Abstract
Tumor dormancy describes a prolonged quiescent state in which tumor cells are present, but disease progression is not yet clinically apparent. Breast cancer is especially known for long asymptomatic periods, up to 25 years, with no evidence of the disease, followed by a relapse. Factors that determine the cell’s decision to enter a dormant state and that control its duration remain unclear. In recent years, considerable progress has been made in understanding how tumor cells circulating in the blood interact and extravasate into secondary sites and which factors might determine whether these cells survive, remain dormant, or become macrometastases. The mechanisms of tumor cell dormancy are still not clear. Two different hypotheses are currently discussed: tumor cells persist either by completely withdrawing from the cell cycle or by continuing to proliferate at a slow rate that is counterbalanced by cell death. Because dormant disseminated tumor cells may be the founders of metastasis, one hypothesis is that dormant tumor cells, or at least a fraction of them, share stem cell-like characteristics that may be responsible for their long half-lives and their suggested resistance to standard chemotherapy. Therefore, knowledge of the biology of tumor cell dormancy may be the basis from which to develop innovative targeted therapies to control or eliminate this tumor cell fraction. In this review, we discuss biological mechanisms and clinical implications of tumor dormancy in breast cancer patients.
Introduction
The theory of hematogenous spread of solid malignancies and its role in metastasis development was originated by several researchers in the 19th century.Citation1 In breast cancer, the presence of single disseminated tumor cells (DTC) in bone marrow is a common phenomenon, observed in up to 40% of patients at primary diagnosis.Citation2 Therefore, DTC detection is increasingly regarded as a clinically relevant prognostic factor for breast cancer; a pooled analysis of bone marrow samples from more than 4,700 patients revealed that their presence is correlated with a poor outcome.Citation2 Further, DTC are able to survive chemotherapy, and their persistence is a strongly negative prognostic factor.Citation3,Citation4 Beyond detection in bone marrow, single tumor cells are frequently encountered in peripheral blood. These circulating tumor cells (CTC) are routinely found, depending on stage of the disease and detection method, in 10% to 80% of breast cancer patients. Conclusive data on the clinical relevance of CTC are pending; nevertheless, recent studies have shown a prognostic potential of CTC in both primary and metastatic settings.Citation5,Citation6
Every day, large numbers of tumor cells are shed into the circulation of cancer patients.Citation7,Citation8 Many of these CTC are already apoptotic or dead; less than 0.1% will give rise to secondary growth.Citation9,Citation10 Most of the still-viable CTC are supposed to be eliminated in the bloodstream by shear forces or simply by neglect, as they have lost vital contact with stromal cells supporting primary tumors.Citation7,Citation11,Citation12 This phenomenon, described as “metastatic inefficiency,” is consistent with the observation that detection of tumor cells in blood or bone marrow does not inevitably predict metastasis; 50% of initially DTC-positive patients stay disease-free and do not experience a relapse.Citation2 Studies suggest that 0.01% of CTC can ultimately produce a single bone metastasis, and at least 10,000 CTC are required for the development of a metastatic colony.Citation13,Citation14 Nonetheless, approximately one-third of patients fail to clear tumor cells from the blood following removal of primary tumor.Citation15,Citation16 Only a small fraction of CTC may be viable and able to enter secondary sites by mechanical entrapment or active migration through the endothelial cell layer.Citation17–Citation20 Factors supporting extravasation are highly heterogeneous, including platelets; leucocytes; macrophages and factors secreted by them; selectins and their ligands expressed on endothelial cells and tumor cells, respectively; integrins; and fibrin deposition.Citation17
Our picture of the colonization of secondary sites by tumor cells has developed from the “seed and soil” hypothesis developed in 1889 by Paget, emphasizing the interactions between cancer cells and the microenvironment of secondary homing sites.Citation21 However, whether tumor cells have developed a tropism for a certain secondary organ or whether tumor cells are seeded as DTC throughout the body but grow only in certain organs has not yet been finally solved. Recently, the concept of the premetastatic niche – a microenvironment permissive to the development of metastases – has gained much interest.Citation22 It has been shown that DTC preferentially localize to these sites.Citation23,Citation24 Interestingly, the primary tumor itself appears to determine in which organs premetastatic niches will form by secreting soluble factors, such as vascular endothelial growth factor (VEGF)-A and placenta growth factor; in this way, the primary tumor will finally determine the distribution of metastases in the body.Citation23 A further component of the premetastatic niche seems to be fibronectin, which may assist in recruitment of bone marrow cells.Citation25
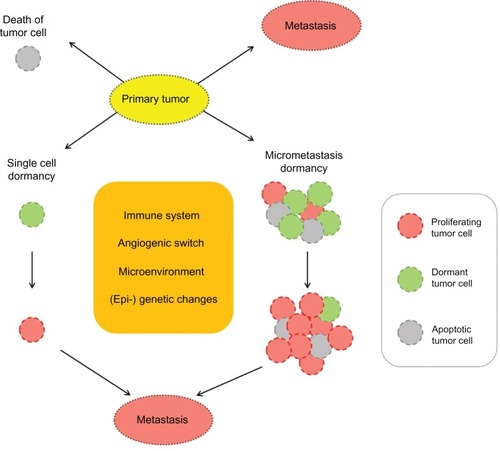
Although DTC, as stated above, can be found in a high percentage of breast cancer patients, and although they are relevant to prognosis, not all patients with DTC develop metastases or a relapse. This suggests that a significant proportion of tumor cells in secondary sites are in a dormant state. shows the possible fates of CTC and DTC.
