Abstract
Aim: We aimed to evaluate early versus delayed removal of the indwelling urethral catheter (IUC) following transurethral resection of prostate (TURP). Methods: In this clinical trial conducted between July 2016 and June 2020, 90 patients underwent TURP were randomized equally into: group A, early IUC removal (24 h), and group B, delayed IUC removal (72 h). Results: The mean length of hospital stay was longer among the patients in group B. There were no significant differences in recatheterization, secondary bleeding, or UTI between groups A and B. The mean VAS score and CRBD were higher in group B. Conclusion: Early IUC removal following TURP is safe approach with favorable clinical outcomes.
Clinical Trial Registration: NCT04363970 (clinicaltrials.gov)
Plain language summary
Urethral catheter insertion is an important step after prostate surgery. It may cause urinary infection and distressing symptoms. In this study we evaluated early versus delayed catheter removal, and we found that early IUC removal is safe approach with favorable clinical outcomes.
Tweetable abstract
For patients undergoing transurethral resection of prostate due to benign prostate hyperplasia, early urethral catheter removal after 24 h is safe approach with favorable clinical outcomes.
Transurethral resection of the prostate (TURP) remains the gold standard surgical procedure worldwide and is a common, minimally invasive approach in urology practice.
The insertion of an indwelling urethral catheter (IUC) is essential for continuous urinary bladder irrigation to reduce the risk of clot formation and retention.
IUC use is associated with an increased risk of developing urinary tract infection (UTI), prolonged hospital stays, aggravated distressing symptoms.
The timing of IUC removal following TURP is controversial and depends mainly on individual clinical practice.
Early urethral catheter removal after 24 h is safe approach with favorable clinical outcomes.
The clinician can take a decision of early IUC removal following TURP, in order to reduce the hospital stays, cost of treatment and morbidities.
Benign prostate hyperplasia (BPH) is a common urological disease among elderly men and one of the major causes of lower urinary tract symptoms (LUTS). The prevalence of BPH increases with age, as approximately 80% of men older than 70 years suffer from BPH, with a significant negative impact on quality of life [Citation1,Citation2].
Globally, alpha 1 adrenergic receptor antagonists, alone or in combination with 5-alpha-reductase inhibitors, are commonly used as oral medical therapy for LUTS secondary to BPH. Guidelines indicate the need for surgical treatment for patients with severe LUTS or for those suffering from undesirable adverse effects or intolerance to medical therapy [Citation3]. Although several surgical approaches have been developed for the treatment of LUTS secondary to BPH, transurethral resection of the prostate (TURP) remains the gold standard surgical procedure worldwide and is a common, minimally invasive approach in urology practice [Citation4].
Following TURP, the insertion of an indwelling urethral catheter (IUC) is essential for continuous urinary bladder irrigation to reduce the risk of clot formation and retention. However, IUC use is associated with an increased risk of developing urinary tract infection (UTI). Catheter-associated urinary tract infection (UTI) is one of the most common hospital-acquired infections worldwide. Approximately 20% of hospital-acquired bacteremias arise from the urinary tract and are associated with a mortality rate of approximately 10% [Citation5-7]. Furthermore, catheter-associated UTIs have been associated with a longer hospital stay, and the economic burden is estimated at $676–12,000 per UTI case and $340–450 million annually [Citation8].
Similarly, prolonged IUC time, UTI and local catheter trauma increase the risk of urethral stricture formation after TURP [Citation9]. Catheter-related bladder discomfort (CRBD) and urethral catheter-related pain (UCRP) are the most common distressing symptoms of TURP [Citation10,Citation11]. CRBD is characterized by symptoms similar to those of overactive bladder, such as urinary frequency and urgency, with or without urge incontinence [Citation12]. Despite diverse medications, many controversies persist in clinical practice and no effective treatment for UCRP and CRBD without adverse events has been established yet [Citation13].
Currently, the optimal time for IUC removal after TURP has not been established. However, it is based on clinical practice rather than evidence-based knowledge, and varies considerably. Moreover, most of the available clinical studies are inconclusive, with variable results [Citation14]. Hence, we conducted this randomized, double blinded, clinical trial to evaluate the effect of early versus delayed IUC removal on the re-catheterization rate, length of hospital stays, secondary bleeding, risk of UTI, UCRP and CRBD.
