Abstract
Background
Apoptosis can be used as a reliable marker for evaluating potential chemotherapeutic agents. Because wortmannin is a microbial steroidal metabolite, it specifically inhibits the phosphatidyl inositol 3-kinase pathway, and could be used as a promising apoptosis-based therapeutic agent in the treatment of cancer. The objective of this study was to investigate the biomolecular mechanisms involved in wortmannin-induced cell death of breast cancer-derived MCF-7 cells.
Methods and results
Our experimental results demonstrate that wortmannin has strong apoptotic effects through a combination of different actions, including reduction of cell viability in a dose-dependent manner, inhibition of proliferation, and enhanced generation of intracellular reactive oxygen species.
Conclusion
Our findings suggest that wortmannin induces MCF-7 cell death via a programmed pathway showing chromatin condensation, nuclear fragmentation, reactive oxygen species, and membrane blebbing, which are characteristics typical of apoptosis.
Introduction
Breast cancer is the most common human malignancy and causes considerable cancer-related mortality in the Western world, where approximately one-third of women develop metastases.Citation1–Citation5 Systemic breast cancer therapies have limitations in terms of nonspecific targeting, toxicity to normal tissues, and increased drug resistance, resulting in only short-term efficacy.Citation2,Citation6 Using conventional therapy, 5-year and 10-year survival rates for locally advanced breast cancer are approximately 55% and 35%, respectively.Citation7,Citation8 However, 10 years after diagnosis and removal of the primary tumor, a 40% recurrence rate has been reported.Citation8,Citation9 Therefore, novel and advanced alternative therapeutic strategies are needed.
Apoptosis is a programmed cell death process and a natural phenomenon that is important in both normal physiological and pathological conditions.Citation2 An ideal anticancer drug would inactivate cancer cells without much more effect in normal cells.Citation10 By inducing apoptosis specifically in cancer cells, this ultimate goal can be achievable. Phosphatidyl inositol 3-kinase (PI3K) helps cancer cells to avoid apoptosis and promotes cell cycle progression and proliferation as well as angiogenesis by modulating proapoptotic molecules, such as Bad and p53,Citation11–Citation15 enabling cancer cells to grow in an uncontrolled manner. Furthermore, mutated PI3K activation is abnormally expressed or overexpressed in several cancers, including stomach, colon, breast, lung, ovarian, and pancreatic cancer, with activation of its main downstream gene, Akt.Citation11,Citation16,Citation17 A study has concluded that PI3K-Akt plays a major role in this cell line with regard to increased drug resistance.Citation18,Citation19 Deregulated PI3K-Akt activity has also been reported in breast malignancies associated with increased resistance to multiple chemotherapeutics and radiotherapies.Citation18,Citation20–Citation22
The MCF-7 cell line has been widely used as a model breast cancer cell line due to its inherent lack of functional caspase 3.Citation23,Citation24 Because MCF-7 cells lack this pivotal crucial effector protease, they become highly resistant to most chemotherapeutic drugs and subsequently survive because of resistance to apoptosis,Citation1,Citation25 and do not undergo classical apoptogenic mechanisms during programmed cell death.Citation26 Studies that have used the MCF-7 cell line with conventional breast cancer chemotherapeutic agents, such as paclitaxel, doxorubicin, 5-fluorouracil, etoposide, and camptothecin, have reported resistance within a few months to a few years.Citation18,Citation19 Therefore, the PI3K-Akt pathway is of great interest as a novel target in the treatment of breast cancer.
Wortmannin (C23H24O8, ) is a highly cell-permeable, antifungal-antibiotic agent similar to the viridian group, and a potent and irreversible PI3K inhibitor that blocks the PI3K-Akt signaling pathway involving cell cycle progression and apoptosis.Citation12,Citation17 Therefore, we hypothesized that wortmannin could induce apoptosis in MCF-7 breast cancer cells. Various modifications and conjugations with wortmannin have been reported, establishing it as an effective antitumor agent.Citation16,Citation27 However, in the present study, the direct apoptotic effect of wortmannin at various concentrations was investigated selectively in the MCF-7 cell line. Thus, the primary goal of these experiments was to determine the apoptotic effects of wortmannin in a widely used MCF-7 cell model.
