Abstract
The role of pharmaceutical physicians who are the experts working in pharmaceutical companies has progressed over the last few decades, from supervising research and development (R&D) studies and/or providing support to marketing teams to serving an independent critical function. In this review, we focus on pharmaceutical physicians serving medical affairs functions in the pharmaceutical industry. Historically, members of the medical affairs team mainly provided a bridge between commercial teams and the R&D sector and between the organization and external stakeholders. Such teams may even have been managed by other departments, with an emphasis on acquiring and generating data for regulatory purposes. In recent years, the role of medical affairs has broadened due to a change in focus and the increasingly stringent regulatory landscape. Strict regulations require the detachment of commercial from medical activities within pharmaceutical organizations. This change provides an opportunity for a different type of partnership, allowing scientifically minded and medically driven initiatives. This article summarizes the key role of pharmaceutical industry-based physicians in medical affairs and discusses the emerging and evolving role of medical affairs for value creation in evidence generation and medical education.
Role of medical affairs in the pharmaceutical industry
Internal: bridge between research and development (R&D) and commercial functions
As regulatory bodies increase scrutiny on the promotion of therapeutic products, the medical affairs department has played an important role in separating R&D and commercial functions to reduce the commercial influence on R&D efforts.Citation1,Citation2 Medical affairs serves as a liaison between the two functions, facilitating the transition of drugs from R&D to commercial.Citation3 Although the marketing department designs and provides pharmaceutical sales representatives (PSRs) with visual aids to engage physicians, medical affairs play a vital role in approving and providing feedback on such information tools.Citation4 Pharmaceutical industry-based physicians play an important role in ensuring that key messages are backed by sound scientific evidence and no misleading claims are made.Citation5 The medical affairs department is also involved in training PSRs to be knowledgeable about the company’s products.Citation6 This is relevant because one of the roles of physician–PSR interactions is to inform time-poor physicians about the latest therapeutic area and drug data, although these interactions generally tend to be more promotional.Citation7
External: bridge between the organization and external stakeholders
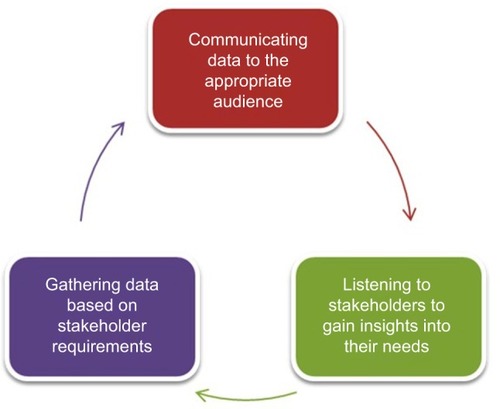
Although PSRs have historically been considered valuable sources of medical information, their perceived importance to physicians has been steadily decreasing. Many physicians now choose to rely on other sources, such as manufacturer websites and peer-reviewed journals, for their information.Citation8 This may have been one of the reasons why almost half of all new drug launches over the last 8 years have failed to fulfill their financial expectations.Citation9 One approach used by pharmaceutical companies to address this underperformance has been to bolster their medical affairs teams.Citation5 Given that evidence-based information is of high interest to physicians, medical affairs will play an important role in gathering and presenting scientific data alongside information about potential patient outcomes. There has also been a shift in focus from broad therapeutic areas covering large patient groups to therapy involving smaller populations, targeted therapy, and personalized medicine.Citation10 This shift has placed more emphasis on the role of medical affairs in establishing connections with health care professionals and providing education about potential individualized therapeutic options.Citation10 In essence, the external-facing role of medical affairs can be summarized using the medical affairs external communication cycle ().Citation10
Companies are increasingly deploying Medical Science Liaisons (MSLs) in the field to communicate scientific information to key stakeholders, including physicians, and to capture relevant medical insights.Citation11 The academic qualifications of MSLs range from medical and pharmacy degrees to PhDs, although most companies prefer postgraduate, doctorate, or specialist qualifications.Citation12 Senior positions in medical affairs, eg, medical manager/medical director roles, generally require at least a basic medical degree and offer dynamic and challenging careers for physicians beyond patient interaction.Citation13 Effective communication skills and commercial acumen as well as management and leadership skills are the key attributes required to be successful in these roles.Citation13
Medical affairs may also be involved in organizing medical advisory boards, Phase IV clinical trials, preparation of medical responses to enquiries, and preapproved early drug access programs.Citation6 Medical affairs departments also play an important function in providing responses to off-label enquiries, which must be handled independently of commercial teams.Citation14
Medical advisory boards provide pharmaceutical companies with valuable insights on various topics, ranging from trial protocols to regulatory submissions.Citation15 Advisory boards can be held at any stage during product development and facilitate the generation of data, the development of products, and the creation of trustworthy educational content.Citation16 However, to make sure that advisory boards provide a return on investment, medical affairs teams need to ensure the optimal allocation of resources.Citation17 This includes having a clear objective for the meeting, selecting the appropriate key opinion leaders (KOLs) or therapeutic area experts, ensuring adequate preparation, and facilitating the implementation of meeting action points.Citation18
Early access programs (EAPs)
EAPs are one way in which pharmaceutical companies can facilitate access to newer, unapproved therapies for patients with rare and/or severe diseases in an ethical manner.Citation19,Citation20 Adoption of EAPs in pharmaceutical companies is becoming increasingly prevalent due to the variety of benefits of these programs.Citation20 In Europe, there are two main types of EAPs: Compassionate Use Programs (CUPs) and Named Patient Programs (NPPs).Citation21 In CUPs, patients in selected health care institutions with severe, debilitating, or life-threatening illnesses who cannot be treated adequately with existing medical treatments are granted access to compassionate (free of cost) use of preapproved drugs by pharmaceutical companies.Citation21 In contrast, NPPs grant access to preapproved drugs to specific “named” patients in response to requests from physicians who ultimately bear the costs of these treatments.Citation21 The emergence of EAPs means that there is a greater need for the mobilization of medical affairs teams to oversee their planning and execution.Citation21 The importance of EAPs cannot be underestimated. For example, the WHO approved the provision of the investigational monoclonal antibody, ZMapp, to six Ebola patients for therapeutic purposes and to prevent the spread of the virus; four of the six patients were saved.Citation20
Investigator-initiated research (IIR)
An IIR is a research led by an independent investigator and that is related to a pharmaceutical company’s area of interest.Citation22 In IIR, the principal investigator is involved in the execution of the study and oversees its regulatory management; the latter is otherwise often the responsibility of pharmaceutical or biotechnology companies.Citation22 However, the investigator may request support from pharmaceutical companies in the form of funding and/or product supply without the company assuming the responsibilities of a “sponsor”, as defined by the International Conference on Harmonization-Good Clinical Practice (ICH-GCP).Citation22,Citation23 Medical affairs teams within pharmaceutical companies are tasked with overseeing IIRs, with an increasingly large proportion of companies using MSLs to support IIR initiatives.Citation24
Evolving role of medical affairs in generating medical evidence
Phase IV real-world clinical studies
Randomized controlled trials (RCTs) are the gold standard for the comparison of different clinical interventions.Citation10,Citation25 Systematic reviews and meta-analyses may also be conducted later to combine results from multiple studies in an effort to increase the statistical power of the results.Citation26 However, RCTs are performed in clinical settings with stringent controls on many variables because they are designed to establish a causal relationship between an intervention and an outcome.Citation25 In addition, RCTs may not be able to detect long-term adverse events.Citation27 Therefore, there is some skepticism about the real-world clinical practice applicability of data from RCTs.