Cancer dormancy
The phenomenon of tumor dormancy has long been recognized clinically; this term describes a prolonged quiescent state in which tumor cells are present, but disease progression is not clinically apparent. Breast cancer is especially known for long asymptomatic periods – up to 25 years – with no evidence of the disease, followed by a relapse.Citation26,Citation27 After this interval, the mortality rate is comparable to that of the general population.Citation28 Tumor dormancy has also been observed in renal, prostate, and thyroid cancers, as well as in melanoma and B-cell lymphoma, whereas late recurrences are rare in lung and colon cancers. Based on autopsy studies conducted to analyze the metastatic spread of human cancers, Rupert Willis coined the term “dormant tumor cells.”Citation29 Little is known regarding the mechanisms underlying this state, and – despite the clinical importance of tumor dormancy – the biology of dormant cells is poorly understood.
Meng et al detected CTC in 36% of breast cancer patients who showed no evidence of the disease for 7 years or more after treatment. A major fraction of patients with dormant CTC will not experience a relapse during their lifetimes.Citation30 CTC were able to persist in breast cancer patients for as long as 22 years; however, their tumorigenic potential seems limited. Breast cancer patients with tumor cell dormancy present with an apparent balance between replication and cell death. Interestingly, similar findings were reported for murine B-cell leukemia 1 lymphoma. Based on animal models, Holmgren et al suggested that angiogenesis suppression could result in such a balance.Citation31 Dormant tumor cells appear to be quite stable. In prostate cancer patients who had no evidence of disease, the proportion of patients with dormant DTC in their bone marrow less than 1 year after surgery was 64%; this proportion remained similarly high 1–5 years postsurgery (63%) and fell only slightly to 45% more than 5 years after surgery.Citation32 In animal models, the viability of dormant tumor cells was found to be more than 6 months.Citation33
Many hypotheses have been developed to explain the cellular homeostasis observed in cancer dormancy by mechanisms such as insufficient angiogenesis, an effective immune response that keeps the carcinoma population stable at low levels, or cross-talk with cells or proteins released in the microenvironment arresting cancer cells in G0–1. Dormant tumor cells may persist in a quiescent state for many years as single cells that are resistant to therapies that eradicate proliferating cells.Citation34,Citation35 Meng et al performed a comprehensive analysis of the half-life of circulating tumor cells in patients whose primary tumor had just been removed and concluded that the half-life is very short (estimated 1–2.4 hours).Citation30 This is consistent with previous findings that epithelial cells separated from the stroma and neighboring cells enter an apoptotic program.Citation36 As the half-life of CTC is measured in hours and many of the detected cells are apoptotic, it might be hypothesized that a source of replicating tumor cells at secondary homing sites is necessary to constantly replenish the CTC and keep them at the same low level for many years.Citation30 The number of persisting tumor cells might be kept in balance by a tight regulation of proliferation and cell death. This could be accomplished by asymmetric cell division, which gives rise to a similar undifferentiated daughter cell responsible for propagation of DTC and another daughter cell replenishing the CTC pool or eventually undergoing cell death. This is a scenario that has been suggested for adult stem cells, further supporting the idea that DTC generating metastases might have a stem cell-like phenotype.
Potential mechanisms of tumor cell dormancy
Factors that determine the length of the dormancy period remain unclear.Citation37 Current data have led to various experimental models that address the phenomenon of tumor dormancy. It appears that dormant cancer cells can persist either by completely withdrawing from the cell cycle (mitotic arrest) or by continuing to proliferate at a slow rate that is counterbalanced by cell death ().Citation38,Citation39 These two types of dormancy are not mutually exclusive; both forms of latency could coexist in the entire population of DTC of a particular cancer patient.