Patients & methods
Study design & approval
This randomized, double blinded clinical trial was conducted between July 2016 and June 2020. The study protocol was approved by the Institutional Review Board at Jordan University Hospital and was registered at ClinicalTrials.gov (NCT04363970). All participants were informed of the study design and signed a written informed consent form in accordance with the Declaration of Helsinki.
Patient recruitment & randomization
Ninety patients with BPH who underwent TURP were randomized into two equal groups using a computer-generated randomization list. Group A comprised patients in whom the urethral catheter was removed 24 h after the procedure (early removal), and Group B comprised patients in whom the urethral catheter was removed 72 h after the procedure (delayed removal). The randomization order was blinded to the patients, primary surgeons and the post-operative independent observers.
Inclusion & exclusion criteria
Patients eligible for inclusion in the study were men aged ≥45 years with LUTS secondary to BPH. Patients with large amounts of PVR urine, prostate size more than 100 g, urethral stricture, UTI, simultaneous optical urethrotomy or cystolithotripsy, bleeding diathesis, spinal cord injury, cerebrovascular accident, neurogenic urinary bladder, capsular or urinary bladder perforation or severe bleeding during or after surgery were excluded.
Technique
All procedures were performed under general or spinal anesthesia by the same experienced surgeon (SA), who was blinded to the randomization order. Intravenous antibiotics were administered to all patients at the time of anesthesia induction and maintained throughout the hospital stay. The patient was then switched to oral antibiotics for 3 days. Monopolar TURP was performed, and the hyperplastic prostate tissue was removed from the surgical capsule. At the end of the procedure three-way urethral catheter 20 Fr was inserted for irrigation with normal saline 0.9%, and the balloon was inflated with 20 cc normal saline. Bladder irrigation was reduced as soon as feasible and stopped if the drainage was clear. Post-operatively, the urethral catheter was removed once the effluent was clear without irrigation, vital signs were stable, and laboratory tests, such as CBC, creatinine and electrolytes, were normal.
Assessment & outcome measures
All patients were admitted to the hospital and assessed preoperatively by history and physical examination, including International Prostate Symptom Score (IPSS) and digital rectal examination. Laboratory data collected included full blood counts; serum prostate-specific antigen (PSA); kidney function tests, including serum creatinine, urea, sodium and potassium; urine analyses; and urine cultures. Transabdominal ultrasonography was used to assess prostate size and post-void residual urine (PVR). Uroflowmetry tests were performed to measure the maximum flow rate (Qmax).
The outcomes of interest in this study were inability to void re-catheterization, secondary bleeding, UTI, length of hospital stay, CRBD and UCRP. IPSS, Qmax, and PVR were assessed at baseline, 2 weeks, 1 month and 3 months after TURP. UCRP and CRBD were evaluated before and after IUC removal. UCRP was assessed using the VAS score (0–10), with 0 indicating no pain and 10 indicating maximum unbearable pain. CRBD was assessed based on three grades and questionnaire items: grade I (mild 1–3); reported by the patient only on questioning; grade II (moderate 4–6); expressed by the patient without questioning and not accompanied by any behavioral responses; and grade III (severe 7–10); expressed by the patient and accompanied by any behavioral responses. If the VAS score was ≥4, tramadol (1 mg/kg) was administered at the maximum dose of 400 mg/24 h. After urethral catheter removal, all patients were evaluated on the floor by an independent observer before discharge for urinary retention and hematuria. The patients were discharged after they were able to void satisfactorily several times. Adverse events prompting re-catheterization include urinary retention and bleeding. Patients were instructed to visit the emergency room if they developed urinary retention, severe hematuria, or signs and symptoms of urinary tract infection. Patients were assessed at the urology outpatient clinic at different time points during the follow-up for urinary retention, hematuria, UTI, IPSS, PVR and Qmax. At any time point of follow-up, if the patient developed urinary retention and severe hematuria, IUC was reinserted. Patients who developed UTI were treated accordingly. The length of the hospital stay was assessed from the day of admission to the date of discharge.
Statistical analysis
Descriptive and inferential statistics were calculated using IBM SPSS Statistics for Windows sciences (IBM, 25). According to the level of measurement of the variables, t-test and chi-square tests were used to compare the participants' characteristics and clinical outcomes based on the time of catheter removal after TURP. An independent samples t-test was used to compare the sample means of the two independent groups for an interval-scale variable when the distribution was approximately normal. The criteria utilized were a power of 80%, a moderate effect size of 0.55, and an alpha of 0.05. The sample size was calculated using G*Power software (Faul et al.) [Citation15]. The required sample size for each group is 42. The actual sample size was 90 (45 patients per group).