Real-world data (RWD) include information on patients’ health status and outcomes that are obtained regularly from sources such as electronic health records (EHRs) and disease registries. RWD can aid in identifying long-term adverse events and guide the development of specific guidelines in clinical practice.Citation28 Obtaining RWD from pragmatic clinical trials and observational studies is important for demonstrating the “worth” of health care solutions to the various stakeholders.
Medical teams are also increasingly involved in the execution of postlaunch clinical trials, including Phase IV real-world studies.Citation6 The scope of this work is still evolving but is currently the subject of increased attention and may allow the exploration of new questions such as potential new drug indications for smaller patient groups.Citation29
Health economics and outcomes research
As the complexity of the medical landscape increases and the number of treatment options available to patients continues to expand, there is growing demand for the value of medications to be demonstrated.Citation30 This emphasis on the “value” of health care solutions, rather than just the price, has put pressure on pharmaceutical companies to demonstrate the worth of their health care solutions in terms of cost-effectiveness as well as direct and indirect benefits.Citation31 Thus, there is an increased focus on health economics and outcomes research (HEOR) that requires relevant data. Medical affairs, in collaboration with market access colleagues, may conduct HEOR to understand and communicate the value of products to external stakeholders, including Health Technology Assessment (HTA) bodies.Citation31
HTA
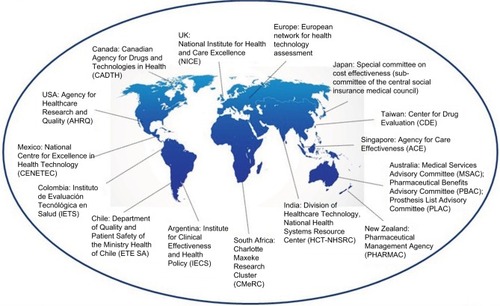
There are a number of different HTA bodies around the world, and these are highly relevant to pharmaceutical companies ().Citation31–Citation39 The role of HTA organizations is to enable efficient allocation of finite resources to meet the almost infinite demands for better medical technologies, including pharmaceuticals, medical devices, and other medical interventions.Citation34,Citation40
The HTA-driven global shift toward evidence-based value analysis has seen the expansion of the medical affairs function to consider HEOR aspects. Medical affairs plays an important role in ensuring that HTA requirements are met, sometimes even before the medical therapy has been approved for marketing.Citation41,Citation42 For example, the UK HTA agency, the National Institute for Health and Care Excellence (NICE), receives information about potential new therapies up to 20 months before they have been authorized to enter the market.Citation42 This liaison with HTA bodies ensures that pharmaceutical companies are able to fulfill the criteria required to meet increasingly stringent HTA requirements. This is beneficial because earlier collaboration will decrease delays to market access for products, thereby allowing patients to have timely access to new therapies.
Scientific publications
Medical affairs teams are increasingly being involved in the communication of nonpromotional scientific content via a variety of channels, including scientific publications.Citation3,Citation43 Original articles (eg, from RWD, market research on educational needs, and practice gaps), review articles, pharmaceutical-independent consensus/expert opinions, and letters to editors are key types of publications authored or led by medical affairs personnel.
Several studies have highlighted gaps between actual practice and scientific evidence in the clinical setting.Citation44–Citation47 Narrowing of these gaps has proven beneficial in reducing patient morbidity and mortality and decreasing the cost of health care.Citation48–Citation51 Medical affairs professionals may be involved in identifying and communicating these gaps through cross-sectional research.Citation52–Citation55 They can also collaborate with health providers on narrative review manuscripts that address gaps in current clinical practice or educational needs.Citation56–Citation58 Identifying and communicating practice gaps could provide support to convince government health divisions to take action and update clinical practice guidelines.Citation59
Addressing inconsistencies in trial results by combining data using systematic review and/or meta-analysis techniques is another important approach to facilitate better understating of clinical data.Citation26 Medical affairs has an important, and currently under-utilized, role in leading these publications in collaboration with health providers.
In the absence of local guidelines, pharmaceutical-independent consensus/expert opinion publications play an important role in bridging existing knowledge and practice gaps.Citation60 A “medical consensus” document is generally regarded as a credible and evidence-based publication by health professionals.Citation61 Medical affairs teams can lead the publication of consensus papers (eg, based on medical advisory board meetings and clinical forums) to support the adoption of evidence-based practices in local clinical settings.Citation62
However, it is extremely important for medical affairs personnel to adhere to good publication practice standards while leading such activities. A series of reporting guidelines has been developed and motivated primarily by concerns about the quality of publications ().Citation63
Table 1 Recommendations from key guidelines for drafting scientific publications
Evolving role of medical affairs in medical and public education
Continuing medical education (CME) for health providers
CME is a cornerstone of current medical practice.Citation64 Although CME assessments and regulations differ between countries, almost all require physicians to undergo some form of continuous education during their practice.Citation65,Citation66 In countries that mandate CME for physicians, regulatory institutions stipulate the expected standards and practices for such activities. Should a physician fail to meet these requirements, the consequences depend on the regulations of each specific country.Citation66–Citation68
Despite often being mandatory, the costs of attending CME events are usually high and can act as a deterrent to physician participation.Citation69 It is therefore not surprising that commercial support contributes to a large percentage of CME funding.Citation70 While the proportion of total commercial funding has decreased from more than half in 2006 to slightly above a quarter in 2016, it still makes a sizeable contribution ($704 million in 2016).Citation71,Citation72 This commercial funding is vital because many CME events could not proceed without financial backing.Citation73 Pharmaceutical companies see this as a key opportunity to present the latest findings and to educate physicians about new products or even established therapeutic areas.Citation74 Physicians benefit by gaining insight into medications and therapy options that may be useful for their clinical practice.Citation75 However, it is important to note that there is a major difference between industry-led and industry-independent CME events. The former includes events where a pharmaceutical company has substantial influence over the presented content, and these are generally not allowed to contribute to CME credit in most developed countries.Citation76 These events are also often perceived as marketing ventures by companies to introduce new drugs or studies to physicians.Citation68
The pharmaceutical industry supports independent CME events through unrestricted educational grants and sponsorships.Citation77 Most of these may still relate to education about a specific drug or treatment area of commercial relevance.Citation78 Sponsorships differ from educational grants in that they generally provide direct, tangible benefits to the sponsor while grants have no strings attached.Citation78 For example, sponsorship may be provided to subsidize the cost of entry to CME events for physicians, where permitted by law. In addition, conference sponsors are often able to set up booths and promote their brand through the placement of product logos, promotional materials, and handouts directly outside the event venue. In contrast, independent grants come with no requirement for the payer’s direct benefit.Citation79,Citation80 Many companies offer independent grants to a variety of official institutions.Citation81–Citation83 These include universities, medical schools, patient associations, and professional bodies.Citation82,Citation84 Grants are given with the goal of ensuring continuous improvement in health care provision and patient outcomes.Citation85 The defining difference between independent grants and sponsorship is that grant recipients have full control over the content of their communications.Citation86 This means that grant-supported events are less likely to be under industry influence and more likely to promote the communication of unbiased information. As a result, grant-funded events are often perceived as having greater credibility than sponsored events.Citation87 In fact, many have suggested that industry grants should be overseen and managed by independent committees that would be responsible for decisions about which specific course or event this funding would be disseminated to.Citation88–Citation90
In the pharmaceutical industry, there has been a recent trend for responsibility for CME activities to be transferred from the marketing department to medical affairs.Citation91 This reflects the shift in pharmaceutical strategy driven by stakeholder demand for credible, accurate, and evidence-based information. The growing role of medical affairs in the management of CME activities is an important step toward mitigating the “stigma” associated with industry-sponsored events and building trust between the pharmaceutical industry and its key stakeholders.
