Abstract
Bisphosphonates inhibit osteoclast-mediated bone resorption, thereby inhibiting the release of growth factors necessary to promote cancer cell growth, differentiation, and tumor formation in bone. These agents have demonstrated efficacy for delaying the onset and reducing the incidence of skeletal-related events in the advanced breast cancer setting, and have been shown to prevent cancer therapy-induced bone loss in the early breast cancer setting. Emerging clinical data indicate that the role of bisphosphonates in advanced and early breast cancer is evolving. Retrospective analyses and recent clinical trial data show that zoledronic acid may improve outcomes in some patients with breast cancer. Data from ABCSG-12 and ZO-FAST suggest that zoledronic acid may improve disease-free survival in the adjuvant breast cancer setting in postmenopausal women or women with endocrine therapy-induced menopause, and recent data from a predefined subset of the AZURE trial added to the anticancer story. However, the overall negative AZURE trial also raises questions about the role of bisphosphonates as an anticancer agent in patients with breast cancer. Overall, these data suggest that the addition of zoledronic acid to established anticancer regimens may have potential anticancer benefits in specific patient populations, although more studies are required to define its role.
The burden of bone metastases in women with advanced breast cancer
The impact of breast cancer continues to be felt worldwide with more than 1 million new cases identified each year,Citation1 and 254,650 new cases in 2009 in the United States alone.Citation2 Although the primary tumor is often effectively treated with surgical resection and chemotherapy or endocrine therapy, cancer cells that escape the local site have a predilection for metastasis to bone, an environment that may help them survive during adjuvant therapy. As a result, distant metastases can develop in bone. Approximately 65% to 75% of women with advanced breast cancer will ultimately develop bone metastases, which can lead to skeletal-related events (SREs) such as pathologic fractures, spinal cord compression, hypercalcemia of malignancy, bone pain requiring palliative radiotherapy, and orthopedic surgery.Citation3 Skeletal-related events can be severely debilitating, and may result in a significant reduction in functional independence and quality of life. Furthermore, SREs are associated with increased morbidity and mortality, and pathologic fractures have been associated with a significant increase in the risk of death in women with advanced breast cancer (32%; P < 0.01).Citation4
Managing bone metastases with bisphosphonates
Women with bone metastases from breast cancer often require palliative therapy (radiotherapy and analgesics) to prevent further bone destruction and to manage the pain associated with malignant bone disease. The presence of bone lesions frequently leads to an increase in the rate of osteoclast-mediated bone resorption. Bisphosphonates, antiresorptive agents that inhibit osteoclast function, have demonstrated efficacy for delaying the onset and reducing the incidence of SREs and controlling pain.Citation5–Citation8 During bone resorption, bisphosphonates bind to mineralized bone surfaces and are ingested by osteoclasts, wherein they block activation signals and can induce apoptosis.
Clodronate, ibandronate, pamidronate, and zoledronic acid (ZOL) are bisphosphonates that have demonstrated efficacy for delaying the onset and reducing the incidences of SREs and reducing the pain associated with bone metastases.Citation9 These bisphosphonates have demonstrated varying activity in SRE-prevention trials in patients with multiple myeloma, metastatic breast cancer, prostate cancer, and lung cancer ().Citation10–Citation29 However, it should be noted that pamidronate and ZOL are the only bisphosphonates approved in the metastatic breast cancer setting in the United States.Citation30,Citation31 Recently, the antiresorptive agent denosumab also gained approval in the United States for the prevention of SREs in patients with bone metastases from solid tumors, including breast cancer.Citation32 Current National Comprehensive Cancer Network (NCCN) guidelines for breast cancer recommend initiation of antiresorptive therapy (ZOL, pamidronate, or denosumab) if there is plain radiographic evidence of bone destruction, and continuation of antiresorptive therapy until there is substantial decline in patient performance status.Citation33 Antiresorptive therapy is not currently recommended for the prevention of bone metastases.
