Abstract
Purpose
Connective vascular diseases (CVD), including scleroderma, are reported to represent for some researchers a relative contraindication and for others absolute contraindication for radiotherapy. The purpose of our study is to add four new cases to the existing body of international literature and to determine whether women with pre-existing scleroderma who have been surgically treated for early breast cancer could undergo postsurgical radiotherapy without serious early and late complications.
Patients and methods
From May 1998 to November 2010, we irradiated for early breast cancer four patients suffering from pre-existing scleroderma; after conservative surgery, we performed whole breast postoperative radiotherapy of 50.4 Gy total dose to the whole breast plus a 9 Gy boost to the tumor bed. We reviewed the records of all four patients and evaluated the early and late reactions using acute radiation morbidity scoring criteria (Radiation Therapy Oncology Group [RTOG], American College of Radiology, Philadelphia, PA) and late radiation morbidity scoring scheme (European Organisation for Research and Treatment of Cancer [EORTC], Brussels, Belgium and RTOG).
Results
After a median follow-up of 105 months (range 12–155 months) the early and late toxicity concerning the skin, the subcutaneous tissues, the lungs, and the heart have been acceptable and are in full accordance with what have been reported in international literature.
Conclusion
This study matches global experience, which shows that patients with scleroderma and breast cancer must be discussed by the multidisciplinary tumor board in order for a personalized treatment strategy to be formulated. Radiation therapy can be proposed as a postsurgical therapeutic option in selected cases.
Introduction
Adjuvant radiotherapy to the breast is of tremendous value in preventing local failure in women with early-stage breast cancer. Many clinical trials bear this out. They repeatedly show that adjuvant radiotherapy reduces the likelihood of local recurrence and allows for higher rates of breast preservation, and also show how adjuvant breast irradiation should be performed.Citation1–Citation8 Many studies in which long-term cosmetic outcome after breast conserving surgery followed by radiotherapy was examined have produced results ranging from good to excellent with most patients.Citation9,Citation10
High total dose radiation has been shown to lead to acute or delayed and partly irreversible skin and breast tissue effects (eg, inflammation or pigmentation change, telangiectasias, fat tissue necrosis, breast tenderness). Also, pulmonary and heart complications can be observed.Citation11,Citation12 Even morphea, a localized type of scleroderma, can be a late complication of breast radiotherapy in non-sclerodermic patients.Citation13–Citation15 The long-term effects generally become evident more than 6 months after irradiation.
Moreover, in those rare cases of patients with underlying solitary collagen vascular disease (CVD, eg, scleroderma) or mixed CVD, the treatment may result in considerable fibrosis and retraction of the breast towards the axilla.Citation11 It seems indeed that CVD is associated with an increased risk of late radiation-induced normal tissue reactionCitation16 and many papers have been published concerning this issue.Citation17–Citation31 Some of these included very few cases (one or two) while others reported details of a greater number of subjects but lacked uniformity in terms of both CVD types and cancer sites.Citation18–Citation31 The information in these articles is not consistent, with tolerance reported as being anything from good to very poor.Citation17–Citation31 Of these studies, the largest one (209 cases/16 with scleroderma) concluded that the decision to perform irradiation on those suffering from scleroderma must be made on a patient-by-patient basis.Citation28
Material and methods
From May 1998 to November 2010, six patients suffering from clinically and laboratory diagnosed scleroderma were operated on for invasive T1–2N0M0 (five cases) or TisN0M0 (in situ – one case) breast cancer. They all underwent conservative surgical excision and were then assessed by the tumor board for adjuvant treatment. Five of them underwent adjuvant chemotherapy whereas hormonal anti-estrogen treatment only was chosen for the patient with the in situ lesion. All patients were candidates for postoperative radiotherapy but two of them were excluded and underwent secondary mastectomy – the first one due to multifocal disease confirmed by the pathological specimen and the second one due to a long history of a predisposition towards skin allergies.
