Abstract
Axillary web syndrome (AWS) is a common condition occurring in up to 86% of patients following breast cancer surgery with ipsilateral lymphadenectomy of one or more nodes. AWS presents as a single cord or multiple thin cords in the subcutaneous tissues of the ipsilateral axilla. The cords may extend variable distances “down” the ipsilateral arm and/or chest wall. The cords frequently result in painful shoulder abduction and limited shoulder range of motion. AWS most frequently becomes symptomatic between 2 and 8 weeks postoperatively but can also develop and recur months to years after surgery. Education about and increased awareness of AWS should be promoted for patients and caregivers. Assessments for AWS should be performed on a regular basis following breast cancer surgery especially if there has been associated lymphadenectomy. Physical therapy, which consists of manual therapy, exercise, education, and other rehabilitation modalities to improve range of motion and decrease pain, is recommended in the treatment of AWS.
Introduction
Axillary web syndrome (AWS) is a common but often overlooked condition that most commonly occurs in patients following breast cancer surgery with axillary lymph node dissection (ALND).Citation1–Citation4 It also occurs in patients with other shoulder and axillary pathology, including trauma, infection, sentinal node lymphadenectomy,Citation5 and melanoma surgery with axillary lymphadenectomy.Citation6 In the literature, various synonyms for AWS have included cording, lymphatic cording, fibrous banding; and incorrectly, Mondor’s disease. AWS presents as a “tight” cord in the subcutaneous tissue in the axilla.Citation3 The cord may extend down the medial or medial-volar surface of the ipsilateral upper arm and/or down along the ipsilateral lateral chest wall. The cord tightens, often painfully, with shoulder abduction. AWS most commonly develops 2–8 weeks following breast cancer surgery.Citation1–Citation4,Citation7 However, recent studies have shown that AWS can occur months to years later and can also resolve and then relapse.Citation2,Citation7
The reported incidence of AWS ranges from 6% to 86% following breast cancer surgery. The wide range arises from several reasons, the most important of which appears to be whether or not AWS was specifically sought on postoperative examinations. The incidence is also dependent on the type of surgery and the length of follow-up.Citation1–Citation4,Citation7–Citation10 Two recent careful prospective studies that included evaluations of postoperative breast cancer patients demonstrated that the actual value may lie somewhere between these two extremes. One study had a prevalence of AWS of 50% at 18 months,Citation2 and the other had a 51% incidence in the 8 weeksCitation11 following surgery. The incidence is higher in surgery with ALND (36%–72%)Citation1,Citation2,Citation4,Citation7,Citation9,Citation10 compared with surgery with sentinel lymph node biopsy (SNB) (11%–58%).Citation1,Citation4,Citation7,Citation10 For reasons that are not clear, the incidence of AWS is highest in patients who have a prior or contemporaneous contralateral prophylactic mastectomy (86%).Citation2 The incidence is also higher in patients who have a lower body mass index,Citation1,Citation10,Citation12 who are younger age,Citation1,Citation8,Citation10,Citation11 who have more education,Citation11 who exercise more frequently,Citation11 who have a greater number of lymph nodes removed,Citation1,Citation7 who receive more extensive surgery,Citation1,Citation7,Citation11 or who receive adjunctive chemotherapyCitation7 or radiation therapy.Citation7
In our local experience, AWS is currently getting diagnosed in the Breast Cancer Center by dedicated physical therapists doing research on AWS. AWS had previously been diagnosed less frequently than currently because providers and patients were unfamiliar with the signs and symptoms. In addition, some providers who were knowledgeable about AWS did not refer their patients for evaluation because they were unaware of the treatment options available from physical therapy. The primary focus of care following surgery is on cancer treatment planning, not on the less threatening physical impairments of conditions like AWS. Patients see multiple providers after surgery including surgeons, radiation oncologists, medical oncologists, and primary care providers making it difficult for the patient to know to whom they should report their symptoms and to whom they should turn for advice on how to manage their symptoms. Providers are each addressing symptoms with which they are familiar and may assume others are assessing and addressing less pressing physical symptoms such as AWS. The purpose of this article is to describe the signs and symptoms, diagnosis and management, and potential complications of AWS.