Table 1 Summary of BP SRE prevention trials outcomes
However, evidence suggests that the benefits of bisphosphonates may extend beyond the reduction of SREs in patients with breast cancer. Preclinical trials have demonstrated that this class of agent has anticancer effects.Citation34 Anticancer activities include direct inhibition of cancer cell proliferation, induction of apoptosis, synergy with cytotoxic anticancer therapies, inhibition of angiogenesis, and activation of antitumor T-cell immunity. Moreover, modifying the bone microenvironment surrounding cancer cells may have powerful anticancer effects.Citation35,Citation36 Within the bone marrow, the release of bone matrix-derived growth factors by cancer cells during osteoclast-mediated bone resorption can promote cancer cell growth, differentiation, and tumor formation in bone. Inhibition of osteoclast-mediated bone resorption by bisphosphonates can prevent the release of these growth factors, thereby potentially preventing cancer recurrence.
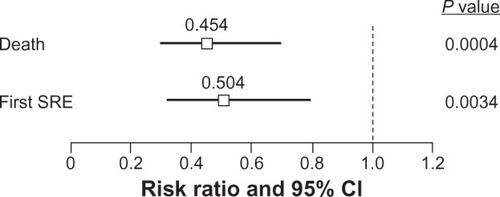
Additional insights into the effects of the bisphosphonates ZOL and pamidronate on clinical outcomes have been reached through the retrospective analyses of biochemical markers of bone turnover. These markers may indicate the severity of metastatic bone disease. For example, elevated urinary N-telopeptide of type I collagen (NTX) levels are associated with increased risk of SREs, disease progression, and death.Citation37,Citation38 An exploratory analysis of patients with breast cancer, prostate cancer, myeloma, and non-small cell lung cancer found that approximately 30% of women with bone metastases from breast cancer have high NTX levels (≥100 nmol/mmol creatinine).Citation38 Patients with high and moderate NTX levels had a 2-fold increase in their risk of skeletal complications and disease progression compared with patients with low NTX levels (P < 0.001 for all). High NTX levels in each solid tumor category were associated with a 4- to 6-fold increased risk of death on study, and moderate NTX levels associated with a 2- to 4-fold increased risk compared with low NTX levels (P < 0.001 for all).Citation38 In a retrospective subset analysis of a phase III trial comparing ZOL with pamidronate in patients with breast cancer,Citation39 60% of patients had elevated baseline NTX levels. Most patients (76%) treated with ZOL normalized their NTX levels after 3 months of therapy, and normalization of NTX significantly reduced the risk of SREs and improved survival ().Citation39
Figure 1 Kaplan–Meier estimates of survival by baseline and 3-month N-telopeptide of type I collagen (NTX) levels in women with bone metastases from BC. Reprinted with permission from Lipton et al. Oncologist. 2007;12(9):1035–1043.Citation39
Similar effects of ZOL were observed in a retrospective analysis of 3 large, phase III trials (N = 1341) in patients with breast cancer (n = 578), prostate cancer (n = 472), or non-small cell lung cancer and other solid tumors (n = 291) and elevated baseline NTX levels, which found that ZOL normalized or maintained normal NTX levels in most patients with bone metastases.Citation40 Indeed, normalization of NTX levels within 3 months of treatment was associated with reduced risks of skeletal complications and death compared with persistently elevated NTX.Citation40
In addition to studies in breast cancer, ZOL has also demonstrated survival benefits in several prospective studies in other tumor types. Notably, ZOL has shown improved survival in small pilot studies with patients with metastatic bladder cancerCitation41 or multiple myeloma,Citation42 and in a large phase III clinical trial in patients with multiple myeloma (N = 1960).Citation43 Together, these data support the potential survival benefits of ZOL in patients with bone metastases from advanced cancer.