In total, four patients were submitted for postoperative whole breast irradiation with a three-dimensional conformal technique using two opposite tangential 6 MV photon-wedged fields plus a supplementary boost to the tumor bed with a single electron-beam field. All four patients provided informed consent.
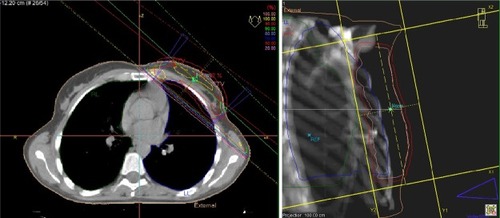
A CT-based three-dimensional treatment plan was formulated for all patients. The treatment plans were produced using the PLATO® and Oncentra Master Plan® (Nucletron BV, Veenendaal, the Netherlands) treatment planning systems with pencil beam algorithms and with correction for tissue inhomogeneities (). All patients received a total dose of 59.4 Gy (50.4 Gy to the whole breast plus a boost of 9 Gy to the tumor bed and the surgical scar) with a conventional fractionation of 1.8 Gy/day, 5 days per week. We managed to achieve dose homogeneity of 95%–105%. According to our quality assurance checks, the correlation between the planned and the delivered doses was well within a 3% margin.
The quality assurance program of our department includes: (a) full quality control of the linacs with daily dosimetry and verification, (b) quality control of the treatment planning system and verification of the accuracy of the dose calculation using thermoluminescent dosimetry on phantoms, (c) monitor unit calculation for each patient using an independent algorithm for verification purposes, and (d) port films for positioning verification. Finally, in vivo dosimetry studies with TLDs and diodes were performed for the breast irradiation (entrance and exit dose) which showed a good correlation between the doses calculated and the doses actually delivered.
Of the four patients in question, one did not undergo chemotherapy due to in situ cancer. The remaining three each underwent a different chemotherapy regimen, ie,
Patient 1: CMF (cyclophosphamide, methotrexate, fluorouracil) − 6 cycles,
Patient 2: CMF-3 cycles + FEC (epirubicin, fluorouracil, cyclophosphamide) − 3 cycles,
Patient 3: AC (adriamycin, cyclophosphamide) − 4 cycles + TXT (docetaxel) − 4 cycles.
We clinically followed up these four patients once a month for the first 2 years, then once every 3 months for the next 3 years and, finally once every 6 months until the submission of this paper.
Using acute radiation morbidity scoring criteria (Radiation Therapy Oncology Group [RTOG], American College of Radiology, Philadelphia, PA) and late radiation morbidity scoring scheme (European Organisation for Research and Treatment of Cancer [EORTC], Brussels, Belgium and RTOG), we evaluated early and late adverse effects on the skin and subcutaneous tissues (clinically), the lung (clinically and by chest CT scans every 3 months for the first 2 years, then after every 6 months) and the heart (clinically, by electrocardiogram [ECG] and by chest CT images).
Results
Early skin reactions were as follows: grade 1 toxicity – two patients (), grade 2 – one patient, and grade 3 – one patient. No grade 4 toxicity was observed and all patients completed the radiotherapy regimen without delays or gaps. No lung or heart toxicity was observed ().
After a median follow-up of 105 months (ranging from 12 to 155 months) late toxicity of the skin was as follows: grade 0 toxicity – one patient (after a follow-up of 83 months); grade 1 (slight atrophy) – two patients (after a follow-up of 12 and 127 months); grade 2 – in one patient (after a follow-up of 155 months). There was no grade 3 or 4 toxicity. Regarding subcutaneous tissue toxicity, we had two patients with grade 1, one patient with grade 2, and one patient with grade 0-toxicity. Only one patient developed grade 1 lung toxicity. Minor increase in the lung density within the radiation portals was noted in one out of four patients at CT-scan evaluation. No case presented with clinical respiratory symptomatology. None of the patients developed heart toxicity ().