Signs and symptoms
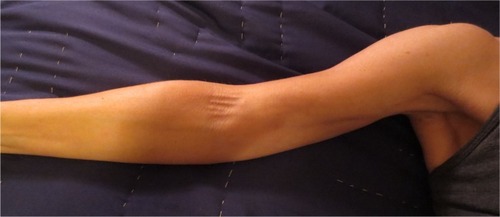
AWS presents as a tight, ~1 mm wide, linear singular cord or multiple cords of tissue in the subcutaneous tissue of the axilla (). The cords may extend “down” the medial or medial-volar ipsilateral upper arm () or “down” along the lateral edge of the ipsilateral chest wall. The cords become visible and/or palpable when the arm is fully extended (straight) and then abducted. One study suggested that in >50% of patients the cords will not be visible requiring careful palpation to detect it.Citation1 Another study indicated that >70% of cords are palpable, with the implication being that the remainder were only visible.Citation13 If the arm is straightened at the elbow and then abducted adequately essentially all cords are palpable and many are visible as a linear “tenting” or “furrowing” of the skin. When the arm is not in the “straightened” (elbow extended) and abducted position, tension is taken off the cord, and the cord may not be evident.
Figure 1 Axillary web syndrome of the left axilla.
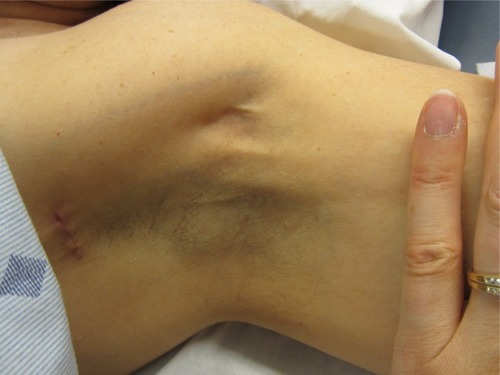
Figure 2 Axillary web syndrome of the right extremity.
AWS is usually diagnosed 2–8 weeks following breast cancer surgery.Citation2,Citation3,Citation6,Citation7 A recent prospective studyCitation11 suggested that 94% of patients who developed AWS diagnosed it within the first 4 weeks using a self-assessment tool. Another prospective studyCitation13 found that careful practitioner evaluation was able to detect 66% of AWS cases within 7 days of surgery. In addition, AWS can present months to years laterCitation2,Citation7,Citation8,Citation14,Citation15 and may also reoccur after resolution.Citation2 Therefore, assessment for AWS should be performed on a frequent basis in the first 3–6 months after surgery, and then less frequently but still regularly for up to 3 years.
At the onset of AWS, patients experience restricted upper extremity movement, primarily during shoulder abduction, and pain or discomfort whenever arm movement increases the tension on the cord. Patients who have not been educated about AWS may be unaware that they have developed a cord. In our experience, patients in whom the diagnosis was delayed often believed that their symptoms were a “normal” part of the postoperative recovery. The full gamut of patient symptoms is dependent on the cord location. Some patients may be unable to extend their elbow because the cord extends across the antecubital fossa. In these cases, the patients may come to the clinic with their arm adducted across their trunk in a sling-like position. Cord extension below the elbow occurs more commonly in patients who have had ALND compared with those who have had SNB.Citation4
Patients with less rigid or shorter cords may have minimal cord tension and minimal symptoms until they approach full extension and abduction. In our experience, they frequently describe arm movement as feeling “different” or “not normal” but their movements are not painful and they are often unaware of the cord. We have seen patients who “learned to live” with their symptoms and later developed chronic shoulder mobility and strength issues.
On occasion, subcutaneous nodules may appear to be connected to AWS cords. Two studies have addressed the concern that the nodules represent metastatic disease.Citation16,Citation17 Biopsies of the nodules demonstrated enlarged lymph vesselsCitation16 and lymph vessels surrounded by fatty tissue.Citation17 It was speculated that the nodules are due to engorgement of the obstructed lymphatic vessels. At this time, no postoperative AWS cord-related nodules have histologically demonstrated metastatic disease.
AWS has been reported to occur prior to breast cancer surgery, likely due to lymphatic involvement by metastatic disease.Citation5 In this study, the patients had subsequent documentation of metastatic spread to axillary lymph nodes. The authors speculated that an aggregation of cancer cells leads to lymphatic obstruction that causes elevated pressures similar to the pressure increase due to surgical interruption. The pathophysiological explanation for preoperative AWS remains a hypothesis, but based on our experience and the literature, it seems prudent to consider the possibility of metastatic spread in making decisions about patient management.