Adjuvant zoledronic acid therapy in early breast cancer
Current techniques for screening and early diagnosis of breast cancer and refinement of first-line and adjuvant treatments for breast cancer have led to increased survival in this setting. However, chemotherapy often causes ovarian failure and early menopause, which leads to osteoporosis.Citation44 The use of hormone replacement therapy is successful in preventing bone loss after natural and surgical menopause, but it cannot be utilized in breast cancer survivors because of its potential effect on dormant tumor cells.Citation45 Moreover, estrogen-depleting regimens are common adjuvant therapies for early breast cancer, and have been associated with significantly increased risk of fractures.Citation6,Citation46,Citation47 The use of antiresorptive therapy is beneficial in this setting for preventing bone loss, and ZOL has consistently protected against bone loss associated with ovarian ablative and adjuvant hormonal therapies for early breast cancer. Furthermore, ZOL therapy may have effects beyond bone health in patients with early breast cancer.Citation48,Citation49
In preclinical models, ZOL has been shown to block breast cancer metastasis to bone,Citation50 thereby preventing the vicious cycle of bone resorption and tumor formation. Data from translational clinical trials suggest that ZOL may affect the viability of cancer cells via its effects on bone metabolism.Citation51–Citation54 Indeed, ZOL may modify the bone microenvironment surrounding cancer cells through indirect effects on the ability of disseminated tumor cells (DTCs) to survive and/or reactivate to initiate tumor recurrence.Citation36 This concept has been supported by studies of the effects of ZOL on DTCs. The detection of DTCs in the bone marrow of women with early stage breast cancer is prognostic of early relapse.Citation55,Citation56 DTCs can lie dormant in bone marrow for extended periods of time before becoming active and metastasizing to secondary sites.Citation36
In pilot clinical trials in patients with breast cancer, ZOL reduced DTC persistency ().Citation51,Citation53,Citation54,Citation57 In one study, 120 patients with newly diagnosed breast cancer received neoadjuvant chemotherapy with or without ZOL (4 mg every 3 weeks) for 1 year. Of the women who were DTC-positive at baseline, 70% of ZOL-treated patients became DTC-negative by 3 months, versus 53% in the chemotherapy-alone group (P = 0.054).Citation51 Furthermore, 87% of ZOL-treated patients who were DTC-negative at baseline remained DTC-negative at 3 months compared with 60% of patients receiving chemotherapy alone (P = 0.03).Citation51 Patients who were DTC-positive after completing adjuvant chemotherapy for breast cancer (N = 45) who received monthly ZOL for 2 years experienced a significant reduction in the prevalence of DTCs at 12 and 24 months compared with baseline (P ≤ 0.001).Citation52,Citation57 In each of these 3 studies, ZOL was generally well tolerated.Citation51–Citation54,Citation57 The ability of ZOL to reduce DTCs in patients with breast cancer may result from anticancer synergy between endocrine therapy and ZOL, which has been demonstrated in preclinical studies.Citation58
Table 2 Effect of ZOL on DTC levels in patients with early breast cancer
Data from 3 phase III clinical trials suggest that ZOL may have anticancer benefits in the adjuvant breast cancer setting ().Citation48,Citation59,Citation60 In the ABCSG-12 trial, premenopausal women with early stage, endocrine-responsive breast cancer (N = 1803) received goserelin and tamoxifen ± ZOL or goserelin and anastrozole ± ZOL for 3 years. Analyses after a median follow-up of 48 months showed that the addition of ZOL to adjuvant endocrine therapy reduced the risk of disease progression by 36% compared with endocrine therapy alone.Citation48 Overall, ZOL improved disease-free survival (DFS) by 32% (hazard ratio [HR] = 0.68; 95% confidence interval [CI] = 0.51, 0.91; P = 0.009), decreased the risk of disease progression 36% (P = 0.01),Citation48 and produced a trend toward improved overall survival (OS) versus no ZOL (HR = 0.66 [0.41, 1.09]; P = 0.1).Citation49 Furthermore, at 48 and 62 months’ follow-up, the DFS benefits of ZOL remained significant compared with hormonal therapy alone, suggesting a long-term carryover benefit from the initial 3 years of ZOL treatment.Citation49
Figure 2 Forest plot of DFS outcomes in adjuvant ZOL BC trials. The ZO-FAST, E-ZO-FAST, and ABCSG-12 phase III clinical trials in patients with early BC showed that ZOL improved DFS.
Abbreviations: BC, breast cancer; DFS, disease-free survival; NR, not reported; ZOL, zoledronic acid.