Materials and methods
A MCF-7 breast cancer cell line, Eagle’s Minimum Essential Medium (EMEM), bovine insulin, penicillin-streptomycin, and trypsin-ethylenediamine tetra-acetic acid (TE) was obtained from the American Type Culture Collection (ATCC, Rockville, Maryland). Trypan blue, Hoechst 33342, acridine orange, ethidium bromide, propidium iodide, H2O2, and Phosphate-buffered saline (PBS) was sourced from Amresco Inc (Salon, OH). 2′, 7′-Dichlorofluorescein diacetate (DCF-DA), and Yo-PRO®-1 and propidium iodide (PI) double staining kits were purchased from EMD Chemicals (Gibbstown, NJ) and Invitrogen (Life Technologies, Carlsbad CA), respectively. MTT [3-(4, 5-dimethylthiazol2-yl) – 2.5-diphenyltetrazolium bromide], dimethyl sulfoxide, fetal bovine serum, and a standard tissue culture (75 cm2) flask with a filtered cap was purchased from BioExpress (Kaysville, UT).
MCF-7 cell culture
MCF-7 cells were obtained from the ATCC and maintained in EMEM 10% fetal bovine serum, 0.01 mg/mL bovine insulin, 100 U/mL of penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2. Cells were grown in a standard tissue culture (75 cm2) flask with a filtered cap, and the growth media were changed every 2–3 days as per the ATCC protocol. Cells were subcultured with 0.25% TE after reaching 70% confluence. For all experiments, the cells were seeded on 6-well, 12-well, and 96-well plates at a density of 5 × 104 cells/well, 2.5 × 104 cells/well, and 1 × 104 cells/well, respectively, and allowed to attach before applying various treatments.
Viability, cell death, and cytotoxicity using Trypan blue exclusion assay
The Trypan blue exclusion method was used with some modifications to determine total cell numbers and the proportion of live and dead cells. Briefly, MCF-7 cells were grown on 6-well plates at a density of 5 × 104 cells/well. Replacement of starvation media was performed as usual at 24 hours before treatment with wortmannin. The cells were treated with different concentrations of wortmannin for 24 hours and then trypsinized centrifuged redispersed in 0.5 mL of media, mixed thoroughly with 50 μL of 0.4% Trypan blue (w/v), and allowed to stain at room temperature for 5 minutes. The cells were then counted using a hemocytometer. Each experiment was repeated three times with triplicate samples. In statistical analysis, the percentage of cell death was calculated by counting at least 200–300 cells per sample in several randomly selected fields using the formula of percentage of cell death = number of dead cells/number of total cells × 100.
MTT assay
Cell metabolic activity was determined using a modified MTT assay. Briefly MCF-7 cells were seeded at a density of 1.25 × 104 cells/well in 24-well plates. EMEM was replaced by 0.5 mL of starvation medium on the day before the experiments. Different concentrations of wortmannin were then applied with incubation at 37°C in 5% CO2 for 24 hours. The cells were then labeled with 20 μL of MTT solution (5 mg/mL in PBS) added into each well, followed by 5 minutes of orbital shaking, after which the cells were incubated in the dark for 4 hours. The medium was then removed dimethyl sulfoxide (DMSO) was added to dissolve the formazan, and photographs were taken using a phase-contrast microscope. Three separate experiments were conducted.
Phase-contrast microscopy for morphological analysis
MCF-7 cells were seeded in triplicate into 6-well plates at a density of approximately 5 × 104 cells/well and treated with various concentrations of wortmannin. For qualitative assessment, images were taken randomly using a Zeiss Axiovert 40 CFL inverted phase-contrast microscope (Zeiss, Oberkochen, Germany).