No breast pain either acute or chronic has been reported and all four patients were recorded as alive and without locoregional relapse or progression of disease at the time of the study (November 2011).
Discussion
The current approach to the treatment of early breast cancer is to administer conservative surgery (ie, lumpectomy, quadrantectomy, or partial mastectomy) plus postoperative adjuvant irradiation and, when indicated, adjuvant chemotherapy. This approach is preferable for esthetic reasons, particularly with younger patients and is therapeutically as effective as older more invasive surgical treatments.
On the other hand, CVDs, including scleroderma, are reported to represent for some researchers a relative contraindication and for others an absolute contraindication for radiotherapy.Citation16 Therefore, in young people with early breast cancer and a history of scleroderma, the therapeutic decision (conservative excision plus radiotherapy versus total mastectomy) is something which needs serious consideration.
In the past, it has been shown that patients with CVD present increased radiation sensitivity.Citation26,Citation30,Citation31 Some authors proposed decreasing the doses of radiotherapy in order to improve tolerance.Citation28 However, their articles suffer from a lack of homogeneity in terms of both cancersCitation18–Citation31 and types of CVDCitation18,Citation22,Citation23,Citation29,Citation35 that makes it difficult to draw a solid conclusion in a specific setting.
Morris and PowelCitation28 looked at 209 patients with CVD (collagen vascular disease), 16 of whom presented with scleroderma. The authors concluded that radiation therapy was feasible, though the higher risk of early and late reactions must always be taken into account. They emphasized the importance of adapting the treatment protocol to each patient. The drawback of their results is that they referred to the whole population of CVD patients, which makes it difficult to know the treatment tolerance in those suffering from scleroderma.Citation28
Lin et alCitation29 published a study which focused on 86 courses of RT for 73 patients with CVD, nine of them suffering from scleroderma. The researchers concluded that the treatment is generally well tolerated. However, when RT is administered to patients suffering from CVD, there seems to be a greater possibility of late toxicity. RT administered to the pelvis or administered when systemic lupis erythematosus or scleroderma is present may involve an even higher level of risk as far as severe toxicity is concerned. Such issues should be taken into account when RT is being considered as a treatment option for these patients.
Ross et alCitation23 reported a series of 61 patients with CVD (including three with scleroderma) treated for different types of tumors with a follow-up of 18 months. Sixty percent of the patients received doses higher than 40 Gy. They concluded that there were more early and late reactions.
Chen et alCitation18 reported a series of 36 patients (four with scleroderma), with a median follow-up of 12.5 years. The results for the 36 patients compared with a control group were as follows: early reactions: 14% vs 8%, respectively and a significantly higher rate of late reactions (17% vs 3%, respectively) but as the study involved such a small number of cases, no statistical significance can be attached to the results.
Finally, a very interesting study by Gold et al from Mayo Clinic (Rochester, NY) suggests that the number of organ systems involved in CVD may have some predictive value for late complications of breast radiotherapy in these patients (ie, the greater the number of systems involved, the higher the likelihood of late complications may be).Citation32
Accelerated partial breast irradiation (APBI) with either breast brachytherapy or intraoperative radiotherapy (IORT) could be a good option for women with a history of CVD who are suffering from early-stage breast cancer. Most studies have concluded that, although the results need to be confirmed, APBI appears to be equally as safe and well tolerated as external-beam radiotherapy (EBRT).
