DFS outcome when we used either ROC curve value, the median or the mean serum IL-18 level as cut-off points. Conversely, the patients with low serum sIL-2R had a significantly better 3-year DFS rates than patients with higher serum sIL-2R (75.4 vs 35.4%; p = 0.007; ). On the other hand, none of the conventional prognostic factors was predictive of DFS (), except for clinical stage (p = 0.008; ).
DFS: Disease-free survival.
Multivariate analyses employing strong conventional prognostic factors and serum cytokine levels demonstrated that serum sIL-2R was independent prognostic factor for OS, and that clinical stage and sIL-2R were marginal independent prognostic factors for DFS ().
Table 4. Multivariate analyses on overall survival and disease-free survival.
Discussion
Many investigators have attempted to identify prognostic factors to discriminate NHL patients who are likely to have favorable or worse outcomes [Citation21].
Since the introduction of rituximab, the utility of IPI has been questioned [Citation5]. To more strictly select appropriate therapeutic options, poorer prognostic groups need to be further discriminated.
Cytokines are small proteins that play an important role in immune regulation, and might reflect tumor growth. Currently, it is well known that an altered cytokine milieu exists in DLBCL [Citation22].
In this study, we investigated pretreatment serum IL-18 and sIL-2R levels in DLBCL patients treated with R-CHOP as prognostic factors with respect to OS, DFS, CR rates. The thresholds used to define the prognostic groups constitute a source of debate in any study on serum cytokines. We evaluated the optimal cut-off values for serum IL-18 and sIL2R levels to predict CR using the ROC curve. With the use of these cut-off values, we divided the patients into low and high groups.
In the current study, the ROC curve generated cut-off value of serum IL-18 was 11.3 pg/ml, and this value was much lower than that reported by Goto and collegues (590 pg/ml) [Citation18]. Correspondingly, cut-off value of sIL-2R in our patients was 592.4 U/ml, and this value was also lower than cutoff described by precedent investigators [Citation4,Citation20,Citation23]. These observed variations might be explained by distinctive nature of the disease in Egyptian patients including variable etiologic, genetic and biological factors.
We conducted univariate analysis of the effect of serum IL-18 upon disease outcome end points.
Utilizing ROC curve cut-off value, elevation of serum IL-18 was not predictive of CR (p = 0.339), 3-year DFS (p = 0.279) or 3-year OS (p = 0.153) rates. This might be referred to the impact of other predictors of outcome in DLBCL that might have been overlooked in our study (immunoblastic histology; molecular markers such as BCL2, c-myc, stromal signatures, gene expression profile subtypes) [Citation24]. Additional potential confounders include the IL‐18 binding protein (IL‐18BP) which is known affect the concentrations of active free form of IL‐18 [Citation25,Citation26].
The aforementioned findings are in line with the results of a Turkish group who evaluated changes in pre- and post-treatment levels of serum IL-18 in 46 aggressive NHL patients (87% of cases were DLBCL). Serum levels of serum IL-18 remained unchanged after chemotherapy. Consequently, their conclusion was that serum IL-18 measurements have no prognostic value in aggressive NHLs including DLBCL [Citation27].
Moreover, we probed utility of mean serum IL-18 level as a predictive cut-off value. Elevation of serum IL-18 was not predictive of remission (p = 0.465), or 3-year DFS rates (p = 0.127). Nevertheless, the 3-year OS rates for patients with high and low serum IL-18 levels were significantly different (p = 0.008). Earlier, Goto and fellows reported that serum IL-18 levels were predictive of CR, 4-year PFS, and OS rates in patients treated with R-CHOP (p = 0.0048, p < 0.0001, p < 0.0001, respectively) [Citation18]. Given these finding, serum IL-18 level seems to have a probable propensity to affect OS in DLBCL patients treated in rituximab era. However, a greater number of cases need to be followed-up for longer periods of time to reach a clearer understanding of the prognostic value of serum IL-18 level in DLBCLs in rituximab era.