Fluorescence microscopy for analysis of apoptosis
Apoptotic cell death was quantified using acridine orange and ethidium bromide as fluorescent probes. Briefly, cells treated or not treated with wortmannin were trypsinized centrifuged pelleted and stained with 20 μL of dye mixture containing 15 mg/mL of acridine orange and 50 mg/mL of ethidium bromide in 1× PBS and allowed to stain for 5–10 minutes at room temperature. Thereafter, 2–3 drops of the stained cells were placed on each slide with a coverslip. Photographs were taken at random locations under a Nikon 90i fluorescence microscope equipped with Nikon BR software. Untreated cells were used as the negative control.
Flow cytometric apoptosis assay using Yo-PRO®-1 and propidium iodide staining
Apoptosis was quantified by Yo-PRO®-1 and propidium iodide double-staining methods using a vibrant apoptosis detection kit. Briefly, the cells were treated with different concentrations of wortmannin for 24 hours. The cells were then collected by trypsinization, and resuspended in ice-cold 1× PBS at a density of about 1 × 106 cells/mL in PBS. The cells were double-stained with Yo-PRO®-1 and propidium iodide according to each manufacturer’s protocol, followed by 30 minutes of incubation on ice. The cells were then analyzed by BD FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) using CellQuest® Pro software.
Nuclear chromatin staining with Hoechst 33342
MCF-7 cells (about 5 × 104 cells/well) were plated on coverslips in 6-well plates and treated with different concentration of wortmannin for 24 hours. After incubation, the cells were stained with 1 μg/mL Hoechst 33342 dye (diluted with distilled water) for 20 minutes in a 37°C incubator with 5% CO2. The cells on the coverslips were then washed in ice-cold PBS (1×) three times, after which the cells were mounted on glass slides and photographed immediately using a 90i Nikon fluorescent microscope.
Measurement of intracellular ROS
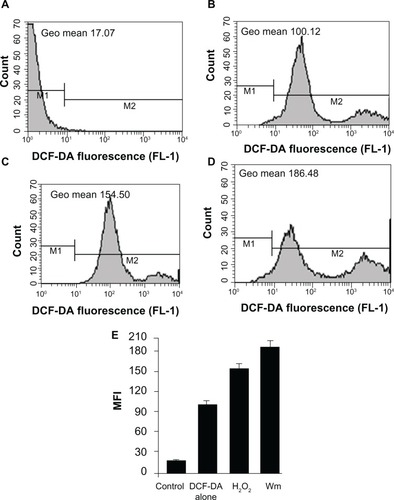
Cells treated with wortmannin were grown on 6-well plates as described in the cell culture section for measurement of intracellular ROS accumulation and quantification. After 24 hours of incubation, the cells were stained with 5 μM ROS-specific H2O2-sensitive DCF-DA dye and incubated at 37°C for 30 minutes. The cells were then trypsinized pelleted and washed three times with ice-cold PBS. Negative control cells are not treated with wortmannin. As a positive control, H2O2 (0.3%) treated cells were used.Citation28 The cells were then analyzed for fluorescence intensity using the FACSCalibur flow cytometer and CellQuest Pro software.
Statistical analysis
Experiments were done in triplicate. The data are expressed as the mean ± standard error. Significance was evaluated by one-way analysis of variance and the Student’s t-test.
Results
Effects of wortmannin on MCF-7 cell growth, toxicity, and viability
The Trypan blue exclusion assay was used to determine the amount of MCF-7 cell death. MCF-7 cells were incubated with 0–500 nM of wortmannin for 24 hours to assess their cellular growth and toxicity using the Trypan blue exclusion assay. As shown in , wortmannin induced cell death in MCF-7 cells compared with untreated controls in a concentration-dependent manner as determined by Trypan blue staining. The 50% inhibitory concentration (IC50) of wortmannin was calculated to be 400 nM over 24 hours ().
Effect of wortmannin on cellular morphology
As shown in , distinctive morphological changes were observed at higher concentrations and over a longer time period compared with controls, indicating induction of apoptosis in a dose-dependent manner. Thus, the phase-contrast micrographs of wortmannin-treated MCF-7 cells show typical apoptotic morphological characteristics.