Complications
Early literature described AWS as a self-limited condition, which resolved within 3 months of onset.Citation3,Citation4 More recent research has demonstrated that AWS does not resolve in all patients, can persist for years after surgery, and may reoccur after resolution.Citation1,Citation2,Citation7 AWS development has been associated with early and delayed physical complications following breast cancer surgery.Citation1,Citation2,Citation7 Patients with AWS experience statistically lower range of motion for shoulder abduction and flexion compared with patients without AWS.Citation1 Reduced function and lymphedema have also been associated with AWS.Citation2,Citation7 The actual incidence of lymphedema in patients with AWS is lower than we initially expected and from a clinical standpoint some recent studies suggest that there may be no direct correlation between the presence of AWS and the development of lymphedema. In one study,Citation18 35.9% of their surgical patients developed AWS and 31.4% developed lymphedema. However, the overlap was small enough that there was no statistically significant association between AWS and the development of lymphedema (OR=0.87, 95% CI 0.65–1.15, P=0.329). In the other study,Citation19 the AWS group had less increase in upper arm volume than the non-AWS group in which upper extremity volumes were increased. Overall, the AWS group had significantly more pain, less active ROM in shoulder abduction, and a lower upper-limb volume at 0–10 cm proximal to the lateral epicondyle. The overall incidence of lymphedema was 9.9% and was not associated with AWS. In our experience following AWS patients for longer than 2 years, it appears that chronic AWS may lead to other secondary problems such as chronic alteration of movement patterns and shoulder dysfunction and pain. This has been substantiated in some other recent studiesCitation2,Citation19,Citation20 but further research is needed to determine the scope and severity of complications associated with AWS. One recent studyCitation21 found non-specific vascular changes in the affected arm, the significance of which are currently unknown.
Receiving a breast cancer diagnosis and undergoing breast cancer treatment is very stressful for patients. In our experience, AWS may lead to a higher level of psychological distress and anxiety due to the additional burdens of loss of function, reduced movement, and pain. Some patients have also related fears that the symptoms might be a sign of cancer recurrence. The small amount of research has been done on this matterCitation5,Citation7 has not found any association between postoperative AWS and cancer recurrence. Patient education and physical therapy treatment for AWS may help to reduce overall levels of patient anxiety.
Assessment
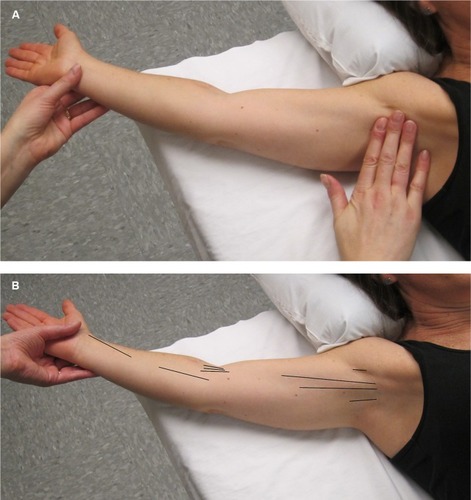
Assessment for AWS does not usually require further testing beyond physical examination to determine if AWS is present or not present (). Description of the location of AWS (axilla, upper arm, elbow, lower arm, wrist/hand, lateral chest wall) and the number of cords present (single/multiple) are also beneficial ().
Figure 3 (A) In order to make an accurate diagnosis of the presence or absence of AWS. The physical examination should be performed in a manner that is designed to facilitate the search for the problem. The first step is to gently but maximally extend the arm at the elbow and then gently but maximally abduct the affected arm at the shoulder. The person performing the evaluation both visualizes and palpates for cords in the locations indicated in (B). (B) This figure illustrates the locations (see black lines) in which a cord or cords may be found, including the axilla, down the upper arm from the axilla to and across the antecubital space, and rarely down the forearm to the base of the thumb.
