Abstract
Aim: To determine overall survival (OS) and glycemic control in patients with cancer and diabetes. Materials & methods: Patients of our institution with breast, colon, lung, pancreas and prostate cancer were retrospectively reviewed. OS was compared between matched patients with and without diabetes, and changes in glucose value over time were assessed. Results: For 3934 patients each with and without diabetes, adjusted analysis showed no difference in OS according to diabetes status (hazard ratio: 1.07; 95% CI: 0.96–1.20). Mean glucose values decreased over time in patients with and without diabetes (p = 0.01). Conclusion: In this large study of patients with five common cancers, the co-occurrence of diabetes did not affect OS. Cancer did not adversely affect glucose levels.
Plain Language Summary
The aim of this study was to evaluate survival and glucose control in patients with cancer and diabetes at three separate geographic locations in a single health system. From an institutional cancer registry, we identified patients with breast, colon, lung, pancreas and prostate cancers. Patients with and without diabetes were matched by age, sex, cancer type, staging, geographic location and year of cancer diagnosis. In this study, the co-occurrence of diabetes did not affect overall survival. Cancer did not adversely affect glucose levels.
Keywords:
Cancer and diabetes are common diagnoses. Diabetes prevalence continues to increase in the US, and current estimates indicate that nearly 34 million people have the diagnosis [Citation1]. Between 2013 and 2017, an estimated 5,511,635 people alive in the US were diagnosed with cancer [Citation2]. Even though cancer rates are decreasing, the actual number of new cases is increasing because of population growth and aging each year [Citation3]. Moreover, up to 18% of patients with cancer may have coexisting diabetes [Citation4]. As the number of cases of diabetes and cancer increase, it will become more likely that these two conditions will coexist in the same patient. When the two are present in the same patient, care becomes more complex and may require additional specialists to assist with diabetes management and education. Therefore, the mechanism of how diabetes and cancer interact to affect a patient’s outcome is of interest.
One area of study has been the effect of diabetes on survival in patients with cancer. The relationship between cancer and diabetes has been previously examined in a single academic practice, and results showed that diabetes was common in patients with certain solid-organ cancers. In a combined population with different cancer diagnoses, the prevalence of diabetes was 6.8% [Citation5]. The relationship between cancer and diabetes also has been examined in many case–control studies of individual solid and hematologic cancers: breast, lung, prostate, colorectal, pancreatic, gastroesophageal, uterine/ovarian, melanoma, lymphoma, leukemia, squamous cell and neuroendocrine cancers [Citation6–17]. With the exception of melanoma and gastroesophageal and squamous cell cancers, diabetes had no significant effect on death or recurrence in any malignant process studied. Moreover, none of the studies showed adverse effects of cancer on glycemic control 1 year after cancer diagnosis among those with diabetes [Citation6–17].
Data on mortality rates in patients with cancer and coexisting diabetes have been inconsistent. Differences in findings most likely represent variations in methodologic approach to analyses [Citation6,Citation18,Citation19]. Given that the association between cancer, diabetes, survival and glycemic control remains unsettled, ongoing analyses are needed so that healthcare teams can better inform their patients regarding risks. The main limitation of the previous analyses was that data were derived from a single institution (located in the Southwestern US), and the studies often had small sample sizes. However, a convergence of the electronic health record (EHR) across three academic campuses of one institution afforded the opportunity to corroborate the findings on a larger sample size of cancer cases with and without diabetes. In this study, we assessed the interaction between cancer and diabetes from three geographic areas to further examine the questions regarding how diabetes affects cancer survival, and whether a diagnosis of cancer affects glycemic control.
Materials & methods
Overview of practice
The cancer center at our institution encompasses academic campuses in the Southeast, Southwest and Midwest (the sites included in this analysis). It is a comprehensive cancer center designated by the National Cancer Institute, with coordinated care and collaboration across the three sites.
