Abstract
Breast cancer is the most common type of cancer affecting women in the United States. Triple-negative breast cancer remains the most aggressive molecular subtype secondary to a lack of therapeutic targets. The search for a target has led us to investigate immunotherapeutic agents. Immunotherapy has recently demonstrated significant breakthroughs in various types of cancers that are refractory to traditional therapies including melanoma and Non-Small Cell Lung Cancer (NSCLC). Breast cancer however remains one of the tumors that was initially least investigated because of being considered to have a low immunogenic potential and a low mutational load. Over the past few years, antiPD1/PDL1 drugs have started to make progress in the triple-negative subtype with more promising outcomes. In this report, we review the treatment of triple-negative breast cancer and specifically shed light on advances in immunotherapy and newly approved drugs in this challenging disease.
Background
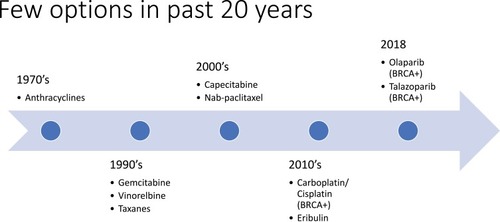
Breast cancer is the most common cancer diagnosed in women, representing 15.3% of all new cancer cases in the United States.Citation1 The rate of new breast cancer diagnoses has remained relatively stable over the last 10 years, and mortality rates have decreased since 2006.Citation1 Prognosis for those with a breast cancer diagnosis is encouraging, with a 5-year survival rate of 89.7%.Citation1 However, not all subtypes of breast cancer have made significant therapeutic advances. Triple-negative breast cancer (TNBC) applies to breast cancers that are low in expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).Citation2–Citation4 TNBC accounts for approximately 10–15% of all breast cancers diagnosed and is associated with a worse prognosis than ER-positive, PR-positive, or HER2-positive breast cancers.Citation5–Citation9 In a study of over 50,000 women with breast cancer, 5-year survival was found to be 77% in TNBC compared to 93% for other breast cancer subtypes.Citation5,Citation10 Additionally, in a 2012 study of over 12,000 women, patients with TNBC experienced worse breast cancer-specific survival (hazard ratio 2.88, 95% CI 2.59–3.45) and worse overall survival (hazard ratio 2.72, 95% CI 2.39–3.10).Citation9 The poorer prognosis in TNBC is explained by early recurrence rates of 10–15% per year for the first several years after initial surgery, compared to 3–5% per year in ER-positive and PR-positive breast cancer, which can recur decades after diagnosis.Citation5,Citation6 Despite remarkable progress with multiple novel agents targeting HER2 or ER, treatment options in TNBC have been limited to cytotoxic chemotherapy as the mainstay of systemic therapy, and few options have been available over the past 20 years ().Citation5,Citation11,Citation12
The search for therapeutic targets in this challenging disease has led us first to PARP inhibitors. The advent of PARP inhibition in the BRCA1/2 mutation carriers has recently brought some progress into treating this small subpopulation of triple-negative breast cancer. The EMBRACA study which randomized to talazoparib (a parp inhibitor) vs physician choice of standard therapy (capecitabine, eribulin, gemcitabine, or vinorelbine) in patients with locally advanced or metastatic breast cancer with a germline BRCA1/2 mutation revealed significantly longer progression-free survival (PFS) of 8.6 months with talazoparib versus 5.6 months with physician’s choice (HR 0.54, 95% CI 0.41–0.71, p<0.001).Citation13 Median overall survival at the interim analysis was also statistically significant, 22.3 months in the talazoparib group versus 19.5 months in the standard therapy group (HR 0.76, CI 0.55–1.06), p=0.11). Remarkably, there was also a total of 5.5% of patients in the talazoparib group that had a complete response (CR) compared with no patients in the standard therapy group. More importantly, the safety profile of talazoparib was better tolerated compared to standard chemotherapy, which was supported by the patient-reported quality-of-life outcomes. The OLYMPIAD study which randomized olaparib (another parp inhibitor) to physician’s choice of standard therapy (capecitabine, eribulin, or vinorelbine) also revealed significantly improved efficacy and safety profiles of the PARP inhibitor compared to standard chemotherapy in patients with metastatic breast cancer and a germline BRCA mutation.Citation14 The PFS was significantly longer in the olaparib group compared to the standard therapy group (7.0 months vs 4.2 months; HR 0.58; 95% CI 0.43–0.80; p<0.001). Additionally, olaparib was better tolerated compared to standard chemotherapy. Rates of grade 3 adverse events were lower in the olaparib group compared to the standard therapy group (36.6% vs 50.5%, respectively). Although PARP inhibitors appear to be a promising therapeutic target, only approximately 5% of patients with breast cancer carry a germline BRCA mutation, and even fewer patients with triple-negative breast cancer carry the mutation. Therefore, this does not address most triple-negative breast cancer patients who are actually non-BRCA carriers.