Biochemical and morphological analysis of apoptosis
To confirm whether the reduced cell viability was due to apoptosis, acridine orange and ethidium bromide fluorescence staining was used to detect apoptotic cells. Using fluorescence microscopy, uniform normal green cells were observed in the control group (), whereas an increased number of dark orange or red cells with increased wortmannin concentrations were detected (–). Results are from three independent experiments.
Qualitative MTT assay
Reduced cell viability was qualitatively determined by MTT assay after the cells were treated with various concentrations of wortmannin, as detected by phase-contrast microscopy (see ). The morphological features of wortmannin-treated MCF-7 cells demonstrated reduced purple formazan growth. Wortmannin inhibited proliferation of MCF-7 cells in a dose-dependent manner. The results indicated that cytotoxicity of wortmannin in cancer cells was significant when compared with the control. The cytotoxic MTT assay result shows dose-dependent death of MCF-7 cells with wortmannin treatment.
Wortmannin quantitatively induces apoptosis in MCF-7 cells
To elucidate further the mechanisms of cell death in wortmannin-induced MCF-7 cells, we analyzed the quantitative flow cytometry data from FACSCaliber using Yo-PRO®-1 and propidium iodide double-staining. The bottom right quadrant (LR) defined the percentage of early apoptotic cells with Yo-PRO®-1 staining; the top right quadrant (UR) defined the percentage of late apoptotic cells (Yo-PRO®-1 and propidium iodide-stained cells). In the quadrant histogram shown in , Yo-PRO®-1-positive cells and propidium iodide-negative cells in the LR quadrant were considered to be early apoptotic cells, and Yo-PRO®-1-positive and propidium iodide-positive cells in the UR quadrant were considered to be late apoptotic cells. MCF-7 cells treated with wortmannin for a 24-hour period are shown in . The percentage of cells in the early stages of apoptosis gradually increased, ranging from 4.44% to 36.38% in a dose-dependent manner compared with the control group (0.21%). Furthermore, the total percentage of cells (early and late apoptotic) also increased from 63.82% to 74.42% in a dose-dependent manner compared with the control group (8.41%).
Nuclear chromatin condensation with Hoechst 33342
The effect of wortmannin on nuclear morphology using fluorescence microscopy is demonstrated in . We observed that the nuclei of wortmannin-treated cells showed hypercondensed chromatin strongly bound to fluorescent dyes, with a brighter appearance along with shrunken and fragmented nuclei. This allowed clear discrimination from untreated nonapoptotic MCF-7 cells with smooth, flattened nuclear morphology and normal nuclei, as well as uniformly dispersed chromatin. Thus, wortmannin-treated breast cancer MCF-7 cells demonstrated a chromatin condensation effect and confirmed that the mechanism of cell death was via apoptosis.