Single-cell dormancy
In the single-cell dormancy model, isolated tumor cells detached from the primary tumor arrive at the future metastatic organ and enter a prolonged state of mitotic arrest. This model of arrested apoptosis contrasts with the micrometastatic dormancy model, in which proliferation in micrometastatic foci is counterbalanced by cell death. Speculations about the metabolic status of minimal residual disease (MRD) during dormancy are not yet sufficiently investigated. Cell cycle regulation mechanisms are highly complex, with various stimuli interacting at numerous cell cycle checkpoints to determine the proliferative status. The presence of tumor cell dormancy due to a growth arrest of cancer cells is supported by evidence that within tissues where primary tumors are developing or tissues that harbor disseminated cells and have a functional vasculature, tumor cells are found to be in a nonproliferative mode.Citation40–Citation42 In several studies, dormant cancer cells have been demonstrated to be in a G0–1 arrest; this was linked to negative staining for proliferation markers (eg, Ki67, PCNA).Citation43,Citation44 Dormant tumor cells may gain a survival advantage by blocking the receptors for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which would result in arrested apoptosis. Two exemplary mechanisms of TRAIL-receptor blocking have been described and may be of relevance to dormant tumor cells. TRAIL receptors in cancer cells can be blocked by osteoprotegerin, an important member of the tumor necrosis factor receptor superfamily.Citation45,Citation46 Interestingly, bone marrow stromal cells from breast cancer patients secrete enough osteoprotegerin to inhibit apoptosis in vitro.Citation47 More recently, c-Src (a tyrosin-specific kinase involved in breast cancer progression) was demonstrated to support cancer cell survival in the bone marrow microenvironment by conferring resistance to TRAIL.Citation48
Micrometastatic dormancy
In contrast to dormancy due to mitotic arrest, dormancy of a micrometastasis seems to be caused by a balance of cell proliferation and apoptosis, such that the tumor does not increase in size. This constant balance is regulated by proangiogenic proteins and angiogenic inhibitors produced by tumor and stromal cells, as well as immunologic, hormonal, or other microenvironmental switches.Citation49 According to Naumov et al, a failure to activate the angiogenic switch can maintain a group of cancer cells in a dormant state.Citation50 Indraccolo et al reported that a short-term perturbation in the tumor microenvironment, in the form of a transient angiogenic burst, could suffice to interrupt tumor dormancy.Citation49 Genetic data support the interpretation that minimal residual cancer might be divided into “active” and “dormant” groups, in which an advantageous mutation is acquired shortly before a highly aggressive metastatic clone appears.Citation51
At present, there is no definite answer to the question of which model best represents tumor dormancy in breast cancer. Hussein and Komarova hypothesized that indolent breast cancers might fit into the single-cell dormancy model, while more aggressive diseases are linked to the micrometastatic dormancy model.Citation39 Indeed, in a series of experiments, Barkan et al demonstrated that more aggressive basal-type cell lines, such as MDA-MB-231, proliferated readily, while estrogen receptor (ER)-positive MCF7 remained in a state of mitotic arrest, potentially linking the dormancy type associated with arrested growth with the less aggressive disease phenotype.Citation52
Clinical implications of tumor dormancy
The phenomenon of clinical cancer dormancy describes a persistent disease without symptoms or signs (chronic disease), unless the balance between tumor cells and their microenvironment is disturbed and a relapse occurs. In breast cancer, 20% of clinically disease-free patients relapse 7–25 years after mastectomy, and from 10–20 years, the rate of relapse is relatively steady at about 1.5% per year.Citation26,Citation28,Citation53 These recurrences are thought to arise from interruption of the dormant state attributed to DTC.
Recent advances in the field of systemic chemotherapy have yielded improvements in relapse-free and overall survival. Chemotherapy optimally targets highly proliferative cells; however, dormant tumor cells are mostly either slowly proliferating or in a state of arrested growth, explaining the failure of conventional cytotoxic regimes in some breast cancer patients and the need for additional treatment strategies. Further, a large proportion of DTC in breast cancer patients display stem cell-like features, such as ALDH1 positivity or presence of CD44 and absence of CD24.Citation54 These stem cell characteristics – eg, immunophenotype, growth characteristics, and low proliferation rate – may determine their resistance to cytostatic therapy.Citation3,Citation6 These cells are currently considered to be the persisting, dormant cells and represent a surrogate marker for MRD. New therapeutic options that emerge from understanding tumor cell dormancy include the ability to induce or maintain dormancy and induce apoptosis in residual dormant cells. Studies of tumor dormancy might help determine whether a patient has dormant disease and what type of mechanism is active (single-cell dormancy versus micrometastatic dormancy). Potential therapeutic strategies that arise from dormancy studies include: (1) targeting the microenvironment, (2) targeting angiogenesis, (3) targeting signal transduction, and (4) activating the immune system.