The first proposition that the immune system and cancer are associated was in the 19th century, based on the frequent appearance of tumors at sites of chronic inflammation and the presence of immune cells in tumor tissues.Citation15,Citation16 The use of immune therapy and specifically checkpoint inhibition made a first impression initially in lung cancer and melanoma. Several immunotherapy treatments have been heavily utilized in melanoma with promising outcomes such as interferon (INF) a-2b, peginterferon a-2b, interleukin-2 (IL-2), as well as checkpoint inhibition.Citation15 In 2010, a trial with ipilimumab, a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitor was conducted and was the first treatment to show improvement in overall survival in patients with metastatic melanoma.Citation17 Following, this landmark and groundbreaking trial, several trials in melanoma with combination immunotherapies, nivolumab and ipilimumab were conducted as well as single-agent nivolumab, and single-agent pembrolizumab.Citation18–Citation25 In lung cancer, several checkpoint inhibitors have been approved in those with PD-L1 expression, which is defined as PD-L1 levels of at least 50%.Citation26 The PD-1 inhibitors used to treat metastatic non-small cell lung cancer (NSCLC) are nivolumab and pembrolizumab, and the PD-L1 inhibitors are atezolizumab and durvalumab. Additionally, in NSCLC, combination chemotherapy, immunotherapy, and targeted therapy have been studied and obtained FDA approval.Citation26–Citation35
Although immunotherapy has been successfully studied in melanoma and lung cancers, when checkpoint inhibitors were tested as monotherapy in breast cancer patients, those studies were not very promising. Early studies with Phase 1, 2 clinical trials evaluating PD1 protein blockade in advanced triple-negative were very disappointing. Response rates were less than 10%.Citation36–Citation41 Those studies proved that breast cancer is not a highly immunogenic disease and patients with breast cancer need to be selected for a preexisting predisposition to mount an immune response. This highlighted the importance of the host as well as the tumor in selecting the best treatment approach. In October 2018, the IMPASSION 130 study led to the approval of Atezolizumab in a preselected population with advanced triple-negative breast cancer.Citation42 This approval was the first meaningful step in moving immunotherapy into the breast cancer arena. Since then, multiple efforts have been generated to advance immunotherapeutic agents in triple-negative breast cancer. In this report, we will review the main advances and shed light on the emerging data in this exciting field.
Checkpoint Inhibition in Triple-Negative Breast Cancer
Triple-negative breast cancer is a highly heterogeneous breast cancer subtype that has been defined by the lack of a target. It has been subdivided into 6 different subgroups based on its molecular heterogeneity that include basal-like, mesenchymal-like, mesenchymal stem-like, luminal androgen receptor expression, immunomodulatory and an unstable type.Citation43 For years it was thought that this disease is resistant to immunotherapy, however recent studies have shown evidence of significant immune infiltration of TILs (tumor-infiltrating lymphocytes) in a subset of patients with triple-negative breast cancer. Triple-negative breast cancer seems to have a high expression of PDL1 and harbors a strong infiltration by immune cells in the actual tumor bed. TILs seem to have both a prognostic as well as predictive power, with high numbers correlating with better outcome and better response to therapy. Elevated TIL scores were proven to correlate with increased pathological complete response to neoadjuvant chemotherapy.Citation44–Citation47 This proves that the immune system plays a pivotal role in this subgroup of patients.
Based on that, efforts were generated to prime the immune system to elicit an immune response capable of fighting off those cancer cells. Immune checkpoint blockade exploited this mechanism at its best through targeting the PD1/PDL1 pathway. PD1 (programmed cell death-1) receptor is a cell surface membrane protein, member of the B7 family of checkpoints that is expressed on the surface of activated T cells. PD1 is activated by its ligands PD-L1 and PD-L2 that are commonly expressed on the surface of dendritic cells or macrophages as well as on tumor cells. When activated, the PD1/PDL1 pathway leads to the suppression of the T-cell-mediated immune response, which normally can minimize states of chronic inflammation and help control autoimmune diseases.