Case selection
Methods similar to those in previous analyses were used for this larger study [Citation6–17], which was approved by our institutional review board. Data on cancer cases from calendar years 2012 through 2018 were retrospectively retrieved from the institutional cancer registry. Cases were identified by pathology results and by International Classification of Diseases, Ninth Revision codes. Annual review of the registry is performed, including updated survival information. This analysis was restricted to cancers of the colon, breast, lung, prostate and pancreas to maintain consistency with the previous analysis. Patients younger than 18 years old or who had more than one primary cancer diagnosis were excluded.
This initial cancer dataset was linked to the EHR to identify patients who had a coexisting diagnosis of diabetes (diagnostic code 250.00) corresponding around the time of cancer diagnosis. Data on hemoglobin A1c (HbA1c) and glucose levels during the first 12 months after cancer diagnosis were extracted. The glucose detection method uses hexokinase and is the same at all sites [Citation20]. Patients with diabetes (cases) were matched 1:1 using a greedy algorithm to patients with cancer but no diabetes (controls) to yield the final analytic dataset. Variables included in the matching algorithm were cancer type, stage, geographic location of care, age, sex and year of cancer diagnosis [Citation6–17]. EHR claims during the preceding and concurrent year from the date of cancer diagnosis were used to calculate the Charlson comorbidity index (CCI) [Citation21]. The CCI was modified to exclude diabetes from the calculation to capture the prevalence of comorbid conditions other than diabetes.
Previous studies have shown no significant association of diabetes related therapies with cancer outcomes, so these were not included in this analysis [Citation7–13]. In addition, cancer therapies are varied (e.g., cytotoxic chemotherapy, targeted therapies, immunotherapies, radiation and surgery) and also vary by cancer type. Moreover, cancer therapeutic protocols are standardized across all three sites, and little difference would be expected in outcomes on the basis of treatment. Therefore, these were not reviewed.
Statistical analyses
Statistical analyses were similar to those described in prior reports [Citation6–17]. Demographic and clinical variables were compared between matched groups with and without diabetes by use of paired t-tests for continuous variables and McNemar tests for categorical variables. For comparisons between geographic locations, χ2 tests or analysis of variance were used. Overall survival (OS) was calculated from time of cancer diagnosis until death. OS was estimated with the Kaplan–Meier method, and survival curves were compared between diabetes and non diabetes groups by use of the log-rank test for overall and cancer types separately. Cox proportional hazards models were used to assess OS and included matched pairs as a strata variable. An additional Cox model was constructed that included CCI (without diabetes) as a covariate. Linear mixed models evaluated HbA1c levels during the first year after cancer diagnosis for patients with diabetes only, because values were unavailable for most patients without diabetes. A similar approach was used for modeling glucose values during the same time frame in both groups. Fixed effects included time since cancer diagnosis (days), designation of case or control, an interaction term (days × case–control designation) and patient-specific random effects. SAS version 9.4 (SAS Institute Inc.) was used for analysis, and p-value less than 0.05 were considered statistically significant. Data were analyzed and reported in aggregate (overall for all cancers and all locations combined).
Results
Patient characteristics
A total of 7868 patients were analyzed from all the three geographic locations (3934 with diabetes, matched with 3934 without diabetes) (). The mean (SD) age was 67 (10) years at diagnosis. The majority of patients (60.0%) were from the Midwest location, with 21.5% from the Southeast and 18.6% from the Southwest location. A greater proportion of patients with diabetes were of race other than White (12.5 vs 7.9%; p < 0.001), and patients with diabetes had a significantly higher CCI score (6.2 vs 5.4; p < 0.001). Prostate cancer was the most common cancer type (n = 2656; 33.8%), followed by lung (n = 1794; 22.8%), breast (n = 1422; 18.1%), pancreas (n = 1344; 17.1%) and colon cancer (n = 652; 8.3%). Race, CCI, and age at cancer diagnosis differed significantly across geographic locations ().
Table 1. Patient demographics by diabetes status for total cohort.
Table 2. Patient demographics by geographic location for total cohort.