A comprehensive assessment for AWS was established for research purposesCitation22 but could also be used in clinical practice to objectively assess AWS. The length, width, and depth of each cord can be measured at each location to assess change over time.Citation22 A tape measure can be used to measure the length of AWS cords (in cm) but it is difficult to measure the width and depth with a tape measure, therefore subjective judgment can be used to assess width (<, >, or = to 1 mm) and depth (superficial, middle, deep). Using a marking pen to outline the cord on the surface of the skin will allow the cords to be measured more easily if the cords are palpable but not visible.Citation22 Pictures are also beneficial to document changes. Goniometric shoulder movements (active and passive range of motion) should be used to assess for movement restrictions with specific attention to shoulder abduction as it is the most affected movement followed by flexion.Citation1 Elbow and hand motion should also be measured if affected. Physical therapists, who are movement specialists, can provide a thorough shoulder assessment on postural changes and shoulder kinematics, including scapular dysfunction and altered movement patterns. Location and intensity of pain should be assessed during upper extremity movements since tension on the cord occurs during movement especially shoulder abduction. The Disabilities of the Arm, Shoulder, and Hand questionnaire has been frequently used to assess upper extremity shoulder function in individuals with AWSCitation1,Citation2,Citation7,Citation20,Citation22 but other questionnaires may also be useful to assess pain, function, and/or quality of life in individuals with AWS.Citation2,Citation7,Citation11,Citation20 Lymphedema assessment is also warrantedCitation1,Citation2,Citation7,Citation8,Citation15,Citation20 using available measuring methods such as girth measurements, perometer, bioimpedance, and/or tissue dielectric constant.
Although rare, there are cases, listed below, in which further testing may be indicated to rule out other potential causes of cording.
Chest wall cording: AWS-related cording on the chest wall may be difficult to detect and differentiate from Mondor’s disease (or syndrome). When cords are located on the chest wall, some doctors appear to consider AWS and Mondor’s disease synonymous.Citation23 Mondor’s disease is a thrombosis of a superficial chest wall vein. Mondor’s disease is often described as having a linear or curvilinear vessel-like appearance with occasional redness or swelling, which may appear as a protuberance or furrow, and is often thicker than the AWS cord. Mondor’s disease may be present following breast augmentation and is often located along the inferior fold of the breast. In our experience, high frequency ultrasound (15 MHz or greater) or high resolution MR imaging is able to reliably determine if the band is a thrombosed superficial vein. If either of these two imaging tests fail to find a vascular structure, our results and those of one other study indicate that the cord is due to AWS since AWS is not associated with a consistent pathological structure using ultrasound imaging.Citation24,Citation25
AWS with nodules: Currently, there is no evidence that nodules attached or adjacent to AWS cords following surgery are associated with metastatic disease. Nonetheless, we would agree that a biopsy of the nodule should be considered if there is any concern for potential metastatic disease.
AWS prior to cancer surgery: Cases of AWS prior to breast surgery may be a sign of metastatic disease to the lymph nodes.Citation5 A thorough examination of the axillary lymph nodes should be performed prior to or during surgery. Additionally, if it is felt that it may impact the type or extent of the surgery preoperative MRI or axillary ultrasonography can be used to identify and potentially biopsy suspicious lymph nodes.
AWS unrelated to cancer surgery: Individuals who experience AWS unrelated to cancer surgery may need to undergo further testing to determine the cause of the condition. Some cases of AWS have been related to infection and strenuous activity.Citation5,Citation26 If there is any concern about metastatic disease involving the axillary lymph nodes, diagnostic imaging such as PET-CT, mammography, ultrasound, or MRI of the breast and axilla should also be considered.
Treatment strategies
Based on our experience and results from several other studies,Citation4,Citation14,Citation15,Citation17,Citation20,Citation27–Citation31 physical therapy is recommended as a safe and effective primary treatment for AWS. These studies support the hypothesis that physical therapy resolves cording more rapidly than no treatment. Physical therapy treatment at our institution consists of an initial process of patient education, supervised and at-home exercises and tissue “manipulations” including a variety of adjunctive rehabilitation interventions to improve range of motion and decrease pain, and therapist-performed, in-clinic manual therapy including myofascial release, soft tissue mobilization, and cord manipulation and stretching while the arm is abducted. Non-steroidal anti-inflammatory drugs and opioids have also been recommended based on the severity of associated pain.Citation32 If, as we currently believe, AWS is due to lymphatic pathology, treatment by a physical therapist specialized in lymphedema and/or cancer rehabilitation would be advantageous. Other physical therapy techniques that have a positive effect on the outcome of AWS include compression bandaging and manual lymphatic drainage. Scar release manipulation and massage of adhesions can assist with releasing the “adhesive” tissue that seems to be an integral part of the AWS cords. Joint mobilization can lessen secondary joint restrictions in the shoulder, scapula, rib, clavicle, and upper back. Stretching and strengthening exercises can improve range of motion and muscle strength. In those patients with the capability and understanding, the at-home program can even be expanded to include the exercises and manual techniques directed at the cord and associated adhesions mentioned above.