Unfortunately, Tumor cells can exploit this pathway to evade the immune detection system or what is called the cancer immunity cycle. Tumor cells overexpress PDL1 and trigger the PD1/PDL1 pathway, which leads to the inhibition of the cytotoxic T cells. These deactivated T cells remain inhibited in the tumor microenvironment that leads to the unopposed proliferation of cancer cells.
Anti-PD-1 antibodies (like Pembrolizumab and Nivolumab) and anti-PDL-L1 antibodies (like Atezolizumab and Durvalumab) have been developed and are currently being investigated. Those monoclonal antibodies aim to restore the immune system by disrupting the PD1/PDL1 interaction.Citation48 However, it remains to be seen whether PD1 or PDL1 blockade is better.
A similar pathway being investigated is targeting the Cytotoxic T lymphocyte-associated protein 4 (CTLA-4). CTLA4 is a T cell inhibitory receptor that is expressed on activated CD4+ and CD8+ T cells that specifically overexpress CD25 and foxp3. CTLA4 is upregulated by activation of T cell receptor and cytokines such as Il-12 and IFN gamma, which usually forms a negative feedback to, activated T cells leading to a physiologic break of the immune response. CTLA-4 was initially implicated in cancer when in vivo it was demonstrated that blockade of the inhibitory effects of CTLA-4 can release the brake and potentiate the immune response against tumor cells leading to tumor regression in mouse models of sarcoma and colon adenocarcinoma. Anti CTLA-4 drugs are currently being investigated. Ipilimumab has already been approved in melanoma and is currently being investigated in breast cancer.Citation17 Tremelimumab another anti-CTLA4 is also being investigated.
Other targets that are being investigated for potential checkpoint inhibition include the BTLA, VISTA, TIM3, LAG3, and CD47 proteins but are very early in development.Citation49,Citation50 We will review next the active and completed clinical trials exploring those drugs specifically in triple-negative breast cancer.
Clinical Trials Landscape
One of the first trials with checkpoint inhibitor monotherapy in breast cancer was the phase Ib KEYNOTE-012 trial that evaluated pembrolizumab in heavily pretreated metastatic TNBC.Citation51 In this trial, monotherapy pembrolizumab demonstrated an 18.5% overall response rate (ORR) among 27 patients who were evaluable for antitumor activity. Although preliminary evidence of clinical activity was seen from monotherapy pembrolizumab in this trial, compared to the results in melanoma and lung cancer, these results were discouraging. Other early trials with monotherapy pembrolizumab, atezolizumab, or avelumab were also unhopeful and demonstrated ORR of around 10% or less.Citation36,Citation39,Citation41,Citation52,Citation53
The suggestion to combine chemotherapy and immunotherapy emerged from concept from the cancer-immunity cycle. It was hypothesized that immunotherapy activity can be enhanced by the addition of chemotherapy by priming the immune system to elicit an immune response by releasing antigens and danger signals that recruit antigen-presenting cells.Citation42,Citation54 Several chemotherapeutic agents commonly used in breast cancer such as anthracyclines, cyclophosphamide, and taxanes can promote immunogenic cell death resulting in release of antigens.Citation54 However, it was determined that taxanes in particular may additionally activate toll-like receptor activity and promote dendritic cell activity.Citation42 In the IMPASSION 130 trial, the first breakthrough for immunotherapy in breast cancer, atezolizumab was used in combination with nab-paclitaxel in metastatic TNBC.
Atezolizumab is a programmed death-ligand 1 (PD-L1) blocking monoclonal antibody that binds to PD-L1 on tumor cells and tumor-infiltrating immune cells.Citation55 It executes a complete dual blockade of PD-1 and the B7.1 receptor, which is a costimulatory cell surface protein. The blockage of both PD-1 and B7.1 receptors on T cells and antigen-presenting cells suppress cytotoxic T-cell activity, T-cell proliferation, and cytokine production. Ultimately, blocking PD-L1 activity results in decreased tumor growth.