Targeting the microenvironment
Recently, oncologic research has focused increasingly not only on the cancer cell itself but also on complex interactions between malignant cells and their microenvironment. The specific microenvironment determines the extent of cell proliferation, angiogenesis, invasion, and survival. Therefore, systemic treatment should target not only the tumor cell but also the surrounding microenvironment. One such treatment option is bisphosphonates, which are potent inhibitors of osteoclast-mediated bone resorption. There is increasing evidence of their potent anticancer activity in vivo as well as in vitro, supporting a role for these drugs beyond their traditional use in treatment of bone metastases secondary to breast cancer.Citation55,Citation56 Several studies confirmed their efficacy in prophylaxis of bone metastasis and their positive impact on survival in selected subgroups of patients.Citation57 In vitro bisphosphonates were shown to influence the microenvironment by altered secretion of growth factors and cytokines; inhibit tumor cell adhesion, invasion, and proliferation; and induce apoptosis.Citation58 Furthermore, these drugs may act indirectly on cancer cells through microenvironmental changes using immunomodulatory and antiangiogenic effects. Small trials have already demonstrated that dormant DTC in bone marrow of primary breast cancer patients can be eliminated by bisphosphonates.Citation59
Targeting angiogenesis
Once dormant tumor cells leave their quiescent state and their mass reaches a certain size, their growth and survival becomes dependent on the development of a vascular bed and blood vessel recruitment. Angiogenesis is thus a critical feature of tumor growth and its inhibition a potential treatment strategy. Clinical trials on bevacizumab, a monoclonal antibody against VEGF, have shown promising results in metastatic breast cancer patients when this treatment is combined with chemotherapy.Citation60 In terms of eliminating dormant tumor cells, antiangiogenic agents might achieve clinically relevant results by preventing angiogenic activation of growth progression (the “angiogenic switch”). Ongoing clinical trials will help to clarify the role of bevacizumab and other angiogenesis inhibitors (eg, small inhibitors of VEGF receptor tyrosine kinases, such as sunitinib) in breast cancer treatment.Citation61
Targeting signal transduction
There is an increasing body of cell-modulating drugs (eg, antibodies or small molecules) directed at specific targets of the cell cycle and of tumorigenesis. The human epidermal growth factor receptor 2 (HER2) is one of the most prominent targets of such approaches. We and others previously reported a discrepancy between cancer cells from the primary tumor and those in secondary sites, such as blood and bone marrow, especially with regard to HER2 and ER status.Citation15,Citation62–Citation65 HER2 gene amplification can be acquired during disease progression, even if the primary tumor was HER2-negative at the beginning of treatment.Citation66 However, patients with HER2-negative tumors but HER2-positive MRD are not eligible for HER2-based treatment. These patients might potentially benefit from such therapy.Citation67 Inversely, MRD cells are generally ER-negative and progesterone receptor-negative, despite originating from a hormone receptor-positive tumor.Citation15,Citation68 This might be relevant to clinicians when selecting patients for targeted or endocrine therapy. In a cohort of 88 patients with ER-positive primary tumors who presented with DTC in their bone marrow, only 14% had ER-positive DTC.Citation65 The loss of ER-positivity in DTC or CTC may explain the failure of endocrine therapy in a subset of ER-positive patients. Therefore, determining the phenotype of MRD is becoming increasingly important, as DTC and CTC are the targets of all adjuvant therapies. While local treatment can adequately deal with the primary tumor and local lymph node metastases, the definitive success of the therapy depends on its ability to eradicate occult tumor cells that persist after primary surgery, before they become clinically evident.
Recent studies suggest that a selected subgroup of patients may benefit from extended adjuvant treatment. Regarding all the validated prognostic factors, monitoring of MRD is the only one available after the primary tumor has been removed. A large pooled analysis demonstrated a strong negative impact of persistent DTC on both disease-free and overall survival.Citation4 Recently, Rack et al presented results of an interventional post-adjuvant trastuzumab-based pilot trial.Citation69 Ten recurrence-free asymptomatic breast cancer patients with persistent HER2-positive DTC received trastuzumab once every 3 weeks for 12 months. All patients completed chemotherapy at least 6 months prior to entering the study. HER2-targeted therapy eradicated HER2-positive DTC in all patients and significantly reduced the number of DTC-positive patients. Similar results were previously reported by Bozionellou et al.Citation70 Trastuzumab effectively targeted HER2-positive MRD in 90%–95% of initially DTC- or CTC-positive patients.