Results
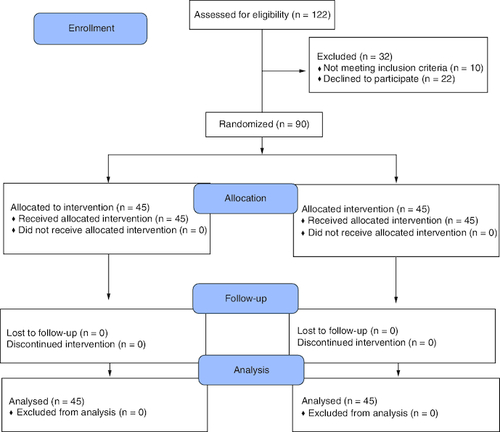
summarizes the patient enrollment, allocation, follow-up, and final analyses in both study groups. A total of 45 patients were included in each group. The mean baseline data and perioperative characteristics were comparable between patients in both groups, with no significant differences. presents the baseline and perioperative clinical characteristics of patients.
Table 1. Baseline data and perioperative characteristics.
The mean length of hospital stay was longer among patients in group B than among patients in group A, 3.64 ± 0.57 versus 2.83 ± 0.43, (p < 0.001).
Most patients in both groups voided successfully after IUC removal and had no urinary retention requiring urethral re-catheterization. There were no significant differences in re-catheterization, secondary hemorrhage and UTI between groups A and B 0.07 ± 0.25 versus 0.09 ± 0.29 (p = 0.70), 0.13 ± 0.34 versus 0.09 ± 0.29 (p = 0.51), 0.00 ± 0.0 versus 0.07 ± 0.25 (p = 0.08), respectively. Although there was no significant difference between the groups in terms of UTI, patients in group B who had delayed IUC removal were at a greater risk of developing UTI (p = 0.08) ().
Table 2. Comparison of clinical data.
The mean VAS score for UCRP before IUC removal was significantly higher in Group B than in Group A 3.73 ± 1.18 versus 2.67 ± 1.04 (p < 0.001). After the removal of the IUC, the VAS score was still higher among patients in group B 0.56 ± 0.62 versus 0.24 ± 0.61 (p = 0.019). CRBD was significantly higher before and after IUC removal in Group B 5.49 ± 1.20 versus 3.36 ± 1.25 (p < 0.001), 1.11 ± 0.57 versus 0.22 ± 0.47 (p < 0.001), respectively. There were no significant differences between the groups in terms of IPSS, Qmax or PVR at baseline and 3 months after TURP. The mean total dose of tramadol was higher among patients in group B than in group A (129.0 ± 105.26 mg vs 45.44 ± 59.13 mg, respectively); (p < 0.001) ().
Discussion
The ideal time for IUC removal after TURP remains controversial, and the decision is usually based on clinical practice rather than on evidence-based knowledge. The major finding of our double blinded, randomized, clinical trial was that early IUC removal after TURP was a feasible and safe clinical approach. Patients in both groups voided successfully, with no significant differences between them in terms of re-catheterization, secondary bleeding and UTI. In addition, the mean length of hospital stay, VAS score and CRBD were lower among the patients in the early IUC removal group.
Yu et al. [Citation14] reported in meta-analysis that there was no significant difference in the rate of re-catheterization between the early and delayed catheter removal groups (RR: 1.12, 95% CI: 0.73 1.72). In contrast, different studies have reported that early catheter removal leads to an increased re-catheterization rate and clot retention compared with delayed catheter removal [Citation16-18]. In our study, the mean re-catheterization rate was 0.07 ± 0.25, (p = 0.70).
Chander et al. [Citation19] demonstrated in their study that earlier catheter removal reduced the length of hospital stay from 3.1 to 1.28 days. Similarly, Shum et al. [Citation20] concluded that early catheter removal on the first post-operative day was safe, with an overall hospital stay of 1.6 days. However, this study had a small sample size (40 patients) and the energy source for TURP was bipolar, whereas we used mono-polar. Our results confirm the previous finding that the length of hospital stay was significantly reduced in the early catheter removal group to 2.83 ± 0.43 versus 3.64 ± 0.57 (p < 0.001).
Furthermore, several studies have reported that early catheter removal is not only safe but also cost-effective. Mueller et al. [Citation21] reported that the mean cost savings for early catheter removal following TURP were $829 and $1406 for patients aged <70 and >70 years, respectively.