In the IMPASSION 130 trial, patients with previously untreated metastatic TNBC were randomized to receive either atezolizumab plus nab-paclitaxel or placebo plus nab-paclitaxel.Citation42 PFS was found to be significantly longer with those that received atezolizumab plus nab-paclitaxel compared to those that received placebo plus nab-paclitaxel (7.2 months vs 5.5 months, respectively; HR 0.80, 95% CI 0.69–0.92, p=0.0025). In those that were found to have PD-L1 positivity of at least 1% on the tumor-infiltrating immune cells, the difference in PFS between the two groups was more pronounced. Patients receiving atezolizumab and nab-paclitaxel had a 7.5-month PFS, compared to 5.0 months in those receiving placebo plus nab-paclitaxel (HR 0.62; 95% CI 0.49–0.78, p<0.001). Overall response rates were higher with those that received the checkpoint inhibitor, with 7.1% achieving a CR, compared to 1.6% in those that did not receive atezolizumab. In terms of safety, the safety profile was consistent with the toxic effects of each agent, with no new adverse events observed. The most common immune-related toxicity reported in the study was hypothyroidism, 13.7%. However, overall, the combination of atezolizumab and nab-paclitaxel appeared to be well tolerated. These results led to the FDA approval in 2019 in patients with unresectable, locally advanced or metastatic TNBC whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity cover > 1% of the tumor area) in combination with nab-paclitaxel. Pivotal trials are summarized in .
Table 1 Landmark Clinical Trials
There are currently close to 300 clinical trials ongoing trials investigating the use of immunotherapy in breast cancer54. Most of the trials focus on evaluating checkpoint inhibitors, however some trials are exploring the possibility of vaccines as well as adoptive T-cell therapy. Most of the ongoing trials are phase 1 or 2 and about 15% are Phase 3 studies. In terms of subtypes of breast cancer being studied, most trials that are investigating immune checkpoint blockade alone or in various combinations are in TNBC, however exploration with ERBB2 positive breast cancer is being considered as well. About 80% of the studies are being conducted in the metastatic setting, however there are several ongoing trials in the neoadjuvant and adjuvant setting.
Neoadjuvant clinical trials with combination chemotherapy with or without Immunotherapy have shown promising results, as evidenced by the KEYNOTE-173 and ISPY2 trials. The KEYNOTE-173 trial is a 6-cohort, phase 1b trial of pembrolizumab in combination with platinum and taxanes as neoadjuvant treatment in patients with locally advanced TNBC.Citation56 In this small population of 60 patients, overall pCR rate was 60%, and ORR was 100%. Overall, it appears that pembrolizumab in combination with chemotherapy has some promising antitumor activity in the neoadjuvant TNBC setting. The ISPY2 trial is a Phase II trial evaluating pembrolizumab in combination with standard therapy (paclitaxel followed by doxorubicin and cyclophosphamide) as a neoadjuvant treatment for TNBC.Citation57 Findings revealed a signification increase in pathologic complete response rate of 60% with the addition of pembrolizumab versus 20% without.
Several other studies in the neoadjuvant and adjuvant settings, with immunotherapy in the TNBC population are currently ongoing. The ongoing neoadjuvant trials include the Impassion 031, NeoTRIPaPDL1, and Keynote522. In the adjuvant setting, the current ongoing trials include SWOG 1418; IMPASSION 030; A-BRAVE. With these emerging investigations, there is a robust potential for the role of immunotherapy in the breast cancer setting.
Immunotherapy Challenges
Although immunotherapy has shown promising results in treatment for different types of cancer there are still many challenges.
The side effect profile of immunotherapy remains challenging for most medical oncologists. The side effects of chemotherapy are well known to us, chemotherapy in general weakens the immune system causing an increased risk of infection. Immunotherapy on the other hand, hyper activates the immune system causing a more peculiar but a variety of toxicity called immune-related adverse events (IRAEs).Citation58 It can vary from mild symptoms such as flu-like symptom, fatigue, body ache to more pronounce involving many organs such as life-threatening pneumonitis, immune-induced colitis, immune-induced hepatitis, hypopituitarism seen in Melanoma and SCLC studies.Citation59,Citation60
In the IMpassion 130 study of Atezolizumab and Nab-Paclitaxel vs placebo with Nab-Paclitaxel in advanced triple-negative breast cancer, the frequency of nausea cough, neutropenia, pyrexia and hypothyroidism was at least 5 percentage point greater in the Atezolizumab combination arm then the placebo-chemotherapy arm42. The rate of grade 3 or 4 was 48.7%, vs 42.2%. However, fatal adverse events occurred in 6 patients in the Atezolizumab-chemotherapy arm vs 3 patient in the placebo-chemotherapy arm. The 3 deaths in the Atezolizumab paclitaxel group were from autoimmune hepatitis, mucosal inflammation and septic shock. The authors concluded that the unique spectrum of adverse events that are associated with immune checkpoint blockade does necessitate supplementary monitoring and treatment practices beyond those that are required for chemotherapy.