Public education
The internet has become one of the primary sources for consumers to access medical information.Citation92,Citation93 It provides a cost- and time-efficient avenue for seeking medical advice without having to consult a physician. One study found that about 35% of people who search for medical information online use this as a tool to self-diagnose their ailments.Citation94 More alarmingly, over a third of these people do not double-check their self-diagnoses with a health care professional.Citation94
While there are many credible sources of information on the internet,Citation95 a large portion of online resources provide low quality information and can thus pose harm to consumers’ health.Citation96,Citation97 The widespread availability of noncredible medical information is a danger to society, and pharmaceutical companies have a civic duty to protect consumers by being sources of trustworthy information. The European Union (EU) took this into account when they decided to loosen the policy against allowing prescription medication information to be posted online by their manufacturing companies.Citation98 In addition to posting information about their products online, pharmaceutical companies provide health education to the public and carry out real-world public health initiatives.Citation99,Citation100 The importance of medical affairs personnel in these ventures will only grow as more and more patients go online to source health and medical information.
Another pertinent issue is direct-to-consumer advertising (DTCA) of prescription medicines. This is prohibited in most countries, with notable exceptions being Hong Kong, New Zealand, and the USA.Citation101–Citation103 In the USA, the Food and Drug Administration (FDA) imposes restrictions on advertising and mandates that no misleading or misrepresented information is to be provided to consumers.Citation104 While DTCA provides a potential avenue for pharmaceutical companies to educate the general public about their products, many also see a potential risk for public harm.Citation105,Citation106 In fact, the perceived risk is so high that in 2008, 22 of the 27 EU members voted to prohibit even the provision of limited information to consumers via DTCA.Citation107 These fears are largely due to the perception that pharmaceutical companies do not consider public education as the main aim of their advertising but, instead, strive to gain a larger profit margin by acquiring more paying customers.Citation108 As the importance of consumers as stakeholders increases (aptly demonstrated by the rising prominence of patient associations in Europe), medical affairs will have an ever-expanding role in ensuring that patient centricity is at the forefront of any initiative that their company undertakes.Citation109
Collaborations with patient associations
More recently, companies have extended medical engagement activities to patient associations in order to fully leverage the valuable patient insights they can provide.Citation6 The medical affairs function plays an important role in understanding and integrating patient perspectives into the organization. According to the US FDA, patient involvement in drug development is a priority and, therefore, medical affairs should aim to involve patients earlier in the drug development process to better facilitate the identification of meaningful clinical trial endpoints.Citation110 Medical affairs departments also help to educate patients and health care professionals on the organization’s R&D pipeline and current clinical trials. In addition, reaching out to patients can increase the possibility of obtaining funding for orphan drugs from charitable patient organizations given the shift in focus to smaller therapeutic areas.Citation111 Such engagements provide pharmaceutical companies with the opportunity to develop more scientific, accurate, and patient-centric programs to engage their key stakeholders, giving them a competitive edge in the changing pharmaceutical landscape.
Unique conflicts of interest (COIs) for medical affairs professionals
Medical affairs physicians themselves face many challenges in terms of COIs. These arise when a physician’s ability to make decisions in the best interests of their patients becomes affected by their relationship with the pharmaceutical company that employs them.Citation112 Their position within the company is noncommercial in nature and could potentially conflict with marketing objectives.Citation113 However, as both an employee of a company and a physician who has a duty to act in a patient’s best interest, a pharmaceutical physician needs to ensure that his/her obligations to both parties have been fulfilled.Citation114 Therefore, it is important to understand and adhere to the key internal and external check systems that control the quality and credibility of internal compliance governance by medical affairs personnel and their external stakeholder interactions.
Control systems in pharmaceutical industry
In today’s ever-changing world, controls on pharmaceutical industry practices have to evolve to keep pace with its dynamic nature. The four main pillars of control systems are regarded to be internal company protocols, industry codes of practice, regulations, and laws.Citation115 summarizes the different control systems governing pharmaceutical communication around the world. These are of increasing importance to medical affairs departments given that they have an expanding role in communicating both directly and indirectly with physicians, patients, and other key stakeholders.
Table 2 Summary of control systems governing pharmaceutical practices around the world
Laws and regulations
Laws and regulations relating to the communications between the industry and their stakeholders are governed by national and potentially even regional regulatory organizations. For example, member nations of the EU are required to follow European Medicines Agency (EMA) laws.Citation116 However, individual nations within the EU may also impose additional local laws or regulations that they deem necessary to meet the main directives set by the EMA.Citation117
Local laws and regulations are upheld by the regulatory bodies of individual countries. Both the laws and their enforcement can vary from country to country. For example, the advertising of medicines is regulated by the FDA in the United States and by the Therapeutic Goods Administration in Australia.Citation104,Citation118 Furthermore, laws that govern other com mercial entities within the country also apply to pharmaceutical companies. For example, the Foreign Corrupt Practices Act in the USA and the Bribery Act in the UK affect the commercial behavior of local companies’ subsidiaries that are abroad.Citation119,Citation120 Laws and regulations in individual countries can differ greatly, as seen in the above example of DTCA. Relevant laws are usually taken as the basis for which industry and internal policies or guidelines are enacted, although these policies can often be more comprehensive than the rules and regulations of governmental agencies.Citation115
Industry codes
Local trade associations specific to the pharmaceutical industry have been established all over the world and fall under the jurisdiction of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA).Citation121 Members of the IFPMA adhere to a strict set of regulatory measures that ensure high standards of quality and promote ethical practices among pharmaceutical companies.Citation122 Examples of member associations include the Pharmaceutical Research and Manufacturers of America in the USA, the Association of the British Pharmaceutical Industry in the UK, the Singapore Association of Pharmaceutical Industries, Medicines Australia, and the European Federation of Pharmaceutical Industries and Associations, which represents EU nations.Citation123 These member associations determine regulations based on the IFPMA Codes of Practice. All member associations and companies implement these codes. In particular, the IFPMA regulates companies’ interactions with health care professionals, medical institutions, and patient organizations.Citation124
Internal policies
International pharmaceutical companies have a set of global internal policies that govern their communications with stakeholders worldwide.Citation125–Citation127 These are the first point of reference because employees are expected to adhere to company policies by following industry-, national-, or international-level regulations.Citation128,Citation129 These policies often incorporate higher-level regulations and are, thus, more stringent and detailed.Citation115
Additional control systems for medical affairs
The goal of the medical affairs department is to communicate medical data about products with a high level of integrity, so they are seen by key stakeholders and the general public as a credible and transparent source of medical information.Citation6 This could contradict with the promotion-minded approach of marketing colleagues and needs to be managed effectively because both departments have to work together to ethically and informatively educate physicians about their products.Citation113 In order to achieve this balance, there has to be a clear separation between medical affairs and commercial functions. Approaches such as having separate reporting structures (ie, medical affairs does not report to commercial) and separating the funding of both departments (ie, medical affairs does not rely on commercial sales for their funding) help to ensure that medical affairs remains independent of commercial pressures and is able to focus on working in an ethical and patient-centric manner.Citation130 Medical affairs physicians needed to be commercially independent and be able to function without consideration of commercial objectives.