On the other hand, the CR, 3-year DFS and OS rates for our patients with low pretreatment sIL-2R levels were significantly better than individuals with higher levels (p = 0.001, p = 0.007, p = 0.001, respectively). In multivariate analysis, high sIL-2R independently correlated with shorter DFS and OS (HR: 4.1, 95% CI: 1.0–16.5, p < 0.057; and HR: 11.4, 95% CI: 2.5–52.4, p < 0.022, respectively). These obtained results were like those reported by Goto and coworkers who found that high sIL-2R patients had a poorer outcome in terms of CR, PFS and OS for patients with DLBCL treated with R-CHOP (p = 0.0003, p < 0.0001, p < 0.0001, respectively) [Citation5]. Additional subsequent studies identified the prognostic value of sIL-2R in DLBCLs patients from different age and ethnic groups treated with R-CHOP. A report from Tomita and colleagues identified the prognostic value of sIL-2R levels either individually or as a part of SIL index (Stage, sIL-2R, LDH) in predicting poor PFS, and OS outcomes in 572 DLBCL patients treated with R-CHOP [Citation28]. Subsequently, a Spanish group evaluated the prognostic value of different cytokines including sIL-2R in 197 DLBCL patients treated with Rituximab based immunochemotherapy. Elevated levels of serum sIL-2R were significantly associated with lower CR rates and shorter PFS and OS [Citation19]. In a like manner, prognostic impact of pretreatment serum sIL-2R level was identified in elderly patients with DLBCL patients treated with R-CHOP in a Japanese study. This study showed that measurement of the sIL-2R level at diagnosis is clinically beneficial for identifying elderly patients with DLBCL who have a poor prognosis in terms of PFS and OS [Citation20]. Collectively, there is growing body of evidence supporting the proposal that pretreatment serum levels of sIL-2R is a potentially robust prognostic factor that can help stratifying poor risk DLBCL for more aggressive treatment.
The present study has several limitations. First, this was a study from a single institute. Second, the small sample size, and heterogeneity of patients’ characteristics in our study might have limited our ability to identify statistically significant differences in terms of OS, DFS and CR rates with respect to serum IL-18, and other conventional prognostic factors. Third, serum IL-18 and sIL-2R are not specific markers for DLBCL and its level are increased in several inflammatory conditions including viral infection and autoimmune disorders. Further, we did not consider many relevant prognostic factors, such as BCL2, MYC, Ki67 and GEP subtypes in our analyses.
Conclusion
Being potentially effective and applicable, pretreatment serum level of sIL-2R in DLBCL patients deserves to be considered as another important tool for identifying those with poor prognosis, whereas serum IL-18 level should be studied in larger prospective studies. Additionally, the most reliable prognostic factor or the best combination of some prognostic factors for DLBCL should be further clarified in order to assist in selecting the most appropriate immunotherapy-based treatment.
Future perspective
Our present findings have provided evidence for the utility of pretreatment serum levels of sIL-2R as potential prognostic factors in DLBCL patients. However, the most reliable prognostic factor or the best combination of some prognostic factors for DLBCL should be further clarified in order to assist in selecting the most appropriate immunotherapy-based treatment.
Biologic prognostic factors including cytokines are increasingly investigated to stratify diffuse large B-cell lymphoma (DLBCL) patients.
This study aimed to assess the impact of pretreatment serum levels of IL-18 and sIL-2R on the clinical outcome of patients with DLBCL treated with R-CHOP protocol.
Being potentially effective and applicable, pretreatment serum level of sIL-2R in DLBCL patients deserves to be considered as another important tool for identifying those with poor prognosis, whereas serum IL-18 level should be studied in larger prospective studies.
Author contributions
All the authors contributed equally to the conception and design of the study, data acquisition, analysis and manuscript creation.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgments
The authors would like to thank R Allam, NCI, Cairo University, who supervised the statistical work of this study. Special thanks are addressed to M Samra, NCI, Cairo University and H Hamed, Faculty of Medicine, Ain Shams University, for valuable discussions. The authors would like to thank all of their colleagues for sharing their reagents.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Al-HamadaniM , HabermannTM , CerhanJRet al.Non-Hodgkin lymphoma subtype distribution, geodemographic patterns and survival in the US: a longitudinal analysis of the National Cancer Data B se from 1998 to 2011. Am. J. Hematol.90(9), 790–795 (2015).
- EL-BolkainyM , AkramM , GoudaIet al.Lymphoma In: Pathology of Cancer . 4th Edition. 200–204 (2013).
- ShippM. A predictive model for aggressive non-Hodgkin’s lymphoma. The international non-Hodgkin’s lymphoma prognostic factors project. N. Engl. J. Med.329, 987–994 (1993).
- SehnLH , BerryB , ChhanabhaiMet al.The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood109, 1857–1861 (2007).
- GotoN , TsurumiH , GotoHet al.Serum soluble interleukin-2 receptor (sIL-2R) level is associated with the outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP regimens. Ann. Hematol.91(5), 705–714 (2012).
- FukumotoH , NishioM , NishioKet al.Interferon-γ-inducing factor gene transfection into Lewis lung carcinoma cells reduces tumorigenicity in vivo. Jap. J. Cancer Res.88(5), 501–505 (1997).
- KawabataT , IchikuraT , MajimaTet al.Preoperative serum IL-18 level as a postoperative prognostic marker in patients with gastric carcinoma. Cancer92(8), 2050–2055 (2001).