Another challenge remains is the wide variety of atypical clinical response patterns that can be seen across patients with what looks like to be the same type of disease.Citation61–Citation63
Efficacy of immunotherapy varies widely from one patient to the other, even within the same subtype.
This has been well documented in melanoma studies.Citation59,Citation60 Only 15–20% show a complete response while others may have a lesser degree of benefits and even none. Some of those patients who do response have a long-lasting response that can ultimately borderline on cure. It is crucial to be able to predict who would respond, or not respond to therapy, can we alter treatment to obtain a maximal response to immunotherapy?
Also documented data of some patients who respond to therapy after a progression and/or a flare up called pseudoprogression. The pseudo-progression has been reported to be about 4–7% of patients with NSLC treated with PD1 or PD1 inhibitors.Citation64–Citation66 Previously this phenomenon was thought to be the real progression of the tumor. However, it is now known that patients receiving immune checkpoint blockade drugs, the flare up is due to the consequence of treatment-activated immune cells infiltrating the tumor milieu eliciting a radiographic increase in tumor volume, including lesions that were previously not detected in imaging. It is important for the future of immunotherapy that we learn to distinguish between pseudo-progression and actual progression of the disease in order to maximize the effectiveness of treatment. Pseudoprogression has been stated to be around 9% to 29% but not confirmed.Citation66
As more data emerges from immunotherapy studies in triple-negative breast cancer, we hope to have some of these questions answered and find tools to help us better select our patient for the right therapy. It is rewarding to find new therapies in this patient population, but the focus should also be made to be informed and recognize the many challenges with immunotherapy. The goal should be to select the patients who would benefit the most from immunotherapy based on biomarkers and expression.Citation67
Conclusion
While significant improvements have been achieved, immunotherapy has yet to prove its full potential benefit in the field of breast cancer. We still need to improve our understanding of the complex relationship between the host, the tumor and the shared microenvironment between those two. In this era of ultra-genomic testing, we must also prioritize the discovery of the biomarkers that could guide therapy in appropriate patients. And finally, we need to redefine novel endpoints that reflect overall survival benefit that might be very different from one patient to the other. Success however is within reach. Immunotherapy is already transforming the cancer field in ways we have not previously imagined. By customizing immunotherapy to both the disease as well as the individual, breast cancer can become a chronic disease potentially curable as well as preventable.
Disclosure
The authors report no conflicts of interest in this work.
References
- NooneAM, HowladerN, KrapchoM, et al., eds. Cancer Statistics Review, 1975–2015 - SEER Statistics. SEER Cancer Statistics Review.