Continuous professional development for medical affairs professionals
The rapidly changing pharmaceutical industry and the health care sector landscape have increased the importance of medical affairs in pharmaceutical companies. This is reflected by the growing proportion of clinicians entering the pharmaceutical industry,Citation131 with some companies viewing a specialty medical qualification, a PhD, and/or a business-related qualification such as an MBA as helpful additions to a primary medical qualification.Citation131 This shift calls for medical affairs personnel to undertake their own continuing education to address knowledge or experience gaps to be able to respond effectively and efficiently to the dynamic nature of the industry. While traditional roles such as MSL interaction with stakeholders and publishing scientific material remain important, several evolving responsibilities (such as pharmacoeconomic considerations, communicating with patient associations, and planning for prelaunch obligations) are gaining increasing prominence. Pharmaceutical industry physicians need to be trained in these important aspects of medical affairs, necessitating participation in robust training to prepare for the dynamic nature of their roles. To meet these challenges, many educational programs are in place for pharmaceutical physicians to enhance career progression and prepare for their increasingly complex roles.Citation131–Citation133
One example is the International Federation of Associations of Pharmaceutical Physicians and Pharmaceutical Medicine (IFAPP)-Kings College Medical Affairs in Medicines Development Certification Program. This year-long e-learning program was designed by the IFAPP academy in collaboration with industry, professional association, and academic personnel.Citation134,Citation135 The aim of the program is to enhance the skills of medical affairs professionals who have at least a year of experience working in their specific function.Citation135,Citation136 The Duke-National University of Singapore (NUS) Medical School in Singapore also offers the Joint Alliance Duke-NUS Education (JADE).Citation133 JADE provides a similar e-learning platform to the IFAPP-Kings College Program and is designed to develop medical affairs professionals to suit the evolving needs of their roles.Citation133 This program caters not only to medical affairs professionals but also to health care professionals who are keen to develop a career in medical affairs.Citation133
Although the modular, e-learning model is popular in today’s internet age, many medical affairs professionals have limited time to pursue such ventures. Thus, programs offered in the form of short, traditional lecture models do have a role. The Center for Executive Leadership for the Pharmaceutical Industry (C.E.L.forpharma) offers short workshops (1–2 days) with expert speakers and educators.Citation132 These programs are specifically for the betterment of medical affairs executives, enable personnel to develop new skills, and stay relevant in a changing environment.Citation132
The Pharmaceutical Medicine Specialty Training in the UK offers a more intensive, selective, and rigorous program. This 4-year training program for pharmaceutical physicians is overseen by the Joint Royal Colleges of Physicians Training Board and offers physicians the opportunity to be listed on the General Medical Council’s specialist register for pharmaceutical medicine. This program is specifically offered to upgrade licensed medical doctors with positions within the pharmaceutical industry and is more selective than most other programs on offer.Citation131
Conclusion
Pharmaceutical companies are allocating more resources to the medical affairs function in order to increase new product development and postlaunch execution activities. This is vital in addressing the need to improve R&D productivity within the scrutiny of ever-increasing HTA standards and the tightening of laws and regulations globally. The traditional role of pharmaceutical physicians in the medical affairs function is evolving well beyond the usual support to internal commercial functions and collaboration with KOLs. In the future, the focus of medical affairs may be on generating and communicating medical evidence, leading medical education, and empowering patient associations. Increasing responsibility for various facets of the industry, such as CME, public health initiatives, and HEOR, make the field of medical affairs a dynamic venture. A career in medical affairs may offer the right niche, progression, and fulfillment to many health professionals, including physicians, by enabling them to challenge global unmet needs in medical and health communication and education in this exciting and expanding field, with the ultimate goal of improving patient care and outcomes.
Author contributions
All authors were involved in conception, design, analysis, and interpretation of data. All authors were also involved in the preparation of the manuscript, revising it for scientific content, final approval before its submission for publication, gave final approval of the manuscript version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Ms Sonia Menezes, consultant, scientific communications, Pfizer, for her editorial support for this manuscript and Dr Chandrashekhar N Potkar, regional medical affairs lead, Pfizer, for his valuable comments during design of the manuscript. This publication contains personal views and opinions of the authors, and no inference should be derived related to their current or previous employers. Pfizer Pte Ltd paid the publishing fee for this paper.
Disclosure
Dr Sajita Setia was an employee of Pfizer Pte Ltd at the time of submission of this manuscript and Dr Kannan Subrama-niam is an employee of Pfizer Australia. Mr Prasad S Nair and Ms Elma Ching underwent indirect patient care pharmacy training for 3 months at Pfizer, Singapore. The authors report no other conflicts of interest in this work.