Microscopic apoptotic blebbing
To study the mechanisms of cell death, we investigated whether wortmannin induces programmed cell death through the apoptotic pathway. Phase-contrast microscopy was used after treatment with wortmannin for 24 hours. Morphological apoptotic features, such as cell shrinkage, membrane blebbing, rounding up of cells, and a decrease in volume were used as standard apoptosis markers, as shown in .Citation2,Citation10,Citation25
Increased intracellular ROS levels in wortmannin-treated MCF-7 cells
Next we evaluated whether wortmannin-induced apoptotic MCF-7 cells trigger signaling responses via intracellular generation of ROS. As shown in , the level of ROS generation was significantly increased compared with controls in wortmannin-treated MCF-7 cells using the ROS-sensitive 2′,7′-dichlorodihydrofluorescein diacetate probe through FACS analysis. Corresponding geometric means of total fluorescence intensity was calculated for different levels of fluorescence.Citation29–Citation31 Wortmannin treatment increases intracellular ROS accumulation in MCF-7 cell lines. This result suggests that an increase in total cellular ROS generation triggers wortmannin-induced programmed cell death in MCF-7 cells via apoptotic pathways.Citation1,Citation2,Citation28–Citation33
Discussion
Most breast cancer cells become resistant to current chemotherapeutic drugs due to mutation of apoptotic mechanisms.Citation34 It has been reported that MCF-7 cells are resistant to most of the drugs approved by the US Food and Drug Administration, including paclitaxel, doxorubicin, 5-fluorouracil, etoposide, and camptothecin, with the conclusion that the PI3k-Akt pathway plays a major role in increased resistance.Citation18 In addition, another study revealed that the Akt level was higher in MCF-7 cells than in other breast cancer cell lines, rendering these cells resistant to drug-induced apoptosis.Citation19 Several researchers have reported that an abnormal or deregulated PI3k-Akt signaling pathway plays a critical role in breast malignancies associated with multiple chemotherapeutic resistance.Citation18,Citation20–Citation22 Therefore, selectively inhibiting PI3k-Akt expression in breast cancer cells using a small molecule inhibitor, such as wortmannin, might have potential therapeutic value. Thus, our results suggest a novel biomolecular target pathway for apoptosis induction by wortmannin to improve therapeutics for MCF-7 breast cancer cells.
It was determined that wortmannin-treated MCF-7 cancer cells show significant morphological and biochemical apoptogenic characteristics, as indicated by inhibition of growth, reduction in cell viability, condensation of chromatin, DNA fragmentation, and increased percentages of both early and late apoptotic populations and membrane blebbing, and thus they appear to be undergoing apoptotic cell death.Citation2,Citation10,Citation28,Citation35 This appearance is positively correlated with drug concentration and apoptosis induction despite the absence of normal caspase 3 in the cells.Citation23,Citation24 It has been reported that wortmannin toxicity is more potent in DNA damaged cells than in nondamaged cells.Citation36,Citation37 This was confirmed by the finding of an increased amount of chromatin condensation on fluorescence microscopy and early apoptotic phase on flow cytometry-based dot plot analysis.
Wortmannin-induced cell death was confirmed to be apoptotic by FACS dot plot analysis using Yo-PRO®-1 and propidium iodide double-staining. The significant percent-age of cell death detected by flow cytometry is consistent with that detected by Trypan blue staining. In addition, morphological observation revealed typical apoptosis characteristics, including cell shrinkage, membrane blebbing, reduced cellular volume with condensed contents, and a rounded body, further demonstrating wortmannin-induced apoptosis of MCF-7 cells. Moreover, qualitative MTT assay showed reduction of cell viability and inhibition of cellular proliferation by wortmannin in a dose-dependent manner. These effects are not significant at low concentrations, but the effect was more evident and a significant amount of apoptosis induction was observed at higher concentrations. Morphological differences between normal and apoptotic cells can be observed by phase-contrast microscopy, while Trypan blue exclusion is used to evaluate cell death rates. Rapid and sensitive MTT assay qualitatively evaluates mitochondrial activity to test cell growth as well as cell death.Citation38
Several studies have reported that wortmannin has an antitumor effect in human breast cancer cell lines, and are inconsequential in the MCF-7 context, specifically from the programmed cell death perspective.Citation39–Citation43 To the best of our knowledge, there are no further reports on the details of the mechanism of action on MCF-7 cell death. Therefore, we have explored for the first time the possible mechanism of cell death induced by wortmannin in MCF-7 cells by focusing on its ability to induce programmed cell death.
Chromatin condensation is one of the prominent hallmarks of apoptosis, and is brought about by activated caspases. To study whether the cytotoxicity of wortmannin in MCF-7 cells was associated with induction of apoptosis, we used the Hoechst 33342 chromatin condensation staining method after treating cells with wortmannin for 24 hours. Wortmannin-treated cells (–) showed nuclear shrinkage, condensation of chromatin and DNA, and nuclear fragmentation, with a brighter blue-whitish fluorescent appearance compared with controls. The changes in typical apoptotic nuclear morphology, such as DNA fragmentation, nuclear chromatin hypercondensation, and nuclear shrinkage, were identified in treated cells but not in untreated control cells. Normal nuclei contained noncondensed chromatin uniformly dispersed over the entire nucleus, and these cells were recorded as nonapoptotic cells, whereas cells containing condensed chromatin were scored as apoptotic cells.