- HammondMEH, HayesDF, DowsettM, et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi:10.1200/JCO.2009.25.652920404251
- HammondMEH. ASCO-CAP guidelines for breast predictive factor testing: an update. Appl Immunohistochem Mol Morphol. 2011;19:499–500. doi:10.1097/PAI.0b013e31822a8eac22089488
- AllredDC, CarlsonRW, BerryDA, et al. NCCN task force report: estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Compr Cancer Netw. 2009;7:S-1-S-21. doi:10.6004/jnccn.2009.0079
- StoverDG, WinerEP. Tailoring adjuvant chemotherapy regimens for patients with triple negative breast cancer. Breast. 2015;24:S132–S135. doi:10.1016/j.breast.2015.07.03226255198
- DentR, TrudeauM, PritchardKI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi:10.1158/1078-0432.CCR-06-304517671126
- SorlieT, PerouCM, TibshiraniR, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci. 2001;98:10869–10874. doi:10.1073/pnas.19136709811553815
- SorlieT, TibshiraniR, ParkerJ, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci. 2003;100:8418–8423. doi:10.1073/pnas.093269210012829800
- LinNU, VanderplasA, HughesME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi:10.1002/cncr.2758122544643
- BauerKR, BrownM, CressRD, PariseCA, CaggianoV. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi:10.1002/cncr.2261817387718
- PalSK, ChildsBH, PegramM. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627–636. doi:10.1007/s10549-010-1293-121161370
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer. Natl Compr Cancer Netw. 2017. doi:10.1016/j.amepre.2011.02.015
- LittonJK, RugoHS, EttlJ, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi:10.1056/nejmoa180290530110579
- RobsonM, ImS-A, SenkusE, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi:10.1056/NEJMoa170645028578601
- DominguesB, LopesJ, SoaresP, PopuloH. Melanoma treatment in review. ImmunoTargets Ther. 2018;7:35–49. doi:10.2147/itt.s13484229922629
- BalkwillF, MantovaniA. Inflammation and cancer: back to Virchow?Lancet. 2001;357:539–545. doi:10.1016/S0140-6736(00)04046-011229684
- HodiFS, O’DaySJ, McDermottDF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010. doi:10.1056/NEJMoa1003466
- LarkinJ, Chiarion-SileniV, GonzalezR, et al. Combined nivolumab and ipilimumab or monotherapy in untreated Melanoma. N Engl J Med. 2015;373:23–34. doi:10.1056/NEJMoa150403026027431
- PostowMA, ChesneyJ, PavlickAC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi:10.1056/NEJMoa141442825891304
- WeberJS, D’AngeloSP, MinorD, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi:10.1016/S1470-2045(15)70076-825795410
- RobertC, LongGV, BradyB, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi:10.1056/NEJMoa141208225399552
- RibasA, HamidO, DaudA, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600. doi:10.1001/jama.2016.405927092830
- RibasA, PuzanovI, DummerR, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, Phase 2 trial. Lancet Oncol. 2015;16:908–918. doi:10.1016/S1470-2045(15)00083-226115796
- RobertC, SchachterJ, LongGV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi:10.1056/NEJMoa150309325891173
- RobertC, RibasA, WolchokJD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi:10.1016/S0140-6736(14)60958-225034862
- NCCN. NCCN Clinical Practice Guidelines in Oncology-Non-Small Cell Lung Cancer; 2016. doi:10.1016/0011-5029(88)90024-7
- BorghaeiH, Paz-AresL, HornL, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi:10.1056/NEJMoa150764326412456
- BrahmerJR, HammersH, LipsonEJ. Nivolumab: targeting PD-1 to bolster antitumor immunity. Futur Oncol. 2015;11:1307–1326. doi:10.2217/fon.15.52
- BrahmerJ, ReckampKL, BaasP, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi:10.1056/NEJMoa150462726028407
- AntoniaSJ, VillegasA, DanielD, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi:10.1056/NEJMoa170993728885881
- FehrenbacherL, SpiraA, BallingerM, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi:10.1016/S0140-6736(16)00587-026970723
- GadgeelSM, StevensonJ, LangerCJ, et al. Pembrolizumab (pembro) plus chemotherapy as front-line therapy for advanced NSCLC: KEYNOTE-021 cohorts A-C. J Clin Oncol. 2016;34:9016. doi:10.1200/JCO.2016.34.15_suppl.9016
- MokTSK, WuYL, KudabaI, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi:10.1016/S0140-6736(18)32409-730955977
- GandhiL, Rodríguez-AbreuD, GadgeelS, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi:10.1056/NEJMoa180100529658856
- SocinskiMA, JotteRM, CappuzzoF, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi:10.1056/NEJMoa171694829863955
- DirixLY, TakacsI, JerusalemG, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 2018;167:671–686. doi:10.1007/s10549-017-4537-529063313
- DirixL, TakacsI, NikolinakosP, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase Ib JAVELIN solid tumor trial. In: San Antonio Breast Cancer Symposium; 2014; San Antonio, TX, USA.