References
- PharmaForumMedical Affairs – the heart of a data-driven, patient-centric pharma2017 Available from: https://pharmaphorum.com/views-and-analysis/medical-affairs-heart-data-driven-patient-centric-pharma/Accessed April 28, 2018
- JainSBridging the Gap Between R&D and commercialization in pharmaceutical industry: role of medical affairs and medical communicationsInt J Biomed Sci2017334449
- GromTMedical affairs: beyond the scienceShowcase feature2013 Available from: http://www.pharmavoice.com/article/medical-affairs-beyond-the-science/Accessed April 24, 2018
- RollinsBLPerriMPharmaceutical MarketingBurlingtonJones & Bartlett Learning2014
- PlantevinLSchlegelCGordianMReinventing the Role of Medical Affairs2017 Available from: http://www.bain.com/publications/articles/reinventing-the-role-of-medical-affairs.aspxAccessed April 24, 2018
- EversMFlemingEGhatakAPharma Medical Affairs: 2020 and beyondNew YorkMcKinsey & Company2014
- PatwardhanARPhysicians-pharmaceutical sales representatives interactions and conflict of interest: challenges and solutionsInquiry201653004695801666759727637269
- van BisenTWeisbrodJBrookshireMCoffmanJPasternakAFront Line of Healthcare Report 2017: Why involving doctors can help improve US healthcare2017 Available from: http://www.bain.com/publications/articles/front-line-of-healthcare-report-2017.aspxAccessed April 24, 2018
- NatanekRSchlegelCRetterathMEliadesGHow to Make Your Drug Launch a Success Available from: https://www.bain.com/insights/how-to-make-your-drug-launch-a-success/Accessed April 26, 2018
- FirstWorldThe Future of Medical Affairs2016 Available from: http://www.fwreports.com/dossier/the-future-of-medical-affairs/#.W8UdbGgzY2wAccessed October 18, 2018
- RutherfordPSmithNJMedical Science Liaisons: A Key to Driving Patient Access to New TherapiesQuintilesIMS 2016 Available from: https://www.iqvia.com/-/media/library/white-papers/medical-science-liaisons.pdfAccessed April 26, 2018
- Medical Science Liaison SocietyWhat Is a Medical Science Liaison?2018 Available from: http://www.themsls.org/what-is-an-msl/Accessed April 26, 2018
- KausaISA day in the life of a pharmaceutical physician2007 Available from: http://careers.bmj.com/careers/advice/view-article.html?id=2461Accessed April 24, 2018
- International Federation of Pharmaceutical Manufacturers & AssociationsIFPMA Code of PracticeGeneva, SwitzerlandInternational Federation of Pharmaceutical Manufacturers & Associations2012 Available from: https://www.ifpma.org/subtopics/code-of-practice-2/Accessed April 26, 2018
- PressonJAdvisory Board Functions Expanding in Pharma Industry2018 Available from: https://www.cuttingedgeinfo.com/2013/advisory-board-functions-expanding-pharma-industry/Accessed April 24, 2018
- EvensRDrug and Biological Development: From Molecule to Product and BeyondVerlagSpringer2007240274
- Best PracticesNew Study Presents Insights to Advisory Board Effectiveness in the Pharmaceutical Sector Available from: https://www.prnewswire.com/news-releases/new-study-presents-insights-to-advisory-board-effectiveness-in-the-pharmaceutical-sector-300076091.htmlAccessed April 24, 2018
- American Express Open Access10 Steps to forming an effective advisory board2011 Available from: https://www.americanexpress.com/us/small-business/openforum/articles/10-steps-to-forming-an-effective-advisory-board-1/Accessed April 24, 2018
- BalasubramanianGMorampudiSChhabraPGowdaAZomorodiBArun GowdaBZAn overview of Compassionate Use Programs in the European Union member statesIntractable Rare Dis Res20165424425427904819
- PatilSEarly access programs: benefits, challenges, and key considerations for successful implementationPerspect Clin Res2016714826955570
- YazdaniMBoggiFInitiating Early Access Programs: 5 Things to Consider2018 Available from: http://www.executiveinsight.ch/early_access_programsAccessed April 24, 2018
- BerroMBurnettBKFromellGJSupport for investigator-initiated clinical research involving investigational drugs or devices: the Clinical and Translational Science Award experienceAcad Med201186221722321169787
- SuvarnaVInvestigator initiated trials (IITs)Perspect Clin Res20123411912123293757
- Cutting Edge InformationMedical Science Liaisons Aim to Support Investigator Initiated Trials2012 Available from: https://www.businesswire.com/news/home/20121016005277/en/Medical-Science-Liaisons-Aim-Support-Investigator-InitiatedAccessed April 24 2018
- SullivanGMGetting off the “gold standard”: randomized controlled trials and education researchJ Grad Med Educ20113328528922942950
- HaidichABMeta-analysis in medical researchHippokratia201014Suppl 1293721487488
- ChanEWLiuKQChuiCSSingCWWongLYWongICAdverse drug reactions: examples of detection of rare events using databasesBr J Clin Pharmacol201580485586125060360
- McKessonReal-World Evidence from Electronic Health Record Data2017 Available from: http://www.mckesson.com/blog/real-world-evidence-from-electronic-health-record-data/Accessed April 24, 2018
- GrudzinskasCAtkinsonJDesign of Clinical Development ProgramsArthurJAtkinsonJAbernethyDarrell RDanielsCharles EDedrickRobert LMarkeySanford PPrinciples of Clinical Pharmacology2nd ed2007501517
- DekovenMHazardEHGoldbergEPokrasSGot value? Determine it. Demonstrate it. Communicate it. Realise itJ Commerc Biotechnol2008144299306
- Canadian Agency for Drugs and Technologies in HealthWhat Does the Evidence Say?2018 Available from: https://www.cadth.ca/about-cadthAccessed April 24, 2018
- ISPORDirectory of Health Technology Assessment Organizations Worldwide2018 Available from: https://www.ispor.org/HTADirectory/Index.aspxAccessed April 26, 2018
- INHATAINAHTA Members List2018 Available from: http://www.inahta.org/members/members_list/Accessed April 24, 2018
- European Federation of Pharmaceutical Industries and AssociationsThe Use of Health Technology Assessments to Evaluate Medicines2005 Available from: https://www.efpia.eu/media/25170/the-use-of-health-technology-assessments-hta-to-evaluate-medicines-key-principles-2007.pdfAccessed April 24, 2018
- Ministry of Health SingaporeAbout Us2018 Available from: https://www.moh.gov.sg/content/dam/moh_web/ACE-HTA/about-us.html#who-we-areAccessed April 24, 2018
- ShiroiwaTFukudaTIkedaSTakuraTNew decision-making processes for the pricing of health technologies in Japan: The FY 2016/2017 pilot phase for the introduction of economic evaluationsHealth Policy2017121883684128687183
- BarberJMSheehyKPUptake of new medicines in New Zealand: evidence of a waiting listN Z Med J201512814121020
- European Network for Health Technology AssessmentEUnetHTA and the HTA Network2018 Available from: https://www.eunethta.eu/about-eunethta/our-network/Accessed April 26, 2018
- BrockisEMarsdenGColeADevlinNA Review of NICE Methods Across Health Technology Assessment Programmes: Differences, Justifications and ImplicationsOffice of Health Economics2016 https://www.ohe.org/publications/review-nice-methods-across-health-technology-assessment-programmes-differencesAccessed September 28, 2018