Cancer survival
In unadjusted analyses, after a median follow-up of 36.8 months, and when including all cancers aggregated by type and geographic location, patients with diabetes had significantly lower OS than patients without diabetes (hazard ratio: 1.16; 95% CI: 1.04–1.29; p = 0.007) (). A similar finding was noted for breast cancer (hazard ratio: 1.62; 95% CI: 1.09–2.41; p = 0.02). After adjusting for CCI; however, these comparisons were no longer significant. In addition, no differences in OS between patients with and without diabetes were noted for colon, lung, pancreatic and prostate cancers (). Analysis of OS according to geographic location showed no significant differences after adjusting for age, cancer type, race, sex, cancer stage and CCI (data not shown).
Table 3. Unadjusted and adjusted OS for patients with cancer and coexisting diabetes (compared with no diabetes), total cohort.
Glycemic control
Among patients with diabetes, 2049 (52.1% overall; 60% breast, 55% colon, 37% lung, 56% pancreas, 56% prostate cancer) had at least 1 HbA1c measurement during the year after cancer diagnosis; the overall mean (SD) HbA1c value was 6.9% (1.3%). Over a 1-year follow-up period, the overall HbA1c significantly decreased (p = 0.002) (A). HbA1c decreased significantly in all separate cancer diagnoses (p = 0.004), but no group differences were identified between cancer type (p = 0.14).
Data overall (all cancers, all sites) and for individual cancers (aggregate all sites). (A) Hemoglobin A1c (HbA1c) values for patients with diabetes only. (B) Glucose values for patients with and without diabetes.
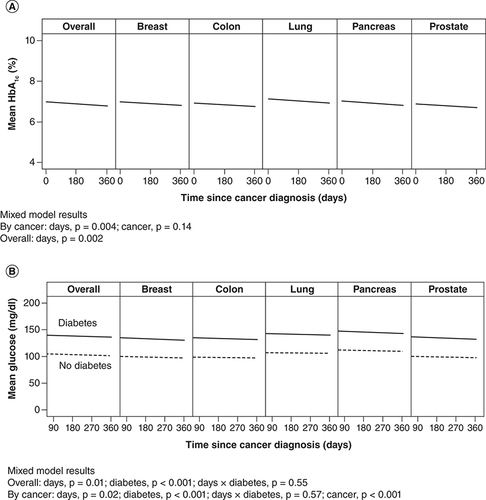
Among patients with diabetes, 1859 (47.3%) had at least one glucose measurement during the year after cancer diagnosis; mean (SD) value was 139 (51) mg/dl. In patients without diabetes, 1656 (42.1%) had at least one glucose measurement; mean (SD) value for these patients was 103 (18) mg/dl, which was significantly lower than in the diabetes group (p < 0.001) (B). Overall, glucose value significantly decreased over time among those with diabetes (p = 0.01). When cancer type was included in the mixed model, observations were similar: a decrease in glucose value over time among diabetes patients (p = 0.02). In addition, mean glucose value differed according to cancer type (p < 0.001) and was highest among those with pancreatic and lung cancers (B).
Discussion
It is still not clear how diabetes and solid-organ cancers interact to affect one another’s outcome. For example, data on survival have been inconsistent, with some studies showing that coexisting diabetes was associated with decreased survival and others not showing this outcome [Citation6,Citation18,Citation19]. A series of case–control analyses, comparing patients with and without diabetes, previously examined site-specific outcomes among patients with newly diagnosed breast, lung, colorectal, pancreas and prostate cancers. With few exceptions (e.g., in gastroesophageal cancer, squamous cell cancers of the head and neck and melanoma), these studies showed that coexisting diabetes was not associated with an increased mortality rate [Citation6–17]. This observation was consistent regardless of the data source – whether from institutional mortality data or the National Death Index [Citation22,Citation23]. In addition, cancer did not affect glycemic control during a 1-year follow-up period in patients with or without diabetes [Citation6–17].