However, there is still much skepticism about this issue because of concerns regarding increased toxicity. As a result, patients suffering from CVD are generally excluded from most clinical trials studying breast APBI (eg, the NSABP B-39/RTOG-0413 study).Citation33
The percentage of women with persistent post-treatment breast pain amounts to 25%–60% depending on the treatment modalities.Citation34 Breast pain is reported even in non-irradiated women undergoing conservative breast cancer treatment, but it is also evident that radiotherapy significantly increases the likelihood of this side effect, which in some cases can be long lasting; in fact, the literature refers to breast pain as a possible side effect of breast-only irradiation at different rates of 8.7%–23.1%Citation35 and up to 58%.Citation36
This side effect possibly reflects a complex pathophysiology involving touching and pressure on the treated breast,Citation37 preoperative, intraoperative, and post-operative risk factors,Citation34 and even the age of the patient (mainly up to 39 years).Citation35 Moreover, it seems that chemotherapy increases the possibility of post-irradiation breast pain.Citation35,Citation36
Finally, in the study by Matthews et al,Citation38 the pain-insomnia-fatigue cluster of symptoms was associated with some individual characteristics such as optimism, self-esteem, and positive and negative mood.
The lack of breast pain in our patients is possibly due to the low fractionation scheme (1.8 Gy). On the other hand, our patients’ age at the time of radiotherapy ranged from 39 to 62 years and our Patient 4 (aged 39 years) did not receive any chemotherapy.
Conclusion
Our small-scale study shows that certain patients with scleroderma may undergo breast radiotherapy for breast cancer without significant side effects, but there is insufficient evidence for us to draw more conclusions safely.
Given the small number of patients with scleroderma who require radiotherapy for breast cancer, only multicenter prospective randomized studies can provide enough results for definite conclusions to be drawn. Until such results are available, the therapeutic proposal must be decided upon in each case by a multidisciplinary tumor board. In reaching its decision, the board must achieve a balance of therapeutic gain, toxicity expectation, and cosmetic results and last, but not least, the wishes of the patient herself, who must provide informed consent.
After discussion by the multidisciplinary tumor board in order for a personalized treatment strategy to be formulated, radiation therapy could be proposed as a postsurgical therapeutic option on condition that:
there is no systemic scleroderma and the patient’s skin is not especially sensitive to sunlight (risk of photodermatitis),
the anatomy of the patient’s breast and chest permits state of the art radiotherapy treatment planning to maximize the protection of the underlying organs at risk (OARs), ie, lung and heart,
an optimal radiotherapy technique adapted to the patient’s anatomy can be applied,Citation30
a thorough follow-up of the patient day-by-day during the course of the treatment is practicable,
the patient is fully capable of taking responsibility to care for her skin and to report any symptom that could be related to the breast irradiation.
Since all but one of our patients (ie, the patient with the in situ cancer) underwent a different chemotherapy regimen, the impact of the chemotherapy in the development of acute and late toxicity cannot be appraised.
Given that a small number of publications report scleroderma-like changes by the use of docetaxel or paclitaxel,Citation39–Citation44 chemotherapy of any regimen should ideally be avoided in patients with CVD until more data from prospective randomized studies are available, as a review publication from Harvard suggests.Citation45 Nonetheless, if chemotherapy is essential, no drugs which can cause radiation recall phenomenon must be prescribed (eg, anthracyclines, mitoxantrone, etc).
Last but not least, the challenge to identify the patients with scleroderma who are at greatest risk for radiation-related toxic effects will be continuous, as a very important clinical investigation for Mayo Clinic concluded.Citation46
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
The authors received no funding for this work.
Figure 2 Early toxicity in our scleroderma Patient 4. Left: grade 1 skin reaction at the end of the breast irradiation. Right: complete healing 6 months later.