If AWS is accompanied by lymphedema, physical therapy utilizing adjunctive manual lymphatic drainage has been shown to significantly reduce arm volume and pain compared with physical therapy alone.Citation20 Cords that extend into the extremity have also been reported to resolve with short-term use (1–2 days) of gradient compression lymphedema bandaging of the extremity.Citation30 Therapists have reported feeling a pop or snap in the cord during manipulative or massage treatment. The sensation feels to the therapist as if the cord is breaking.Citation17,Citation28 The problem has not been studied adequately to determine if “breaking a cord” is safe or effective in the “long term”. In the anecdotal experience at our institution, breaking of the cord is usually followed by a sudden increase in mobility and a decrease in the tension of the cord resulting in partial symptom resolution. It has been speculated that the supporting fibrous or inflammatory tissue that surrounds the cord might be breaking and not the cord itself.Citation28 Our short-term experience indicates that “breaking” a cord does not have any negative effects such as swelling. The intermediate-term effects of “breaking” a cord indicate that the immediate increase in range of motion tends to be maintained. But, if the pathophysiologic basis for the AWS cords is pathology of the lymphatic vessels, such as leakage due to upstream obstruction followed by extra-lymphatic inflammatory or adhesive changes, it is uncertain what effect the “breaking” of the cords might have on the lymphatic system. In general, we feel that gentle manual techniques are recommended. It has also been theorized that AWS cord development could be associated with lymphangiogenesis.Citation5,Citation15,Citation22 If this is true, it is another reason that aggressive manual techniques may not be appropriate.
Conclusion/summary
AWS is a common condition following breast cancer surgery with ALND. It usually presents within 2–8 weeks of surgery but can develop or recur months to years later. It can be associated with later lymphedema in a minority of patients. It presents as a tight subcutaneous cord in the ipsilateral axilla. The cord becomes increasingly evident and tense as the shoulder is abducted. Patients complain of pain with abduction and limited function and range of motion in the affected extremity. Physical therapy and exercise can reduce pain and increase range of motion. Education about and awareness of AWS should be promoted for both patients and providers, and regular assessments for AWS should be initiated by health care providers.
Acknowledgments
The authors would like to thank Mackenzie Dwyer for her assistance in preparing the manuscript and Elena MacDonald and Heather Thompson Buum for their contributions.
Disclosure
The authors report no conflicts of interest in this work.
References
- KoehlerLABlaesAHHaddadTCHunterDWHirschATLudewigPMResearch report movement, function, pain, and postoperative edema in axillary web syndromePhys Ther201595101345135325977305
- KoehlerLAHunterDWBlaesAHHaddadTCFunction, shoulder motion, pain, and lymphedema in breast cancer with and without axillary web syndrome: an 18-month follow-upPhys Ther201898651852729361075
- MoskovitzAHAndersonBOYeungRSByrdDRLawtonTJMoeREAxillary web syndrome after axillary dissectionAm J Surg2001181543443911448437
- LeideniusMLeppänenEKrogerusLvon SmittenKMotion restriction and axillary web syndrome after sentinel node biopsy and axillary clearance in breast cancerAm J Surg2003185212713012559441
- KoehlerLAHunterDWLymphspiration: the axillary web and its lymphatic originLymphology201649418519129908551
- SevereidKSimpsonJTempletonBYorkRHummel-BerryKLeiserowitzALymphatic cording among patients with breast cancer of melanoma referred to physical therapyRehabil Oncol2007254813
- O’TooleJMillerCLSpechtMCCording following treatment for breast cancerBreast Cancer Res Treat2013140110511123813304