There are increasing numbers of other highly specific agents targeting signal transduction, including lapatinib (targeting the HER1/2-dependent tyrosine kinase), enzas-taurin (targeting protein kinase C), lonafarnib (targeting farnesyltransferase), RAD001 (targeting mammalian target of rapamycin), and sunitinib (targeting c-kit, platelet-derived growth factor, and VEGF). The ability to determine the exact nature of MRD cells and to follow changes in their immunophenotype and genotype during disease progression may allow an individual targeted treatment. However, one must bear in mind that elimination of dormant tumor cells may not have a direct impact on survival outcome. Whether patients with persistent MRD benefit from these agents remains to be evaluated in prospective randomized studies.
Activation of immune system
An interesting treatment strategy may be the use of therapeutic cancer vaccines to stimulate an adaptive immune response that could control or destroy existing cancers.Citation71 Various approaches have been developed, including immunization with tumor cells or their antigens and peptides, combined with different adjuvants. The antigens used in breast cancer vaccination strategies can be represented by whole tumor cells (either allogeneic or autologous) or specific tumor-associated antigens, which are delivered as DNA, RNA, protein, or peptide epitopes.Citation72 Cancer vaccines are based on the assumption that the patient’s immune system can be sensitized to tumor-associated antigens of the patient’s own tumor. However, despite decades of investigative efforts, the results are modest. Hypothetically, therapeutic vaccines may be more effective in patients with (dormant) MRD, because the effector-target ratio is more favorable. A limited number of adjuvant trials are in progress.
Role of microenvironment in tumor dormancy
One unresolved question is whether the tumor cell arriving in the bone marrow is already in a dormant state or becomes dormant because of present or missing factors at the site of its seeding. In the first scenario, the hostile environment outside the tumor stroma might induce dormancy in the tumor cell to allow it to survive the voyage through the bloodstream. Alternatively, seeding a nonpermissive microenvironment might favor the activation of a dormant state. In both situations, reduction of oxygenation and induction of factors such as hypoxia-inducible factor (HIF)-1α might favor dormancy. HIF-1α alpha actually induces the expression of genes associated with neoangiogenesis.Citation73 In breast cancer patients, increased expression of HIF-1α is correlated with dissemination of DTC.Citation74 Evidence for this process is still missing, however.
To seed a new environment and to be maintained, a tumor cell must be able to adhere to stromal cells. Such interaction may include β1-integrins and epidermal growth factor receptor.Citation75 Another important player may be the urokinase-type plasminogen activator receptor (u-PAR). Loss of u-PAR or interruption of the u-PAR pathway activates the p38 mitogen-activated protein kinase stress signaling pathway, which is associated with induction of cell dormancy.Citation76,Citation77 The ERK-to-p38 expression ratio seems to regulate the decision to proliferate or to enter a dormant state.
Influence of immune system on tumor dormancy
Despite some evidence from animal experiments that immune surveillance may be able to control tumor growth, data from patient studies are not convincing that the immune system is significantly involved in maintaining tumor dormancy through surveillance mechanisms.Citation78,Citation79 To enable outgrowth, tumor cells are supposed to reduce their immunogenicity by downregulating the expression of antigenic proteins or upregulating ligands, inducing programmed cell death in the immune cells (eg, CD274). A contradicting hypothesis is that the immune system might support the exit of CTC or DTC from the dormant state by cytokines that are released, for example, during inflammation.
Challenges
The analysis of tumor cell dormancy faces several difficulties. The most important obstacle involves detecting, isolating, and characterizing dormant tumor cells. There is no known specific marker for dormant cells, and current isolation methods based on enrichment of tumor cells using epithelial surface proteins might miss the target population, especially if dormant cells have stem cell capacities.