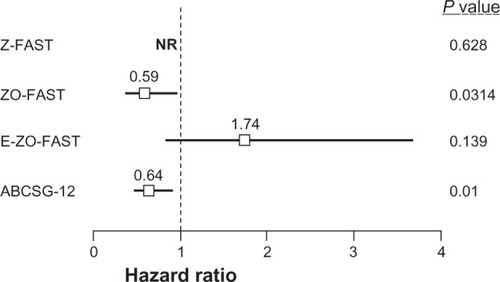
In 3 companion studies, Z-FAST (N = 602), ZO-FAST (N = 1065), and E-ZO-FAST (N = 527), postmenopausal women with early breast cancer receiving letrozole were randomized to receive upfront or delayed ZOL (4 mg via 15-minute infusion every 6 months) for 5 years.Citation60,Citation61 Although disease recurrence was a secondary endpoint in all 3 trials, DFS was an exploratory endpoint that none of the trials were powered to detect.Citation59,Citation60,Citation62 Despite this, the largest of the 3 trials (ZO-FAST, N = 1065) showed improved DFS in patients who received upfront ZOL. After a median follow-up of 36 months, the upfront-ZOL group had a significant 41% reduction in the risk of having a DFS event compared with the delayed-ZOL group (HR = 0.588 [0.361–0.959]; log-rank P = 0.0314),Citation59 and these benefits were maintained through 60 months’ follow-up (HR = 0.66 [0.44–0.97]; log-rank P = 0.0375).Citation63 Furthermore, an integrated 24-month analysis of the Z-FAST and ZO-FAST trials showed a 42.7% improvement in DFS in patients receiving upfront ZOL compared with delayed ZOL (HR = 0.573 [0.358–0.916] log-rank P = 0.0183).Citation61 However, there was no significant difference in DFS between the upfront- and delayed-ZOL groups in the Z-FAST (N = 602) and E-ZO-FAST (N = 527) studies.Citation64
The AZURE trial is assessing the anticancer activity of ZOL in patients with early breast cancer. Patients with high-risk, early stage breast cancer (N = 3360) received anticancer therapy alone or standard therapy plus a tapered dosing schedule of ZOL (4 mg every 3–4 weeks × 6; 4 mg every 3 months × 8; 4 mg every 6 months × 5).Citation65 In the neoadjuvant substudy (n = 205) of the AZURE trial, ZOL plus neoadjuvant anticancer therapy significantly reduced residual invasive tumor size compared with anticancer therapy alone (43%; P = 0.006).Citation66 Furthermore, patients who received ZOL had a 2-fold improvement in their pathologic complete response rates.Citation66 This small substudy suggests that the synergy between ZOL and chemotherapy can have a direct anticancer effect on the primary tumor.
Unlike the other adjuvant breast cancer trials described, the AZURE study was not limited to hormone-responsive breast cancer. The patient population included both premenopausal and postmenopausal women, and standard anticancer therapy included adjuvant chemotherapy as well as endocrine therapy. Overall, the trial results were negative; the adjuvant use of ZOL did not improve the primary endpoint of DFS in the overall patient population (HR = 0.98; P = 0.79) at a median follow-up of 59 months, although there was a nonsignificant trend toward improved OS for ZOL versus control (HR = 0.85; P = 0.07).Citation65 Interestingly, prospective protocol-defined subgroup analyses based on menopausal status showed that ZOL significantly improved DFS (HR = 0.76; P < 0.05) in patients who were at least 5 years postmenopausal at baseline (n = 1041) and OS (HR = 0.71; P = 0.017) when women of unknown postmenopausal status but age >60 years at baseline (n = 1101) were included in the subset analysis.Citation65 Zoledronic acid also reduced each type of recurrence both in and outside bone (HR and P value were not reported) versus control in the postmenopausal subset (n = 1101).Citation65 These results may seem inconsistent with data showing significant DFS benefits from ZOL in premenopausal women in ABCSG-12. However, the premenopausal populations in these 2 studies were markedly different. In ABCSG-12, premenopausal women underwent complete ovarian suppression with goserelin therapy plus either tamoxifen or anastrozole, which resulted in endocrine-therapy induced menopause, while most premenopausal women in the AZURE study received chemotherapy rather than endocrine therapy. The data from these large, prospective clinical trials suggest that ZOL may have anticancer activity in some patient populations, although further studies are needed to clarify which patients may receive the greatest benefit from therapy.
Discussion
Bone health in women with breast cancer is an important concern throughout the disease course. Endocrine therapy for women with early breast cancer combined with ZOL to protect bone health may also have the advantage of reducing the incidence of metastases. In women whose disease has already spread to bone, antiresorptive therapies have established utility for delaying the onset and reducing the incidence of potentially debilitating SREs. Bisphosphonates in general and ZOL specifically have been shown to block multiple steps in the process of tumor metastasis either alone or in combination with anticancer agents.