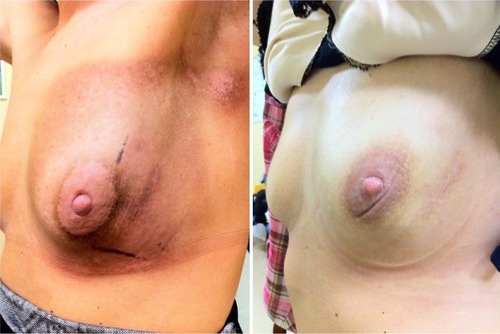
Table 1 Grade of early toxicity after breast radiotherapy in our scleroderma suffering patients
Table 2 Grade of late toxicity after breast radiotherapy in our scleroderma suffering patients
References
- FisherBAndersonSBryantJTwenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy and lumpectomy plus irradiation for the treatment of invasive breast cancerN Engl J Med2002347161233124112393820
- ClarkRMMc CullochPBLevineMNRandomized clinical trial to assess the effectiveness of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancerJ Natl Cancer Inst19928496836891314910
- van DongenJAVoogdACFentimanISLong-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trialJ Natl Cancer Inst200092141143115010904087
- VeronesiUCascinelliNMarianiLTwenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancerN Engl J Med2002347161227123212393819
- JacobsonJADanforthDNCowanKHTen-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancerN Engl J Med1995332149079117877647
- Blichert-ToftMRoseCAndersenJADanish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative GroupJ Natl Cancer Inst Monogr19921119251627427
- ArriagadaRLêMGGuinebretièreJMDunantARochardFTurszTLate local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patientsAnn Oncol200314111617162214581268
- ClarkeMCollinsRDarbySEffects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trialsLancet200536695032087210616360786
- ToledanoAGaraudPSerinDConcurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: long-term results of the ARCOSEIN multicenter randomized studyInt J Radiat Oncol Biol Phys20066532433216542788
- VrielingCColletteLFourquetAThe influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC ‘boost vs no boost’ trial. EORTC Radiotherapy and Breast Cancer Cooperative GroupsRadiother Oncol200055321923210869738
- SassiMLJukkolaARiekkiRType I collagen turnover and cross-linking are increased in irradiated skin of breast cancer patientsRadiother Oncol200158331732311230894
- TrombettaMValakhVJulianTBWertsEDPardaDMammary fat necrosis following radiotherapy in the conservative management of localized breast cancer: does it matter?Radiother Oncol2010971929420227125
- MosterdKWinnepenninckxVVermeulenAvan NeerPAvan NeerFJFrankJMorphea following surgery and radiotherapy: an evolving problemJ Eur Acad Dermatol Venereol20092391099110119470050
- HerrmannTGüntherCCserePLocalized morphea – a rare but significant secondary complication following breast cancer radiotherapy. Case report and review of the literature on radiation reaction among patients with scleroderma/morpheaStrahlenther Onkol2009185960360719756427
- AkayBNSanliHHeperAOPostirradiation linear morphoeaClin Exp Dermatol2010354e106e10819874351
- HölscherTBentzenSMBaumannMInfluence of connective tissue diseases on the expression of radiation side effects: a systematic reviewRadiother Oncol200678212313016445999
- Darras-JolyCWechslerBBlétryOPietteJCDe novo systemic sclerosis after radiotherapy: a report of 3 casesJ Rheumatol1999262265226710529153
- ChenAMObedianEHafftyBGBreast-conserving therapy in the setting of collagen vascular diseaseCancer J20017648049111769860
- TournillacIDandurandMGuillotBBullous lichen sclerosus after radiotherapyAnn Dermatol Venereol19981252121123 French9747229
- MayrNARiggsCEJrSaagKGWenBCPenningtonECHusseyDHMixed connective tissue disease and radiation toxicity. A case reportCancer19977936126189028375
- PhanCMindrumMSilvermanCParisKSpanosWMatched-control retrospective study of the acute and late complications in patients with collagen vascular diseases treated with radiation therapyCancer J20039646146614740974
- HausteinUFExacerbation of progressive scleroderma following roentgen therapyHautarzt1990418448450 German2177048