Secondary bleeding is mainly attributed to surgical techniques and patient factors such as bleeding diathesis, large prostate volume and comorbidity [Citation22]. One variable that can predispose patients to perioperative bleeding is UTI [Citation23]. We ensured that all patients had UTI preoperatively and were treated preoperatively. We also administered prophylactic antibiotics at the time of induction and continued the procedure post-operatively. In addition, Chander et al. [Citation19] did not find significant bleeding or clot retention after early catheter removal. They reported early catheter removal within 7.5 h in 92% of patients and within 10 h in the remaining 8% of their patients. None of the patients required re-catheterization due to bleeding or clot retention. Yu et al. [Citation14] reported in meta-analysis that there were no significant differences in the rate of secondary hemorrhage between the early and delayed catheter removal groups (RR: 1.07, 95% CI: 0.54 2.13).
IUC is a common cause of UTI, as it increases the risk of infection by 5–10% per day of use [Citation24]. The expert panel of the Infectious Diseases Society of America agreed with evidence-based international clinical practice guidelines for procedures and strategies to reduce the risk of catheter-associated asymptomatic bacteriuria and UTI. They concluded that there is strong evidence that the IUC should be removed as soon as it is no longer required to minimize the risk of bacteriuria and UTI [Citation7]. In the present study, the mean UTI in both groups was comparable, but patients in group B who underwent late IUC removal were more likely to have UTI (p = 0.08).
UCRP and CRBD can trigger serious behavioral effects, such as confusion and agitation, which can lead to traumatic attempts to remove the urethral catheter, causing urethral injury and subsequent urethral stricture [Citation10,Citation25-27]. Muscarinic receptor antagonists (MRA) have been shown to be effective in improving tolerance IUC's [Citation10,Citation25]. However, this approach has proven disadvantages related to undesirable adverse effects and pain, which seem to be unrelated to the muscarinic receptors. In our study, the mean VAS and CRBD scores before and after IUC removal were lower in group A, and this is expected because the duration of IUC in group A was shorter (24 h vs 72 h).
This study has some limitations. First, the sample size is small. Second, we did not provide a validated assessment of the cost and quality of life. Finally, there is no validated questionnaire to measure CRBD. Despite these limitations, this study was a randomized controlled trial and all patients, primary surgeons and post-operative independent observers, were blinded to the randomization at the end of the surgery and post-operative follow-up.
Conclusion
Our data indicate that early IUC removal following TURP is a safe and feasible clinical approach that does not increase the incidence of recatheterization, secondary bleeding and UTI. Furthermore, the length of hospital stays, UCRP levels, CRBD and analgesic consumption were significantly reduced. Based on our clinical results, the clinician can take a decision of early IUC removal following TURP, in order to reduce the hospital stay, cost of treatment and morbidities.
Author contributions
All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SA Demour: manuscript writing, data collection, conception and design of the analysis and main conceptual ideas. M Al-Zubi, M Ababneh and S Al-Rawashdah: research design, data collection and manuscript drafting M Ahmad: data analysis and drafting and editing of the manuscript.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained institutional review board approval from Jordan university hospital (IRB #10/2016/14734) for the research described. In addition, they have obtained verbal and written informed consent from the patients for the inclusion of their medical and treatment history within this work.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data (clinical trial registration number NCT04363970). Individual, de-identified participant data and study protocols will be available beginning 9 months and ending 36 months following article publication. The use of this shared data is in accordance with the following terms agreed upon their receipt: investigators whose proposed use of the data has been approve and an independent review committee identified for this purpose and for individual participant dale-meld analysis.
Acknowledgments
The authors are thankful to Dr Sulaiman Al Habib Medical Group's Research Center for their tremendous support.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol. Clin. North Am. 43, 289–297 (2016).
- Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the Health Professionals Follow-up Study. Urology 59, 245–250 (2002).
- Şahin B, Çam HK. The current approach to male patients with lower urinary tract symptoms. J. Urol. Surg. 8, 130–134 (2021).
- Gacci M, Sakalis VI, Karavitakis M et al. European Association of Urology guidelines on male urinary incontinence. Eur. Urol 82, 387–398 (2022).
- Jennifer A, Kaplan JTC. Near-perfect compliance with SCIP Inf-9 had no effect on catheter utilization or urinary tract infections at an academic medical center. Am. J. Surg. 215, 23–27 (2018).