Clinical treatment guidelines that address bone health in women with breast cancer have been developedCitation33 and are continually evolving as new clinical trial data become available. In addition to being a clearly established therapy for the prevention of SREs in patients with bone metastases from breast cancer, ZOL has been shown to reduce disease recurrence, improve DFS and, with longer follow-up, may also improve OS in pre- and postmenopausal women with early endocrine-responsive breast cancer. Data in this setting are promising but still investigational, as trial outcomes have varied for different patient populations.
Furthermore, guidelines for the prevention of SREs in patients with bone metastases support the use of ZOL, pamidronate, or denosumab in patients with breast cancer; ZOL or denosumab in patients with any solid tumor; and ZOL in patients with multiple myeloma. In the advanced cancer setting, prognostic indicators (eg, bone markers) of ZOL activity may ultimately allow for personalization of interventions, potentially providing a greater benefit-risk profile; however, further data are needed. Recent trials also suggest that adding ZOL to adjuvant endocrine therapy in some patient populations may protect bone health and improves clinical outcomes beyond adjuvant therapy alone. However, although ABCSG-12, the Z-/ZO-/E-ZO-FAST companion trials, and the postmenopausal subset analysis in the AZURE study have shown improved outcomes with the addition of ZOL in the adjuvant breast cancer setting, the overall results of the AZURE study were negative. The role of ZOL therapy in the adjuvant setting is evolving and ongoing studies of antiresorptive therapies (ie, ZOL and denosumab) will define this role.
Disclosure
Dr Lipton has served as a consultant for Amgen and Novartis; has received honoraria from Amgen, Novartis, and Genentech; has received research funding from Novartis, Monogram Biosciences, and Oncogene Sciences; and has given expert testimony for Novartis.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. I thank Duprane Pedaci Young, PhD, ProEd Communications, Inc.®, for her medical editorial assistance with this manuscript.
References
- GarciaMJemalAWardEMGlobal Cancer Facts and Figures 2007Atlanta, GAAmerican Cancer Society2007
- American Cancer SocietyCancer Facts and Figures 2010Atlanta, GAAmerican Cancer Society2010
- ColemanRESkeletal complications of malignancyCancer1997808 Suppl158815949362426
- SaadFLiptonACookRChenYMSmithMColemanRPathologic fractures correlate with reduced survival in patients with malignant bone diseaseCancer200711081860186717763372
- ClezardinPFournierPBoissierSPeyruchaudOIn vitro and in vivo antitumor effects of bisphosphonatesCurr Med Chem200310217318012570716
- GnantMMlineritschBLuschin-EbengreuthGAdjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudyLancet Oncol20089984084918718815
- HaTCLiHMeta-analysis of clodronate and breast cancer survivalBr J Cancer200796121796180117325699
- HershmanDLMcMahonDJCrewKDZoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancerJ Clin Oncol200826294739474518711172
- PavlakisNSchmidtRStocklerMBisphosphonates for breast cancerCochrane Database Syst Rev20053CD00347416034900
- BerensonJRLichtensteinAPorterLLong-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study GroupJ Clin Oncol19981625936029469347
- BerensonJRRosenLSHowellAZoledronic acid reduces skeletal-related events in patients with osteolytic metastasesCancer20019171191120011283917
- BodyJJDielIJLichinitserMRIntravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastasesAnn Oncol20031491399140512954579
- BodyJJDielIJLichinitzerMOral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studiesBr J Cancer20049061133113715026791
- BrinckerHWestinJAbildgaardNFailure of oral pamidronate to reduce skeletal morbidity in multiple myeloma: a double-blind placebo-controlled trial. Danish-Swedish co-operative study groupBr J Haematol199810122802869609523
- DearnaleyDPSydesMRMasonMDA double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial)J Natl Cancer Inst200395171300131112953084
- GimsingPCarlsonKTuressonIEffect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trialLancet Oncol2010111097398220863761
- KohnoNAogiKMinamiHZoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trialJ Clin Oncol200523153314332115738536
- KristensenBEjlertsenBGroenvoldMHeinSLoftHMouridsenHTOral clodronate in breast cancer patients with bone metastases: a randomized studyJ Intern Med19992461677410447227
- LahtinenRLaaksoMPalvaIVirkkunenPElomaaIRandomised, placebo-controlled multicentre trial of clodronate in multiple myeloma. Finnish Leukaemia GroupLancet19923408827104910521357451
- LiptonATheriaultRLHortobagyiGNPamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trialsCancer20008851082109010699899
- McCloskeyEVMacLennanICDraysonMTChapmanCDunnJKanisJAA randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC Working Party on Leukaemia in AdultsBr J Haematol199810023173259488619
- MenssenHDSakalovaAFontanaAEffects of long-term intravenous ibandronate therapy on skeletal-related events, survival, and bone resorption markers in patients with advanced multiple myelomaJ Clin Oncol20022092353235911981007
- PatersonAHPowlesTJKanisJAMcCloskeyEHansonJAshleySDouble-blind controlled trial of oral clodronate in patients with bone metastases from breast cancerJ Clin Oncol199311159658418243
- RosenLSGordonDKaminskiMZoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trialCancer J20017537738711693896
- RosenLSGordonDKaminskiMLong-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trialCancer20039881735174414534891
- RosenLSGordonDTchekmedyianSZoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial – the Zoledronic Acid Lung Cancer and Other Solid Tumors Study GroupJ Clin Oncol200321163150315712915606
- SaadFGleasonDMMurrayRA randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinomaJ Natl Cancer Inst200294191458146812359855
- SmallEJSmithMRSeamanJJPetroneSKowalskiMOCombined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancerJ Clin Oncol200321234277428414581438
- Tubiana-HulinMBeuzebocPMauriacLDouble-blinded controlled study comparing clodronate versus placebo in patients with breast cancer bone metastasesBull Cancer200188770170711495824
- Zometa [prescribing information]East Hanover, NJNovartis Pharmaceuticals Corporation2009
- Aredia [prescribing information]East Hanover, NJNovartis Pharmaceuticals Corporation2008
- Xgeva [prescribing information]Thousand Oaks, CAAmgen Inc2010
- National Comprehensive Cancer NetworkNCCN Clinical Practice Guidelines in OncologyBreast Cancer V.2.2011www.nccn.org2010 Updated December 6, 2010Accessed January 5, 2011
- ClezardinPAnti-tumour activity of zoledronic acidCancer Treat Rev200531Suppl 31816225995
- GnantMBisphosphonates in the prevention of disease recurrence: current results and ongoing trialsCurr Cancer Drug Targets20099782483320025570
- MeadsMBHazlehurstLADaltonWSThe bone marrow microenvironment as a tumor sanctuary and contributor to drug resistanceClin Cancer Res20081492519252618451212
- BrownJECookRJMajorPBone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumorsJ Natl Cancer Inst2005971596915632381
- ColemanREMajorPLiptonAPredictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acidJ Clin Oncol200523224925493515983391
- LiptonACookRJMajorPSmithMRColemanREZoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activityOncologist20071291035104317914073
- LiptonACookRSaadFNormalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acidCancer2008113119320118459173
- ZaghloulMSBoutrusREl-HossienyHKaderYAEl-AttarINazmyMA prospective, randomized, placebo-controlled trial of zoledronic acid in bony metastatic bladder cancerInt J Clin Oncol201015438238920354750
- AvilesANamboMJNeriNCastanedaCCletoSHuerta-GuzmanJAntitumor effect of zoledronic acid in previously untreated patients with multiple myelomaMed Oncol200724222723017848748
- MorganGJDaviesFEGregoryWMNational Cancer Research Institute Haematological Oncology Clinical Study GroupFirst-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trialLancet201037697571989199921131037
- PadmanabhanNWangDYMooreJWRubensRDOvarian function and adjuvant chemotherapy for early breast cancerEur J Cancer Clin Oncol19872367457483653192
- PfeilschifterJDielIJOsteoporosis due to cancer treatment: pathogenesis and managementJ Clin Oncol20001871570159310735906