- Torres LacombaMMayoral Del MoralOCoperias ZazoJLYuste SánchezMJFerrandezJCZapico GoñiAAxillary web syndrome after axillary dissection in breast cancer: a prospective studyBreast Cancer Res Treat2009117362563019306057
- LauridsenMCOvergaardMOvergaardJHessovIBCristiansenPShoulder disability and late symptoms following surgery for early breast cancerActa Oncol200847456957518465324
- BergmannAMendesVVde Almeida DiasRdo Amaral E SilvaBda Costa Leite FerreiraMGFabroEAIncidence and risk factors for axillary web syndrome after breast cancer surgeryBreast Cancer Res Treat2012131398799221987036
- BaggiFNevola TeixeiraLFGandiniSAxillary web syndrome assessment using a self-assessment questionnaire: a prospective cohort studySupport Care Cancer20182682801280729508139
- LewisPACunninghamJEDynamic angular petrissage as treatment for axillary web syndrome occurring after surgery for breast cancer: a case reportInt J Ther Massage Bodywork201692283727257446
- FigueiraPVGHaddadCASde Almeida RizziSKLFacinaGNazarioACPDiagnosis of axillary web syndrome in patients after breast cancer surgery: epidemiology, risk factors, and clinical aspects: a prospective studyAm J Clin Oncol2018411099299629045263
- WyrickSLWaltkeLJNgAVPhysical therapy may promote resolution of lymphatic coding in breast cancer survivorsRehabil Oncol20062412934
- KoehlerLAAxillary web syndrome and lymphedema, a new perspectiveNatl Lymphedema Netw2006183910
- ReedijkMBoernerSGhazarianDMcCreadyDA case of axillary web syndrome with subcutaneous nodules following axillary surgeryBreast20061534101413
- JosenhansEPhysiotherapeutic treatment for axillary cord formation following breast cancer surgeryPt Zeitschriftfür Physiother2007599868878
- WarissBRCostaRMPereiraACKoifmanRJBergmannAAxillary web syndrome is not a risk factor for lymphoedema after 10 years of follow-upSupport Care Cancer201725246547027704260
- HuangHCLiuHHYinLYYehCHTuCWYangCSThe upper-limb volumetric changes in breast cancer survivors with axillary web syndromeEur J Cancer Care (Engl)2017262e12637
- ChoYDoJJungSKwonOJeonJYEffects of a physical therapy program combined with manual lymphatic drainage on shoulder function, quality of life, lymphedema incidence, and pain in breast cancer patients with axillary web syndrome following axillary dissectionSupport Care Cancer20162452047205726542271
- FurlanCMatheusCNJalesRMDerchainSSarianLOVascular alterations in axillary and brachial vessels in patients with axillary web syndrome after breast cancer surgeryLymphat Res Biol201816328729328961070
- KoehlerLAxillary Web Syndrome Ongoing Medical Evaluation [dissertation]MinnesotaUniversity of Minnesota2013
- SalmonRJBerryMGHamelinJPA novel treatment for postoperative mondor’s disease: manual axial distractionBreast J200915438138419601943
- KoehlerLAHunterDWHaddadTCBlaesAHHirschATLudewigPMCharacterizing axillary web syndrome: ultrasonographic efficacyLymphology201447415616325915976
- LeducOFumièreEBanseSIdentification and description of the axillary web syndrome (AWS) by clinical signs, MRI and US imagingLymphology201447416417625915977
- RashtakSGambleGLGibsonLEPittelkowMRFrom furuncle to axillary web syndrome: shedding light on histopathology and pathogenesisDermatology2012224211011422508068
- FourieWJRobbKAPhysiotherapy management of axillary web syndrome following breast cancer treatment: discussing the use of soft tissue techniquesPhysiotherapy200995431432019892098
- KepicsJMPhysical therapy treatment of axillary web syndromeRehabil Oncol20042212122
- LuzCMDDeitosJSiqueiraTCPalúMHeckAPFManagement of axillary web syndrome after breast cancer: evidence-based practiceRev Bras Ginecol Obstet2017391163263928701024
- ZutherJENortonSLymphedema Management The Comprehensive Guide for Practitioners4New York, NYThieme2017
- Thompson BuumHAKoehlerLTuttleTMVenturing out on a limb: axillary web syndromeAm J Med20171305e209e21028249666
- ChevilleALTchouJBarriers to rehabilitation following surgery for primary breast cancerJ Surg Oncol200795540941817457830