- Okrainec A, Aarts MA, Conn LG et al. Compliance with urinary catheter removal guidelines leads to improved outcome in enhanced recovery after surgery patients. J. Gastrointest Surg. 21, 1309–1317 (2017).
- Hooton TM, Bradley SF, Cardenas DD et al. prevention, and treatment of catheter-aassociated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious diseases society of America. Clin. Infect. Dis. 50, 625–663 (2010).
- Kang CY, Chaudhry OO, Halabi WJ et al. Risk factors for post-operative urinary tract infection and urinary retention in patients undergoing surgery for colorectal cancer. Am. Surg. 78, 1100–1104 (2012).
- Park JK, Lee SK, Han SH, Kim SD, Choi KS, Kim MK. Is warm temperature necessary to prevent urethral stricture in combined transurethral resection and vaporization of prostate? Urology 74, 125–129 (2009).
- Agarwal A, Raza M, Singhal V et al. The efficacy of tolterodine for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Anesth. Analg. 101, 1065–1071 (2005).
- Tauzin-Fin P, Sesay M, Svartz L, Krol-Houdek MC, Maurette P. Sublingual oxybutynin reduces post-operative pain related to indwelling bladder catheter after radical retropubic prostatectomy. Br. J. Anaesth. 99, 572–575 (2007).
- Hur M, Park SK, Yoon HK et al. Comparative effectiveness of interventions for managing post-operative catheter-related bladder discomfort: a systematic review and network meta-analysis. J. Anesth. 33, 197–208 (2019).
- Zugail AS, Pinar U, Irani J. Evaluation of pain and catheter-related bladder discomfort relative to balloon volumes of indwelling urinary catheters: a prospective study. Investig Clin. Urol. 60, 35–39 (2019).
- Yu JJ, Li Q, Zhang P, Shu B. Early catheter removal adds no significant morbidity following transurethral resection of the prostate: a systematic review and meta-analysis. Int. J. Clin. Exp. Med. 11, 1448–1457 (2018).
- Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39, 175–191 (2007).
- Agrawal SK, Kumar AS. Early removal of catheter following transurethral resection of the prostate. Br. J. Urol. 72, 928–929 (1993).
- Mamo GJ, Cohen SP. Early catheter removal vs conventional practice in patients undergoing transurethral resection of prostate. Urology 37, 519–522 (1991).
- Şahin C, Kalkan M. The effect of catheter removal time following transurethral resection of the prostate on post-operative urinary retention. Eur. J. Gen. Med. 8, 280–283 (2011).
- Chander J, Vanitha V, Lal P, Ramteke VK. Transurethral resection of the prostate as catheter-free day-care surgery. BJU Int. 92, 422–425 (2003).
- Shum CF, Mukherjee A, Teo CPC. Catheter-free discharge on first post-operative day after bipolar transurethral resection of prostate: clinical outcomes of 100 cases. Int. J. Urol. 21, 313–318 (2014).
- Mueller EJ, Zeidman EJ, Desmond PM, Thompson IM, Optenberg SA, Wasson J. Reduction of length of stay and cost of transurethral resection of the prostate by early catheter removal. Br. J. Urol. 78, 893–896 (1996).
- Teng J, Zhang D, Li Y, Yin L, Wang K, Cui X. Photoselective vaporization with the green light laser vs transurethral resection of the prostate for treating benign prostate hyperplasia: a systematic review and meta-analysis. BJU Int. 111, 312–323 (2013).
- Shrestha BM, Prasopshanti K, Matanhelia SS, Peeling WB. Blood loss during and after transurethral resection of prostate: a prospective study. Kathmandu Univ. Med. J. 6, 329–334 (2008).
- Givens CD, Wenzel RP. Catheter-associated urinary tract infections in surgical patients: a controlled study on the excess morbidity and costs. J. Urol. 124, 646–648 (1980).
- Agarwal A, Dhiraaj S, Singhal V, Kapoor R, Tandon M. Comparison of efficacy of oxybutynin and tolterodine for prevention of catheter related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Br. J. Anaesth. 96, 377–380 (2006).
- Tauzin-Fin P, Stecken L, Sztark F. Inconfort lié à la sonde vésicale en période postopératoire. Ann. Fr. Anesth Reanim. 31, 605–608 (2012).
- Zhang Z, Cao Z, Xu C et al. Solifenacin is able to improve the irritative symptoms after transurethral resection of bladder tumors. Urology 84, 117–121 (2014).