- Pichon-RiviereAAugustovskiFRubinsteinAMartíSGSullivanSDDrummondMFHealth technology assessment for resource allocation decisions: are key principles relevant for Latin America?Int J Technol Assess Health Care201026442142720942985
- PMLiveNovo Nordisk’s Klaus Henning Jensen on trends in medical affairs2018 Available from: http://www.pmlive.com/pharma_thought_leadership/novo_nordisks_klaus_henning_jen-sen_on_trends_in_medical_affairs_607002Accessed April 24, 2018
- CianiOJommiCThe role of health technology assessment bodies in shaping drug developmentDrug Des Devel Ther2014822732281
- PCCMedical Affairs Compliance: Analyze the Involvement of Medical Affairs in Commercial Activities2017 Available from: http://www.cbinet.com/sites/default/files/files/Connor_Brian_Pres.pdfAccessed April 26, 2018
- GrolRSuccesses and failures in the implementation of evidence-based guidelines for clinical practiceMed Care2001398 Suppl 24654
- CochraneLJOlsonCAMurraySDupuisMToomanTHayesSGaps between knowing and doing: understanding and assessing the barriers to optimal health careJ Contin Educ Health Prof20072729410217576625
- BuchanHGaps between best evidence and practice: causes for concernMed J Aust20041806 SupplS4815012579
- McglynnEAAschSMAdamsJThe quality of health care delivered to adults in the United StatesN Engl J Med2003348262635264512826639
- SaslowDSolomonDLawsonHWAmerican Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancerCA Cancer J Clin201262314717222422631
- KomajdaMLapuertaPHermansNAdherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER surveyEur Heart J200526161653165915827061
- McculloughMLPatelAVKushiLHFollowing cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortalityCancer Epidemiol Biomarkers Prev20112061089109721467238
- ShapiroDWLaskerRDBindmanABLeePRContaining costs while improving quality of care: the role of profiling and practice guidelinesAnnu Rev Public Health1993142192418323588
- SetiaSSubramaniamKTayJCTeoBWHypertension and blood pressure variability management practices among physicians in Sin-gaporeVasc Health Risk Manag20171327528528761353
- SetiaSSubramaniamKTeoBWTayJCAmbulatory and home blood pressure monitoring: gaps between clinical guidelines and clinical practice in SingaporeInt J Gen Med20171018919728721085
- SetiaSFungSSWatersDDDoctors’ knowledge, attitudes, and compliance with 2013 ACC/AHA guidelines for prevention of ath-erosclerotic cardiovascular disease in SingaporeVasc Health Risk Manag20151130331026082642
- National Institute of Clinical StudiesEvidence-Practice Gaps Report Volume 22005 Available from: https://www.nhmrc.gov.au/guidelines-publications/nic47Accessed April 25 2018
- GweeKAGohVLimaGSetiaSCoprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: risks versus benefitsJ Pain Res20181136137429491719
- LansbergPLeeALeeZVSubramaniamKSetiaSNonadherence to statins: individualized intervention strategies outside the pill boxVasc Health Risk Manag2018149110229872306
- ChadachanVMYeMTTayJCSubramaniamKSetiaSUnderstanding short-term blood-pressure-variability phenotypes: from concept to clinical practiceInt J Gen Med20181124125429950885
- ShekellePEcclesMPGrimshawJMWoolfSHWhen should clinical guidelines be updated?BMJ2001323730515515711463690
- JoshiCConsensus Guidelines: A Tool for Establishing Evidence-Based Practices2017 Available from: https://www.sciformix.com/medical-affairs-blog/consensus-guidelines-tool-establishing-evidence-based-practice/Accessed April 25, 2018
- Council of EuropeDeveloping a Methodology for Drawing Up Guidelines on Best Medical Practices2001 Available from: https://www.leitlinien.de/mdb/edocs/pdf/literatur/coe-rec-2001-13.pdfAccessed April 25, 2018
- Marie-OdileFMedical affairs writing: a key role to relay medical information to everyoneMedical Writing201625268
- JohansenMThomsenSFGuidelines for reporting medical research: a critical appraisalInt Sch Res Notices20161346026
- VarettoTCostaDCContinuing Medical Education Committee and UEMS-EACCMEEur J Nucl Med Mol Imaging201340347047423287995
- World Health Organization (SEAR)Regional Guidelines for Continuing Medical Education (CME)/Continuing Professional Development (CPD) Activities2010 Available from: http://apps.searo.who.int/PDS_DOCS/B4489.pdfAccessed April 25, 2018
- MurgatroydGBContinuing Professional Development: The International Perspective2011 Available from: http://www.gmc-uk.org/CPD___The_International_Perspective_Jul_11.pdf_44810902.pdfAccessed April 25, 2018
- McleodPJMcleodAHIf formal CME is ineffective, why do physicians still participate?Med Teach200426218418615203529
- DelsignoreJLCurrent guidelines regarding industry-sponsored continuing medical educationClin Orthop Relat Res20034124122127
- VenkataramanRRanganathanLPonnishASAbrahamBKRamakrishnanNFunding sources for continuing medical education: An observational studyIndian J Crit Care Med201418851351725136190
- SteinbrookRFinancial support of continuing medical educationJAMA200829991060106218319417
- ACCMEAnnual Report DataAccreditation Council for Continuing Medical Education2006 Available from: http://www.accme.org/sites/default/files/null/395_2006_Annual_report_2006_20070706.pdfAccessed April 26, 2018
- ACCMEACCME Data Report: Growth and Evolution in Continuing Medical Education2016 Available from: http://www.accme.org/news-releases/accme-data-report-shows-growth-and-evolution-accredited-continuing-medical-educationAccessed April 25, 2018
- WietingMWMevisHZuckermanJDThe role of industry in Internet educationClin Orthop Relat Res20034124122832
- WilsonFSContinuing medical education: ethical collaboration between sponsor and industryClin Orthop Relat Res20034124123337
- HolmerAFIndustry strongly supports continuing medical educationJAMA2001285152012201411308442
- US Department of Health and Human ServicesFinal Guidance on Industry-Supported Scientific and Educational ActivitiesFood and Drug Administration1997 Available from: https://www.gpo.gov/fdsys/pkg/FR-1997-12-03/pdf/97-31741.pdfAccessed April 26, 2018
- MehtaPNDrugmakers and continuing medical educationIndian Pediatr200037662663010869142
- American College of Emergency PhysiciansGrants and Sponsorships2016 Available from: https://www.acep.org/Content.aspx?id=42670#sm.0001nd5f2nup5ebxuh32804wind6bAccessed April 25, 2018
- PfizerTransparency in Grants2018 Available from: https://www.pfizer.com/purpose/medical-grants/grant-transparencyAccessed April 25, 2018
- Celgene CorporationGrant Application Requirements2018 Available from: http://www.celgene.com/research-development/celgene-medical-affairs/educational-grant-requests/grant-application-requirements/Accessed April 25, 2018
- Boehringer Ingelheim GmbHApplying for an Independent Medical Education Grant2018 Available from: https://www.boehringer-ingelheim.us/funding-opportunities/medical-education-grants/applying-grantAccessed April 25, 2018
- PfizerDescriptions of Funding Types and Recipient Organizations2018 Available from: https://www.pfizer.com/responsibility/grants_contributions/description_funding_recipient_organizationsAccessed April 25, 2018