- RossJGHusseyDHMayrNADavisCSAcute and late reactions to radiation therapy in patients with collagen vascular diseasesCancer19937111374437528490925
- De NaeyerBDe MeerleerGBraemsSVakaetLHuysJCollagen vascular diseases and radiation therapy: a critical reviewInt J Radiation Oncology Biol Phys1999445975980
- FleckRMcNeeseMDEllerbroekNAHunterTAHolmesFAConsequences of breast irradiation in patients with pre-existing collagen vascular diseasesInt J Radiat Oncol Biol Phys19891748298332777673
- DelanianSMaulard-DurduxCLefaixJLHoussetMMajor interactions between radiation therapy and systemic sclerosis: is there an optimal treatment?Eur J Cancer199632A47387398695286
- WenzelJScleroderma and malignancy. Mechanisms of interrelationshipEur J Dermatol200212329630011978579
- MorrisMMPowelSNIrradiation in the setting of collagen vascular disease: acute and late complicationsJ Clin Oncol1997157272827359215847
- LinAAbu-IsaEGriffithKABen-JosefEToxicity of radiotherapy in patients with collagen vascular diseaseCancer2008113364865318506734
- RansomDTCameronFGScleroderma – a possible contra-indication to lumpectomy and radiotherapy in breast carcinomaAustralas Radiol19873133173183435351
- CooperSGDenhamJWProgressive systemic sclerosis (diffuse scleroderma) and radiotherapyBr J Radio199063754804805
- GoldDGMillerRCPinnMEOsbornTGPetersenIABrownPDChronic toxicity risk after radiotherapy for patients with systemic sclerosis (systemic scleroderma) or systemic lupus erythematosus: association with connective tissue disorder severityRadiother Oncol200887112713118158195
- DragunAEHarperJLOlyejarSEZunzuneguiRGWazerDEThe use of adjuvant high-dose rate brachytherapy in patients with collagen vascular disease: a collaborative experienceBrachytherapy201110212112720678963
- AndersenKGKehletHPersistent pain after breast cancer treatment: a critical review of risk factors and strategies for preventionJ Pain201112772574621435953
- LundstedtDGustafssonMSteineckGRisk factors of developing long-lasting breast pain after breast cancer radiotherapyInt J Radiation Oncol Biol Phys2011 [Epub ahead of print.]10.1016/j.ijrobp.2011.05.065
- GärtnerRJensenMBNielsenJEwertzMKromanNKehletHPrevalence of and factors associated with persistent pain following breast cancer surgeryJAMA2009302181985199219903919
- LundstedtDGustafssonMMalmströmPSymptoms 10–17 years after breast cancer radiotherapy data from the randomised SWEBCG91-RT trialRadiother Oncol201097228128720970212
- MatthewsEESchmiegeSJCookPFSousaKHBreast cancer and symptom clusters during radiotherapyCancer Nurs2011 [Epub ahead of print.]10.1097/NCC.0b013e3182277222
- ClevelandMGAjaikumarBSRegantiRCutaneous fibrosis induced by docetaxel: a case reportCancer20008851078108110699898
- BattafaranoDFZimmermanGCOlderSAKeelingJHBurrisHADocetaxel (Taxotere) associated scleroderma-like changes of the lower extremities. A report of three casesCancer19957611101158630861
- HassettGHarnettPManoliosNScleroderma in association with the use of docetaxel (taxotere) for breast cancerClin Exp Rheumatol200119219720011326485
- KupferIBalguerieXCourvillePChinetPJolyPScleroderma-like cutaneous lesions induced by paclitaxel: a case studyJ Am Acad Dermatol200348227928112582404
- LäuchliSTrüebRMFehrMHafnerJScleroderma-like drug reaction to paclitaxel (Taxol)Br J Dermatol2002147361962112207621
- De AngelisRBugattiLCerioniADel MedicoPFilosaGDiffuse scleroderma occurring after the use of paclitaxel for ovarian cancerClin Rheumatol2003221495212605319
- WoJTaghianARadiotherapy in setting of collagen vascular diseaseInt J Radiat Oncol Biol Phys20076951347135318035210
- GoldDGMillerRCPetersenIAOsbornTGRadiotherapy for malignancy in patients with scleroderma: The Mayo Clinic experienceInt J Radiat Oncol Biol Phys200767255956717236971