The next difficulty in characterizing dormant tumor cells is their low frequency. This issue is acerbated because in vitro or in vivo expansion of dormant cells, firstly, is not yet possible and, secondly, might change the phenotype of dormancy and therefore be counterproductive. These obstacles highlight the need for single-cell analysis technologies using micromanipulation in combination with whole-genome or transcriptome amplification steps and miniaturized PCR approaches. The ultimate aim of functional analysis of dormant tumor cells in transplantation assays still haunts investigators in this field. Finding the right and most sensitive recipient mouse for xenotransplantation may be a crucial factor. For example, implantation of unselected melanoma cells in Matrigel (a mixture of extracellular matrix components) into highly immunocompromised Nod-SCID IL2Rg ko mice resulted in the outgrowth of tumors from single cells.Citation80
How dormant tumor cells are activated
The decision of a tumor cell either to enter a dormant state or to enter metastatic outgrowth is a very important step. Thus, understanding how dormancy is controlled is of major importance. Some key factors have been identified in recent years: The interaction of tumor cells with fibronectin has been suggested to be an important component of the premetastatic niche and may enable tumor cells to exit dormancy. Another extracellular matrix protein, collagen type I, has been shown to promote the transition from dormancy to metastatic growth. In addition to extracellular matrix components, other factors are thought to regulate dormancy, such as the expression of metastasis suppressor genes, angiostasis, loss of ERK activity, and depletion of cytostatic CD8+ T-cells.Citation76,Citation81–Citation84
Conclusion
Tumor dormancy is an important, albeit poorly understood, stage of cancer progression. The mechanisms underlying tumor cell dormancy during asymptomatic periods of solid cancers are yet to be sufficiently investigated; dormant cancer cells can persist either by completely withdrawing from the cell cycle (mitotic arrest), or by continuing to proliferate at a slow rate that is counterbalanced by cell death. Better understanding of the phenomenon of tumor dormancy could lead to development of appropriate targeted strategies to control this step of the disease and thereby prevent the occasional transformation of dormant cells into metastasis. One treatment option might be to wake up dormant tumor cells, thereby sensitizing them to cytotoxic agents that act on proliferating cells. Alternatively, developing strategies to prevent this awakening could transform breast cancer into a chronic but controllable disease.
Disclosure
The authors declare that they have no conflicts of interest.
Figure 1 Possible fates of tumor cells detached from primary tumor.
References
- AshworthTRA case of cancer in which cells similar to those in tumors were seen in the blood after deathAust Med J186914146149
- BraunSVoglFDNaumeBA pooled analysis of bone marrow micrometastasis in breast cancerN Engl J Med2005353879380216120859
- BeckerSSolomayerEBecker-PergolaGWallwienerDFehmTPrimary systemic therapy does not eradicate disseminated tumor cells in breast cancer patientsBreast Cancer Res Treat20071062239243 Epub January 27, 200717260094
- JanniWRackBSchindlbeckCThe persistence of isolated tumor cells in bone marrow from patients with breast carcinoma predicts an increased risk for recurrenceCancer2005103588489115666325
- CristofanilliMBuddGTEllisMJCirculating tumor cells, disease progression, and survival in metastatic breast cancerN Engl J Med2004351878179115317891
- RackBKSchindlbeckCAndergassenUUse of circulating tumor cells (CTC) in peripheral blood of breast cancer patients before and after adjuvant chemotherapy to predict risk for relapse: The SUCCESS trial. ASCO Annual Meeting 2010 [abstract]J Clin Oncol201028Suppl 15s1003
- LiottaLAKleinermanJSaidelGMQuantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantationCancer Res197434599710044841969
- ButlerTPGullinoPMQuantitation of cell shedding into efferent blood of mammary adenocarcinomaCancer Res19753535125161090362
- MehesGWittAKubistaEAmbrosPFCirculating breast cancer cells are frequently apoptoticAm J Pathol20011591172011438448
- LarsonCJMorenoJGPientaKJApoptosis of circulating tumor cells in prostate cancer patientsCytometry A2004621465315472900
- FidlerIJMetastasis: Quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-Iodo-2′-desoxyuridineJ Natl Cancer Inst1970457737825513503
- FrischSMScreatonRAAnoikis mechanismsCurr Opin Cell Biol200113555556211544023
- LiottaLASaidelGMKleinermanJStochastic model of metastases formationBiometrics1976323535550963169
- LiottaLASaidelMGKleinermanJThe significance of hematogenous tumor cell clumps in the metastatic processCancer Res19763638898941253177