The convergence of our EHR platform presented an opportunity to expand the previous analyses to a larger sample size and to assess differences across the geographic campuses of one healthcare system. Some differences were noted between diabetes and non diabetes patients (race and CCI) and across geographic locations (age at cancer diagnosis, race and CCI), although these are largely due to the differences in distribution of cancer types seen at the various locations. For instance, the Southwest site included a larger proportion of patients with prostate cancer than the other two sites. However, in adjusted analyses, no differences in OS were noted between patients with and without diabetes overall or for individual cancer types. Furthermore, no differences were seen in OS between geographic locations. Results of this study corroborate previous single-site findings. At least in this patient population, findings can be reassuring to patients and their healthcare team that the presence of diabetes does not decrease survival.
Several factors may affect glycemic control among patients with diabetes during treatment of cancer. For instance, glucocorticoids, which are often used during chemotherapy, could lead to acute exacerbations of hyperglycemia in patients with and without diabetes. In contrast, weight loss due to decreased appetite could result in decreased glucose levels. Results of analysis of this larger dataset support those of earlier studies indicating that glycemic control does not worsen during the first year after cancer diagnosis.
It is reassuring that OS and glycemic outcomes did not differ according to geographic location of care. Differences in demographics and comorbid conditions across sites could lead to variations in outcome. However, convergence of our EHR also brought a merging of treatment protocols across the healthcare system. These standardized approaches to care could mitigate any influence of differences in other factors on the outcomes examined here.
The results of this study confirm those of previous studies that diabetes does not affect survival in breast, colon, lung, pancreatic and prostate cancers, but similar observations have not been true in other cancer types. For instance, diabetes was associated with decreased OS in gastroesophageal and squamous cell cancers and with shorter progression-free survival in melanoma [Citation11,Citation12,Citation16]. The methods of the current study; however, could be used to reexamine these relationships on a larger and broader geographic scale.
Strengths of this study include the large sample size, inclusion of data from three geographic locations and results controlled for comorbid conditions. Nonetheless, there were several limitations. Although derived from three different geographic areas of care, the data were still from within the same healthcare system, in which cancer treatment protocols have been converged and standardized. It may not be unexpected, therefore, that outcomes would be similar. In addition, the affected population was mostly White. Different results may be obtained when examining OS and glycemic control from healthcare systems with populations that are more ethnically diverse or of lower socioeconomic status, which was not assessed in this analysis.
Conclusion
Results of this three-site study confirmed previous single institution results that diabetes does not affect OS and that cancer does not affect glycemic control in a population of patients with breast, colon, lung, pancreas and prostate cancers. Expanded analyses are needed from other healthcare facilities to determine whether these findings are common or unique to one healthcare system.
Future perspective
These findings should reassure providers that diabetes does not affect OS of patients with breast, colon, lung, pancreas and prostate cancers. Furthermore, cancer does not affect glycemic control in patients with diabetes.
The number of patients with coexisting cancer and diabetes will most likely increase.
Information on the interaction between cancer, diabetes and survival is needed.
In this large study of patients with five cancer types, diabetes did not affect survival.
A diagnosis of cancer did not adversely affect glycemic control.
Continued study from other health facilities is needed to determine whether these findings are common or unique to one healthcare system.
Continued study is needed to confirm findings in more diverse population groups.
Analyses can be extended to hematologic cancers to assess for similar findings regarding diabetes and survival in these populations.
At least in the patient populations analyzed here, findings can be reassuring to patients and their healthcare teams that the presence of diabetes does not decrease cancer survival.
Author contributions
NJ Karlin, HE Kosiorek, PM Verona, KE Coppola, CB Cook contributed in study conception and design; acquisition and analysis of data; drafting and revising the manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Acknowledgments
AB Quiggle, Mayo Clinic, substantively edited the manuscript. The Scientific Publications staff at Mayo Clinic provided proofreading and administrative and clerical support.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report 2020 (2020). http://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Centers for Disease Control and Prevention. United States Cancer Statistics: prevalence (2021). https://gis.cdc.gov/Cancer/USCS/#/Prevalence/
- Centers for Disease Control and Prevention. Changes Over Time: All Types of Cancer (2021). https://gis.cdc.gov/Cancer/USCS/#/Trends/
- HabibSL , RojnaM. Diabetes and risk of cancer. ISRN Oncol.2013, 583786 (2013).