- PagèsF , BergerA , HengleinBet al.Modulation of IL-18 expression in human colon carcinoma: consequences for tumor immune surveillance. Int. J. Cancer84(3), 326–330 (1999).
- TakuboT , KumuraT , NakaoTet al.Comparative study on complete remission rate and overall survival in three groups classified based on the serum IL-18 level in non-Hodgkin’s lymphoma patients. Acta Haematol.104(4), 220–222 (2000).
- O’SheaJJ , GadinaM , SiegelRM. Cytokines and cytokine receptors in: clinical immunology, Fifth Edition. 127–155 (2019).
- WagnerDK , KiwanukaJ , EdwardsBet al.Soluble IL-2 receptor levels in patients with undifferentiated and lymphoblastic lymphomas: correlation with survival. J. Clin. Oncol.5(8), 1262–1274 (1987).
- VivianiS , CameriniE , BonfanteVet al.Soluble IL-2 receptors (sIL-2R) in Hodgkin’s disease: outcome and clinical implications. Br. J. Cancer77(6), 992 (1998).
- SemenzatoG , FoaR , AgostiniCet al.High serum levels of soluble IL-2 receptor in patients with B chronic lymphocytic leukemia. Blood70(2), 396–400 (1987).
- YasudaN , LaiPK , IpSHet al.Soluble IL-2 receptors in sera of Japanese patients with adult T cell leukemia mark activity of disease. Blood71(4), 1021–1026 (1988).
- KitagawaJI , HaraT , TsurumiHet al.Serum-soluble IL-2 receptor (sIL-2R) is an extremely strong prognostic factor for patients with peripheral T-cell lymphoma, unspecified (PTCL-U). J. Cancer Res. Clin. Oncol.135(1), 53–59 (2009).
- GotoN , TsurumiH , TakemuraMet al.Serum IL-18Levels are associated with response to treatment and survival in patients with aggressive non-Hodgkin’s lymphoma (ASH Annual Meeting Abstracts). Blood104, 4543 (2004).
- GotoH , TsurumiH , TakemuraMet al.Serum-soluble IL-2 receptor (sIL-2R) level determines clinical outcome in patients with aggressive non-Hodgkin’s lymphoma: in combination with the International Prognostic Index. J. Cancer Res. Clin. Oncol.131(2), 73–79 (2005).
- GotoN , TsurumiH , KasaharaSet al.Serum interleukin-18 level is associated with the outcome of patients with diffuse large B-cell lymphoma treated with CHOP or R-CHOP regimens. Eur. J. Haematol.87(3), 217–227 (2011).
- DlouhyI , FilellaX , RoviraJet al.High serum levels of soluble interleukin-2 receptor (sIL2-R), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF) are associated with adverse clinical features and predict poor outcome in diffuse large B-cell lymphoma. Leuk.Res.59, 20–25 (2017).
- UminoK , FujiwaraSI , MinakataDet al.Prognostic impact of serum soluble interleukin-2 receptor level at diagnosis in elderly patients with diffuse large B-cell lymphoma treated with R-CHOP. Leuk. Lymphoma 59, 1–8 (2018).
- NicolaidesC , DimouS , PavlidisN. Prognostic factors in aggressive non-Hodgkin’s lymphomas. Oncologist3(3), 189–197 (1998).
- CharbonneauB , MaurerMJ , AnsellSMet al.Pretreatment circulating serum cytokines associated with follicular and diffuse large B-cell lymphoma: a clinic-based case-control study. Cytokine60(3), 882–889 (2012).
- TomitaN , SakaiR , FujisawaSet al.SIL index, comprising stage, soluble interleukin-2 receptor, and lactate dehydrogenase, is a useful prognostic predictor in diffuse large B-cell lymphoma. Cancer Sci.103(8), 1518–1523 (2012).
- RautL , ChakrabartiP. Management of relapsed-refractory diffuse large B-cell lymphoma. South Asian J. Cancer.3(1), 66 (2014).
- NovickD , SchwartsburdB , PinkusRet al.A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine14(6), 334–342 (2001).
- BuflerP , AzamT , Gamboni-RobertsonFet al.A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc. Natl Acad. Sci. USA99(21), 13723–13728 (2002).
- SoydincHO , GuneyN , BasaranMet al.Clinical significance of interleukin-4 and interleukin-18 levels in aggressive non-Hodgkin’s lymphoma patients. Genet. Mol. Res.15(3), Doi: 10.4238/gmr.15038590 (2016).
- TomitaN , SuzukiT , MiyashitaKet al.The SIL index is a simple and objective prognostic indicator in diffuse large B-cell lymphoma. Leuk. Lymphoma57(12), 2763–2770 (2016).