- AdamsS, LoiS, ToppmeyerD, et al. Phase 2 study of pembrolizumab as first-line therapy for PD-L1–positive metastatic triple-negative breast cancer (mTNBC): preliminary data from KEYNOTE-086 cohort B. J Clin Oncol. 2017. doi:10.1200/jco.2017.35.15_suppl.1088
- AdamsS, SchmidP, RugoHS, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J Clin Oncol. 2017. doi:10.1200/jco.2017.35.15_suppl.1008
- AdamsS, SchmidP, RugoHS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019. doi:10.1093/annonc/mdy517
- EmensLA, CruzC, EderJP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a Phase 1 study. JAMA Oncol. 2019;5:74. doi:10.1001/jamaoncol.2018.422430242306
- SchmidP, AdamsS, RugoHS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi:10.1056/NEJMoa180961530345906
- YamC, ManiSA, MoulderSL. Targeting the molecular subtypes of triple negative breast cancer: understanding the diversity to progress the field. Oncologist. 2017;22:1086–1093. doi:10.1634/theoncologist.2017-009528559413
- GaraudS, BuisseretL, SolinasC, et al. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;13:5.
- HidaA, WatanabeT, SagaraY, et al. Diffuse distribution of tumor-infiltrating lymphocytes is a marker for better prognosis and chemotherapeutic effect in triple-negative breast cancer. Breast Cancer Res Treat. 2019;178:283–294. doi:10.1007/s10549-019-05390-x31402409
- DenkertC, von MinckwitzG, Darb-EsfahaniS, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi:10.1016/S1470-2045(17)30904-X29233559
- WimberlyH, BrownJR, SchalperK, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3:326–332. doi:10.1158/2326-6066.CIR-14-013325527356
- LipsonEJ, FordePM, HammersHJ, EmensLA, TaubeJM, TopalianSL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42:587–600. doi:10.1053/j.seminoncol.2015.05.01326320063
- HavelJJ, ChowellD, ChanTA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–150. doi:10.1038/s41568-019-0116-x30755690
- MahoneyKM, RennertPD, FreemanGJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–584. doi:10.1038/nrd459126228759
- NandaR, ChowLQM, DeesEC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib keynote-012 study. J Clin Oncol. 2016;34:2460–2467. doi:10.1200/JCO.2015.64.893127138582
- RugoHS, DelordJP, ImSA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. Clin Cancer Res. 2018;24:2804–2811. doi:10.1158/1078-0432.CCR-17-345229559561
- AdamsS, LoiS, ToppmeyerD, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019. doi:10.1093/annonc/mdy518
- AdamsS, Gatti-MaysME, KalinskyK, et al. Current landscape of immunotherapy in breast cancer: a review. JAMA Oncol. 2019;5:1205. doi:10.1001/jamaoncol.2018.7147
- Tecentriq [package insert]. 2018.
- SchmidP, ParkYH, Muñoz-CouseloE, et al. Pembrolizumab (pembro) + chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): preliminary results from KEYNOTE-173. J Clin Oncol. 2017;35:556. doi:10.1200/jco.2017.35.15_suppl.556
- NandaR, LiuMC, YauC, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35:506. doi:10.1200/jco.2017.35.15_suppl.50628029304
- BrahmerJR, LacchettiC, SchneiderBJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi:10.1200/JCO.2017.77.638529442540
- TawbiHA, ForsythPA, AlgaziA, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi:10.1056/NEJMoa180545330134131
- HarelM, OrtenbergR, VaranasiSK, et al. Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell. 2019;179:236–250.e18. doi:10.1016/j.cell.2019.08.01231495571
- DuffyMJ, CrownJ. Biomarkers for predicting response to immunotherapy with immune checkpoint inhibitors in cancer patients. Clin Chem. 2019;65:1228–1238. doi:10.1373/clinchem.2019.30364431315901
- SambiM, BagheriL, SzewczukMR. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol. 2019;2019:1–12. doi:10.1155/2019/4508794
- Lee VentolaC. Cancer immunotherapy, part 3: challenges and future trends. Pharm Ther. 2017.
- KurraV, SullivanRJ, GainorJF, et al. Pseudoprogression in cancer immunotherapy: rates, time course and patient outcomes. J Clin Oncol. 2016;34:6580. doi:10.1200/jco.2016.34.15_suppl.6580
- Stephen HodiF, BallingerM, LyonsB, et al. Immune-modified response evaluation criteria in solid tumors (imrecist): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018. doi:10.1200/JCO.2017.75.1644
- FerraraR, MezquitaL, TexierM, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543. doi:10.1001/jamaoncol.2018.367630193240
- KwaMJ, AdamsS. Checkpoint inhibitors in triple-negative breast cancer (TNBC): where to go from here. Cancer. 2018;124:2086–2103. doi:10.1002/cncr.3127229424936