- Mallinckrodt PharmaceuticalsIndependent Medical Education Grants Available from: http://www.mallinckrodt.com/research/medical_affairs/independent_medical_educationAccessed April 25, 2018
- Janssen Scientific AffairsRequirements for Grants Requests2018 Available from: https://www.janssenime.com/requirements-for-grant-requestsAccessed April 25, 2018
- PfizerOrganizations Eligible to Apply Directly for Grants and Types of Initiatives Supported2018 Available from: http://www.pfizer.com/files/IGLC_OrganizationEligibility_effJuly2015.pdfAccessed April 25, 2018
- Boston Scientific CorporationIndependent Medical Education Program Grants2018 Available from: http://www.bostonscientific.com/en-US/corporate-citizenship/giving/grants-donations/request-types/educational-conference.htmlAccessed April 25, 2018
- BoardESCThe future of continuing medical education: the roles of medical professional societies and the health care industry: position paper prepared with contributions from the European Society of Cardiology Committees for Advocacy, Education and Industry Relations, Endorsed by the Board of the European Society of CardiologyEur Heart J2018
- MorrisLTaitsmanJKThe agenda for continuing medical education: limiting industry’s influenceN Engl J Med2009361252478248220018969
- RothmanDJMcdonaldWJBerkowitzCDProfessional medical associations and their relationships with industry: a proposal for controlling conflict of interestJAMA2009301131367137219336712
- BrennanTARothmanDJBlankLHealth industry practices that create conflicts of interest: a policy proposal for academic medical centersJAMA2006295442943316434633
- PracticesBContinuing Medical Education: Trends in Structure and Functional Management2018 Available from: https://www.best-in-class.com/bestp/domrep.nsf/products/continuing-medical-education-trends-in-structure-and-functional-management?opendocumentAccessed April 25, 2018
- HesseBWNelsonDEKrepsGLTrust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends SurveyArch Intern Med2005165222618262416344419
- EysenbachGKohlerCWhat is the prevalence of health-related searches on the World Wide Web? Qualitative and quantitative analysis of search engine queries on the InternetAMIA Annu Symp Proc20032003225229
- FoxSDugganMHealth Online2013 Available from: http://www.pewinternet.org/2013/01/15/health-online-2013/Accessed April 25, 2018
- ClineRJHaynesKMConsumer health information seeking on the Internet: the state of the artHealth Educ Res200116667169211780707
- DoupiPvan der LeiJRx medication information for the public and the WWW: quality issuesMed Inform Internet Med199924317117910654811
- LattheMLatthePMCharltonRQuality of information on emergency contraception on the InternetBr J Fam Plann2000261394310781966
- Directorate-General for Health and Food SafetyInformation to Patients: Legislative Approach2018 Available from: https://ec.europa.eu/health/human-use/information-to-patient/legislative_enAccessed April 25, 2018
- Pfizer Medicine Safety Education2018 Available from: https://www.pfizer.com/products/patient-safetyAccessed April 25, 2018
- DaviesNPublic-Private Partnerships Essential for Public Health Education2015 Available from: https://social.eyeforpharma.com/market-access/public-private-partnerships-essential-public-health-educationAccessed April 25 2018
- DonohueJMCevascoMRosenthalMBA decade of direct-to-consumer advertising of prescription drugsN Engl J Med2007357767368117699817
- VentolaCLDirect-to-consumer pharmaceutical advertising. Therapeutic or toxic?P T2011361066968422346300
- ChengKWChongDWKDirect-to-consumer advertising in Hong Kong: possible impact and regulationHong Kong Pharma J2014214130136
- US Food and Drug AdministrationCFR - Code of Federal Regulations Title 21 Code of Federal Regulations2018 Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=202.1Accessed April 25, 2018
- ParekhAMarcusRRobertsMRaischDWRisks and benefits of direct to consumer advertising on patient - provider relationshipsReport of the ISPOR Direct to Consumer Advertisements Working Group Available from: https://www.ispor.org/news/articles/June12/Risks-and-Benefits-of-Direct-To-Consumer.aspAccessed April 25, 2018
- GrazianoLDirect to Consumer Drug Advertisements: A Dangerous Game of Pitching Products to ParentsWright State University2009 Available from: https://corescholar.libraries.wright.edu/oamh_presentations/4/Accessed April 26, 2018
- World Health OrganizationDirect-to-consumer advertising under fireBull World Health Organ2009878565644
- HollonMFDirect-to-consumer advertising: a haphazard approach to health promotionJAMA2005293162030203315855439
- BaggottRForsterRHealth consumer and patients’ organizations in Europe: towards a comparative analysisHealth Expect2008111859418275405
- US Food and Drug AdministrationCDER Patient-Focused Drug Development2018 Available from: https://www.fda.gov/Drugs/Devel-opmentApprovalProcess/ucm579400.htmAccessed April 26, 2018
- RachulCToewsMCaulfieldTControversies with Kalydeco: Newspaper coverage in Canada and the United States of the cystic fibrosis “wonder drug”J Cyst Fibros201615562462927150823
- MuthCCConflict of interest in medicineJAMA201731717181228464142
- MyshkoDThe Medical Affairs-Marketing Connection2004 Available from: http://www.pharmavoice.com/article/137/Accessed August 1, 2018
- GoldacreBEvil ways of the drug companiesBad Science2007 Available from: https://www.theguardian.com/science/2007/aug/04/sciencenewsAccessed August 1, 2018
- FrancerJIzquierdoJZMusicTEthical pharmaceutical promotion and communications worldwide: codes and regulationsPhilos Ethics Humanit Med201491724679064
- European Medicines AgencyAbout Us2018 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/document_listing/document_listing_000426.jsp&midAccessed April 26 2018
- European UnionRegulations, Directives and Other Acts2018 Available from: https://europa.eu/european-union/eu-law/legal-acts_enAccessed April 26, 2018
- Australian Government Department of HealthTherapeutic goods advertising codeTherapeutic Goods Administration2018 Available from: https://www.tga.gov.au/publication/therapeutic-goods-advertising-codeAccessed April 26, 2018
- SecuritiesUSCommissionExchangeForeign Corrupt Practices Act2018 Available from: https://www.sec.gov/spotlight/foreign-corrupt-practices-act.shtmlAccessed April 26, 2018
- Transparency International UKThe Bribery Act2018 Available from: http://www.transparency.org.uk/our-work/business-integrity/bribery-act/#.WuFuxIhubIVAccessed April 26, 2018
- International Federation of Pharmaceutical Manufacturers & AssociationsOur Membership2018 Available from: https://www.ifpma.org/who-we-are/our-membership/full-members/companies/#!/Accessed April 26, 2018
- International Federation of Pharmaceutical Manufacturers & AssociationsCorporate Brochure: Committed to a Healthier FutureGeneva2016 Available from: https://www.ifpma.org/resource-centre/ifpma-corporate-brochure/Accessed April 26, 2018