- GnantMFMlineritschBLuschin-EbengreuthGZoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study GroupJ Clin Oncol200725782082817159195
- EastellRAdamsJEColemanREEffect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230J Clin Oncol20082671051105718309940
- GnantMMlineritschBSchippingerWEndocrine therapy plus zoledronic acid in premenopausal breast cancerN Engl J Med2009360767969119213681
- GnantMMlineritschBStoegerHMature results from ABCSG-12: Adjuvant ovarian suppression combined with tamoxifen or anastrozole, alone or in combination with zoledronic acid, in premenopausal women with endocrine-responsive early breast cancerPresented at 46th Annual Meeting of the American Society of Clinical Oncology (ASCO)June 4–8, 2010Chicago, IL Abstract 533
- OttewellPDDeuxBMonkkonenHDifferential effect of doxorubicin and zoledronic acid on intraosseous versus extraosseous breast tumor growth in vivoClin Cancer Res200814144658466618628481
- AftRNaughtonMTrinkausKEffect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trialLancet Oncol201011542142820362507
- GreenbergSParkJWMeliskoMEEffect of adjuvant zoledronic acid (ZOL) on disseminated tumor cells (DTC) in the bone marrow (BM) of women with early-stage breast cancer (ESBC): updated resultsPresented at 46th Annual Meeting of the American Society of Clinical OncologyJune 4–8, 2010Chicago, IL Abstract 1002
- RackBJuckstockJGenssEMEffect of zoledronate on persisting isolated tumour cells in patients with early breast cancerAnticancer Res20103051807181320592383
- SolomayerEFGebauerGHirnlePInfluence of zoledronic acid on disseminated tumor cells (DTC) in primary breast cancer patientsPresented at 31st Annual San Antonio Breast Cancer SymposiumDecember 10–14, 2008San Antonio, TX Abstract 2048
- JanniWRackBSchindlbeckCThe persistence of isolated tumor cells in bone marrow from patients with breast carcinoma predicts an increased risk for recurrenceCancer2005103588489115666325
- SchindlbeckCKampikTJanniWPrognostic relevance of disseminated tumor cells in the bone marrow and biological factors of 265 primary breast carcinomasBreast Cancer Res200576R1174R118516457698
- LinAYParkJWScottJZoledronic acid as adjuvant therapy for women with early stage breast cancer and disseminated tumor cells in bone marrowJ Clin Oncol200826Suppl20s Abstract 559
- Neville-WebbeHLColemanREHolenICombined effects of the bisphosphonate, zoledronic acid and the aromatase inhibitor letrozole on breast cancer cells in vitro: evidence of synergistic interactionBr J Cancer201010261010101720160726
- EidtmannHde BoerRBundredNEfficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST StudyAnn Oncol201021112188219420444845
- LlombartAFrassoldatiAPaijaOZoledronic acid prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: E-ZO-FAST 36-month follow-upPresented at 2009 ASCO Breast Cancer SymposiumOctober 8–10, 2009San Francisco, CA Abstract 213
- FrassoldatiABrufskyABundredNEffect of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: a 24-month integrated follow-up of the Z-FAST/ZO-FAST trialsPresented at 11th St. Gallen Breast Cancer ConferenceMarch 11–14, 2009St. Gallen, Switzerland Abstract 132
- BrufskyAMBossermanLDCaradonnaRRZoledronic acid effectively prevents aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST study 36-month follow-up resultsClin Breast Cancer200992778519433387
- de BoerRBundredNEidtmannHThe effect of zoledronic acid on aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: the ZO-FAST study 5-year final follow-upPresented at 33rd Annual San Antonio Breast Cancer SymposiumDecember 8–12, 2010San Antonio, TX Poster P5-11-01
- ColemanRBundredNde BoerRImpact of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z-FAST, ZO-FAST, and E-ZO-FASTPresented at 32nd Annual San Antonio Breast Cancer SymposiumDecember 9–13, 2009San Antonio, TX Abstract 4082
- ColemanREThorpeHCCameronDAdjuvant treatment with zoledronic acid in stage II/III breast cancer. The AZURE trial (BIG 01/04)Presented at 33rd Annual San Antonio Breast Cancer SymposiumDecember 8–12, 2010San Antonio, TX Abstract S4–S5
- ColemanREWinterMCCameronDThe effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancerBr J Cancer20101021099110520234364