- KarlinNJ , DueckAC , CookCB. Cancer with diabetes: prevalence, metabolic control, and survival in an academic oncology practice. Endocr. Pract.18(6), 898–905 (2012).
- KarlinNJ , DueckAC , NagiReddy SK , VeronaPM , CookCB. Implications of breast cancer with diabetes mellitus on patient outcomes and care. Diabetes Management4(5), 411–419 (2014).
- KarlinNJ , AminSB , BurasMR , KosiorekHE , VeronaPM , CookCB. Patient outcomes from lung cancer and diabetes mellitus: a matched case–control study. Future Sci. OA4(1), FSO248 (2018).
- KarlinNJ , AminSB , VeronaPM , KosiorekHE , CookCB. Co-existing prostate cancer and diabetes mellitus: implications for patient outcomes and care. Endocr. Pract.23(7), 816–821 (2017).
- KarlinNJ , AminSB , KosiorekHE , BurasMR , VeronaPM , CookCB. Survival and glycemic control in patients with colorectal cancer and diabetes mellitus. Future Sci. OA4(9), FSO335 (2018).
- KarlinNJ , AminSB , KosiorekHE , BurasMR , VeronaPM , CookCB. Survival and glycemic control outcomes among patients with coexisting pancreatic cancer and diabetes mellitus. Future Sci. OA4(4), FSO291 (2018).
- KarlinNJ , BurasMR , KosiorekHE , VeronaPM , CookCB. Glycemic control and survival of patients with coexisting diabetes mellitus and gastric or esophageal cancer. Future Sci. OA5(6), FSO397 (2019).
- KarlinNJ , MangoldAR , AminSBet al.Survival and glycemic control in patients with coexisting melanoma and diabetes mellitus. Future Sci. OA5(3), FSO368 (2019).
- KusneYN , KosiorekHE , BurasMRet al.Mortality and glycemic control among patients with diabetes mellitus and uterine or ovarian cancer. Future Sci. OA7(3), FSO670 (2020).
- RiceBJ , BurasMR , KosiorekHEet al.Survival and glycemic control in patients with coexisting lymphoma and diabetes: a case-control analysis. Future Sci. OA7(1), FSO641 (2020).
- WiedmeierJE , MountjoyLJ , BurasMRet al.Mortality and glycemic control among patients with acute and chronic myeloid leukemia and diabetes: a case–control study. Future Sci. OA7(1), FSO639 (2020).
- EderaineSA , DominguezJL , HarveyJAet al.Survival and glycemic control in patients with co-existing squamous cell carcinoma and diabetes mellitus. Future Sci. OA7(5), FSO683 (2021).
- KusneYN , KosiorekHE , BurasMRet al.Implications of neuroendocrine tumor and diabetes mellitus on patient outcomes and care: a matched case–control study. Future Sci. OA7(5), FSO684 (2021).
- RenehanAG , YehHC , JohnsonJAet al.Diabetes and cancer (2): evaluating the impact of diabetes on mortality in patients with cancer. Diabetologia55(6), 1619–1632 (2012).
- CurrieCJ , PooleCD , Jenkins-JonesS , GaleEA , JohnsonJA , MorganCL. Mortality after incident cancer in people with and without Type 2 diabetes: impact of metformin on survival. Diabetes Care35(2), 299–304 (2012).
- Roche Diagnostics Corporation. Glucose reagent package insert. (2000).
- CharlsonME , PompeiP , AlesKL , MacKenzieCR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis.40(5), 373–383 (1987).
- KarlinNJ , BurasMR , KosiorekHE , CoppolaKE , VeronaPM , CookCB. Assessing the relationship between institutional cancer and diabetes mortality rates using National Death Index data. Future Sci. OA6(10), FSO633 (2020).
- Centers for Disease Control and Prevention. National Death Index (2021). https://www.cdc.gov/nchs/ndi/index.htm