- International Federation of Pharmaceutical Manufacturers & AssociationsGlobal Code Comparison: A Snapshot of IFPMA Member Associations’ Self-Regulation MechanismsInternational Federation of Pharmaceutical Manufacturers & Associations2018 Available from: https://www.ifpma.org/wp-content/uploads/2018/02/IFPMA_GCC_V7.pdfAccessed April 26, 2018
- International Federation of Pharmaceutical Manufacturers & AssociationsIFPMA Code of Practice2012 Available from: https://www.lif.se/globalassets/etik/dokument/ifpma_code_of_practice_2012.pdfAccessed April 26, 2018
- PfizerSales and Marketing Compliance2018 Available from: Pfizer. Sales and Marketing ComplianceAccessed April 26, 2018
- Takeda Pharmaceutical Company LimitedTakeda Global Code of Conduct2017 Available from: https://www.takeda.com/siteassets/system/who-we-are/corporate-governance/compliance/global-code-of-conduct_en.pdfAccessed April 26, 2018
- Astellas Pharma IncPolicies & Position Statements2018 Available from: https://www.astellas.com/en/about/policies-and-position-statementsAccessed April 26, 2018
- AstraZenecaAstraZeneca Global Policy Communications2015 Available from: https://www.astrazeneca.com/content/dam/az/PDF/Global-Communications%20Policy_v6.0_December_2015.pdfAccessed April 26, 2018
- GlaxoSmithKline plcOur Code of Practice for Promotion of Prescription Medicines and for Scientific Engagement2018 Available from: https://www.gsk.com/media/2831/code-of-practice.pdfAccessed April 26, 2018
- WerlingKCarnellHMcCormickDFocus on Life Science Compliance: The Evolution of Medical Affairs Departments2011 Available from: https://www.mcguirewoods.com/news-resources/publications/health_care/focus-life-science-compliance-nov-2011.pdfAccessed August 1, 2018
- British Medical AssociationThe Pharmaceutical Physician2013 Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwiVm43n6J3aAhVCso8KHSLxBYEQFggsMAA&url=https%3A%2F%2Fwww.bma.org.uk%2F-%2Fmedia%2Ffiles%2Fpdfs%2Fabout%2520the%2520bma%2Fhow%2520we%2520work%2Fmedical%2520academics%2Fpharmaceuticalphysican_sept2013_update.pdf&usg=AOvVaw0pemmjh2E-xGyAz54SO7ZfAccessed April 26, 2018
- C.E.L. for PharmaMedical Affairs Training Courses2018 Available from: https://www.celforpharma.com/medical-affairs-training-coursesAccessed April 26, 2018
- Duke NUS Medical SchoolJoint Alliance Duke-NUS Education Certificate Programme in Medical Affairs Available from: https://www.duke-nus.edu.sg/core/page/jade-certification-programme-medical-affairs-brochureAccessed April 26, 2018
- IFAPP AcademyThe IFAPP-Kings College Medical Affairs in Medicines Development Certification Timelines2018 Available from: https://ifappacademy.org/index.php/timelines/Accessed April 26, 2018
- IFAPP AcademyIFAPP Academy2018 Available from: https://ifap-pacademy.org/Accessed April 26, 2018
- IFAPP AcademyIFAPP Academy Student Requirements2018 Available from: https://ifappacademy.org/index.php/ifapp-academy-student-requirements/Accessed April 26, 2018
- International Committee of Medical Journal EditorsRecommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals2017 Available from: http://www.icmje.org/icmje-recommendations.pdfAccessed April 25, 2018
- BattistiWPWagerEBaltzerLGood publication practice for communicating company: sponsored medical research: GPP3Ann Intern Med2015163646146426259067
- CONSORTConsolidated Standards of Reporting Trials Available from: http://www.consort-statement.orgAccessed April 26, 2018
- von ElmEAltmanDGEggerMStrengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studiesBMJ2007335762480680817947786
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200967e100009719621072
- StroupDFBerlinJAMortonSCMeta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) groupJAMA2000283152008201210789670
- Des JarlaisDCLylesCCrepazNTREND GroupImproving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statementAm J Public Health200494336136614998794
- US Food and Drug AdministrationWhat We Do2018 Available from: https://www.fda.gov/AboutFDA/WhatWeDo/default.htmAccessed April 26, 2018
- UK GovernmentServices and informationMedicines and Health-care products Regulatory Agency Available from: https://www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency/services-informationAccessed April 26, 2018
- Government of CanadaHealth Canada Available from: https://www.canada.ca/en/health-canada.htmlAccessed April 26, 2018
- Health Sciences AuthorityCorporate Profile2018 Available from: http://www.hsa.gov.sg/content/hsa/en/About_HSA/Corporate_Profile.htmlAccessed April 26, 2018
- New Zealand Medicines and Medical Devices Safety AuthorityAbout Medsafe Available from: http://www.medsafe.govt.nz/other/about.aspAccessed April 26, 2018
- Japan Pharmaceutical Manufacturers AssociationRegulatory Information Task Force: Information on Japanese Regulatory AffairsPharmaceutical Administration and Regulations in Japan2017 Available from: http://www.jpma.or.jp/english/parj/pdf/2017_contents.pdfAccessed April 26, 2018
- Central Drugs and Standard Control OrganizationAbout Us Available from: http://www.cdsco.nic.in/forms/contentpage1.aspx?lid=962Accessed April 26, 2018
- China Food and Drug AdministrationMain Responsibilities Available from: http://eng.sfda.gov.cn/WS03/CL0756/Accessed April 26, 2018
- RothsteinNAProtecting privacy and enabling pharmaceutical sales on the Internet: a comparative analysis of the United States and CanadaFed Commun Law J2001532 Article 6
- UK Government LegislationThe Medicines (Advertising) Regulations1994 Available from: https://www.legislation.gov.uk/uksi/1994/1932/contents/madeAccessed April 26, 2018
- European Commission Health and Food Safety Directorate-GeneralProcedures for Marketing Authorisation2018 Available from: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-2/vol2a_chap1_rev7_201712.pdfAccessed July 2, 2018
- Singapore Statutes OnlineHealth Products Act2008 Available from: https://sso.agc.gov.sg/Act/HPA2007Accessed April 26, 2018
- Federal Register of LegistlationTherapeutic Goods Act1989 Available from: https://www.legislation.gov.au/Details/C2017C00226Accessed April 26, 2018
- Pharmaceutical Affairs LawJapan Available from: http://www.jouhoukoukai.com/repositories/source/pal.htmAccessed April 26, 2018
- International Comparative Legal Guides. Pharmaceutical Advertising2017Japan Available from: https://iclg.com/practice-areas/pharmaceutical-advertising/pharmaceutical-advertising-2017/japanAccessed April 26, 2018
- International Comparative Legal Guides. Pharmaceutical Advertising2017India Available from: https://iclg.com/practice-areas/pharmaceutical-advertising/pharmaceutical-advertising-2017/indiaAccessed April 26, 2018
- MaFLouNChinese regulation of off-label use of drugsFood Drug Law J201368218920024640468
- China Food and Drug AdministrationProvisions for Drug Advertisement Examination2007 Available from: http://eng.sfda.gov.cn/WS03/CL0768/61649.htmlAccessed April 28, 2018
- Database of Laws and Regulations: National People’s CongressEconomic and Advertisement Law of the People’s Republic of China2018 Available from: http://www.npc.gov.cn/englishnpc/Law/2007-12/12/content_1383782.htmAccessed April 28, 2018