- BanysMKrawczykNBeckerSThe influence of removal of primary tumor on incidence and phenotype of circulating tumor cells in primary breast cancerBreast Cancer Res Treat20121321121129 Epub May 12, 201121562707
- KragDNAshikagaTMossTJBreast cancer cells in the blood: a pilot studyBreast J19995635435811348313
- LuzziKJMacdonaldICSchmidtEEMultistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastasesAm J Pathol199815338658739736035
- CameronMDSchmidtEEKerkvlietNTemporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiencyCancer Res20006092541254610811137
- KienastYVon BaumgartenLFuhrmannMReal-time imaging reveals the single steps of brain metastasis formationNat Med201016111612220023634
- SleemanJPNazarenkoIThieleWDo all roads lead to Rome? Routes to metastasis developmentInt J Cancer2011128112511252621365648
- PagetSDistribution of secondary growths in cancer of the breastLancet18891571
- PsailaBLydenDThe metastatic niche: adapting the foreign soilNat Rev Cancer20099428529319308068
- KaplanRNRibaRDZacharoulisSVEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic nicheNature2005438706982082716341007
- ErlerJTWeaverVMThree-dimensional context regulation of metastasisClin Exp Metastasis2009261354918814043
- KaplanRNPsailaBLydenDBone marrow cells in the ‘pre-metastatic niche’: within bone and beyondCancer Metastasis Rev200625452152917186383
- DemicheliRAbbattistaAMiceliRValagussaPBonadonnaGTime distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancyBreast Cancer Res Treat19964121771858944336
- PocockSJGoreSMKerrGRLong term survival analysis: the curability of breast cancerStat Med198212931047187092
- KarrisonTGFergusonDJMeierPDormancy of mammary carcinoma after mastectomyJ Natl Cancer Inst199991180859890174
- WillisRAThe Spread of Tumors in the Human BodyLondonJ&A Churchill1934
- MengSTripathyDFrenkelEPCirculating tumor cells in patients with breast cancer dormancyClin Cancer Res200410248152816215623589
- HolmgrenLO’ReillyMSFolkmanJDormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppressionNat Med1995121491537585012
- MorganTMLangePHPorterMPDisseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrenceClin Cancer Res200915267768319147774
- GoodisonSKawaiKHiharaJProlonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fuorescent proteinClin Cancer Res2003910 Pt 13808381414506175
- WikmanHVessellaRPantelKCancer micrometastasis and tumour dormancyAPMIS20081167–875477018834417
- BeckerSBecker-PergolaGWallwienerDSolomayerEFFehmTDetection of cytokeratin-positive cells in the bone marrow of breast cancer patients undergoing adjuvant therapyBreast Cancer Res Treat2006971919616319975
- FrischSMFrancisHDisruption of epithelial cell-matrix interactions induces apoptosisJ Cell Biol199412446196268106557
- FehmTMuellerVMarchesRTumor cell dormancy: implications for the biology and treatment of breast cancerAPMIS20081167–874275318834416
- Aguirre-GhisoJAModels, mechanisms and clinical evidence for cancer dormancyNat Rev Cancer200771183484617957189
- HusseinOKomarovaSVBreast cancer at bone metastatic sites: recent discoveries and treatment targetsJ Cell Commun Signal200152859921484191
- TownsonJLChambersAFDormancy of solitary metastatic cellsCell Cycle20065161744175016861927
- WhiteDERaymentJHMullerWJAddressing the role of cell adhesion in tumor cell dormancyCell Cycle20065161756175916880738
- RanganathanACAdamAPAguirre-GhisoJAOpposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancyCell Cycle20065161799180716929185
- Aguirre GhisoJAKovalskiKOssowskiLTumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signalingJ Cell Biol199914718910410508858
- NaumovGNMacdonaldICWeinmeisterPMPersistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancyCancer Res20026272162216811929839
- FisherJLThomas-MudgeRJElliottJOsteoprotegerin overexpression by breast cancer cells enhances orthotopic and osseous tumor growth and contrasts with that delivered therapeuticallyCancer Res20066673620362816585187
- HolenICrossSSNeville-WebbeHLOsteoprotegerin (OPG) expression by breast cancer cells in vitro and breast tumours in vivo – a role in tumour cell survival?Breast Cancer Res Treat200592320721516155791
- Neville-WebbeHLCrossNAEatonCLOsteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosisBreast Cancer Res Treat200486326927915567943
- ZhangXHWangQGeraldWLatent bone metastasis in breast cancer tied to Src-dependent survival signalsCancer Cell2009161677819573813
- IndraccoloSStievanoLMinuzzoSInterruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironmentProc Natl Acad Sci U S A2006103114216422116537511
- NaumovGNBenderEZurakowskiDA model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotypeJ Natl Cancer Inst200698531632516507828
- KleinCABlankensteinTJSchmidt-KittlerOGenetic heterogeneity of single disseminated tumour cells in minimal residual cancerLancet2002360933468368912241875
- BarkanDKleinmanHSimmonsJLInhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeletonCancer Res200868156241625018676848
- UhrJWPantelKControversies in clinical cancer dormancyProc Natl Acad Sci U S A201110830123961240021746894
- BalicMLinHYoungLMost early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotypeClin Cancer Res200612195615562117020963
- HolenIColemanREAnti-tumour activity of bisphosphonates in preclinical models of breast cancerBreast Cancer Res201012621421176176
- FournierPGStresingVEbetinoFHClezardinPHow do bisphosphonates inhibit bone metastasis in vivo?Neoplasia201012757157820651986
- DielIJJaschkeASolomayerEFAdjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: a long-term follow-upAnn Oncol200819122007201118664560
- ClezardinPEbetinoFHFournierPGBisphosphonates and cancer-induced bone disease: beyond their antiresorptive activityCancer Res200565124971497415958534
- SolomayerEFGebauerGHirnlePInfluence of zoledronic acid on disseminated tumor cells in primary breast cancer patientsAnn Oncol Epub3 12012
- RobertNJDierasVGlaspyJRIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancerJ Clin Oncol201129101252126021383283
- MackeyJRKerbelRSGelmonKAControlling angiogenesis in breast cancer: A systematic review of anti-angiogenic trialsCancer Treat Rev201238667368822365657
- SolomayerEFBeckerSPergola-BeckerGComparison of HER2 status between primary tumor and disseminated tumor cells in primary breast cancer patientsBreast Cancer Res Treat200698217918416552629
- KrawczykNBanysMNeubauerHHER2 status on persistent disseminated tumor cells after adjuvant therapy may differ from initial HER2 status on primary tumorAnticancer Res200929104019402419846945
- FehmTHoffmannOAktasBDetection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cellsBreast Cancer Res2009114R5919664291
- FehmTKrawczykNSolomayerEFERalpha-status of disseminated tumour cells in bone marrow of primary breast cancer patientsBreast Cancer Res2008105R7618793387
- MengSTripathyDSheteSHER-2 gene amplification can be acquired as breast cancer progressesProc Natl Acad Sci U S A2004101259393939815194824
- JückstockJRackBSchindlbeckCTreatment with trastuzumab in recurrence free patients with early breast cancer and persistent disseminated tumor cells (DTC) in bone marrowProceedings of the San Antonio Breast Cancer SymposiumDecember 10–14, 2008San Antonio (TX), USA
- AktasBMullerVTewesMComparison of estrogen and progesterone receptor status of circulating tumor cells and the primary tumor in metastatic breast cancer patientsGynecol Oncol20111222356360 Epub May 24, 201121605893
- RackBJuckstockJGunthner-BillerMTrastuzumab clears HER2/neu-positive isolated tumor cells from bone marrow in primary breast cancer patientsArch Gynecol Obstet20122852485492 Epub June 30, 201121717141
- BozionellouVMavroudisDPerrakiMTrastuzumab administration can effectively target chemotherapy-resistant cytokeratin-19 messenger RNA-positive tumor cells in the peripheral blood and bone marrow of patients with breast cancerClin Cancer Res200410248185819415623593
- CuriglianoGSpitaleriGPietriEBreast cancer vaccines: a clinical reality or fairy tale?Ann Oncol200617575076216293674
- SchlomJTherapeutic cancer vaccines: current status and moving forwardJ Natl Cancer Inst2012104859961322395641
- SemenzaGLTargeting HIF-1 for cancer therapyNat Rev Cancer200331072173213130303
- WoelfeUCloosJSauterGMolecular signature associated with bone marrow micrometastasis in human breast cancerCancer Res200363185679568414522883
- WeaverVMPetersenOWWangFReversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodiesJ Cell Biol199713712312459105051
- Aguirre-GhisoJAEstradaYLiuDOssowskiLERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK)Cancer Res20036371684169512670923
- RanganathanACZhangLAdamAPAguirre-GhisoJAFunctional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cellsCancer Res20066631702171116452230
- MatsuzawaATakedaYNaritaMOzawaHSurvival of leukemic cells in a dormant state following cyclophosphamide-induced cure of strongly immunogenic mouse leukemia (DL811)Int J Cancer19914923033091879974
- RabinovskyRUhrJWVitettaESYefenofECancer dormancy: lessons from a B cell lymphoma and adenocarcinoma of the prostateAdv Cancer Res20079718920217419946
- QuintanaEShackletonMSabelMSFullenDRJohnsonTMMorrisonSJEfficient tumour formation by single human melanoma cellsNature2008456722259359819052619
- NashKTPhadkePANavenotJMRequirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancyJ Natl Cancer Inst200799430932117312308
- HorakCELeeJHMarshallJCShreeveSMSteegPSThe role of metastasis suppressor genes in metastatic dormancyAPMIS20081167–858660118834404
- BarkanDGreenJEChambersAFExtracellular matrix: a gatekeeper in the transition from dormancy to metastatic growthEur J Cancer20104671181118820304630
- EylesJPuauxALWangXTumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanomaJ Clin Invest201012062030203920501944