ROS has been reported to have a dual mechanistic role in living cells. At low concentrations, ROS serve as important signaling molecules and at higher concentrations they induce cell death.Citation2,Citation29,Citation44–Citation49 Several studies have reported that increased ROS generation can selectively kill cancer cells by a more rapid increase in the toxic threshold compared with normal cells.Citation44,Citation45,Citation50,Citation51 In light of this, we investigated the possible role of ROS in wortmannin-treated MCF-7 cell lines using FACSCaliber. Our results show that wortmannin induced significant accumulation of ROS in cells. Thus, our results confirm the essential role of ROS in induction of apoptosis in MCF-7 cells.
Our morphological and biochemical analysis indicates that increased intracellular ROS production also subsequently promotes apoptotic cell death.Citation49,Citation51,Citation52 These data are consistent with observations made by earlier researchers,Citation11,Citation17,Citation34,Citation53–Citation55 and establish the anticancer properties of wortmannin in MCF-7 breast cancer cells. These findings could be used to translate the anticancer effects of wortmannin into other cancer cell lines too. Thus, our study could be useful for developing anticancer therapeutics based on programmed cell death in cancer cells resistant to apoptosis induced by other common chemotherapeutic drugs.
Overall, the present study shows the cytotoxic effects of wortmannin, in particular its effects on cell growth and induced apoptotic cell death in the MCF-7 breast cancer cell line. To the best of our knowledge, this is the first report of wortmannin-induced breast cancer cell toxicity and programmed cell death via the apoptosis pathway in a MCF-7 cancer cell line in vitro. Wortmannin has apoptotic properties that suggest potential use specifically in the MCF-7 cancer cell line. However, further investigation would be necessary to elucidate in detail the molecular signaling mechanisms involved in these apoptotic cell death pathways in MCF-7 and other cancer cell lines.
Disclosure
The authors report no conflicts of interest in this work.
Figure 2 Cell death determination by Trypan blue exclusion assay.
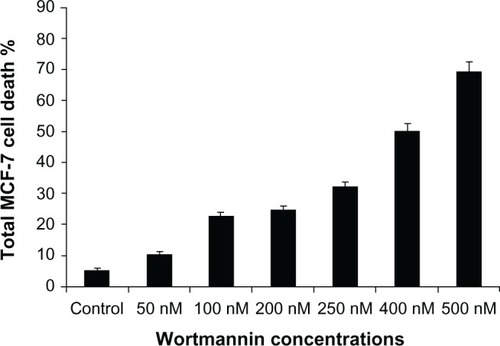
Figure 3 Phase-contrast micrographs of wortmannin treated MCF-7 cells incubated for 24 hours.
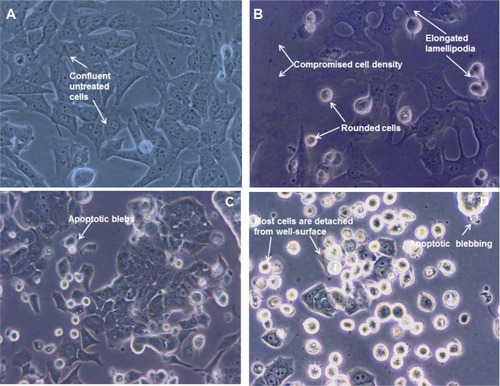
Figure 4 Fluorescence micrographs of MCF-7 cells treated with different concentrations of wortmannin and stained with acridine orange and ethidium bromide. Viable cells show green fluorescence and apoptotic cells show dark orange or red fluorescence. (A) Control cells not treated with wortmannin, and treated with wortmannin (B) 500 nM, (C) 1 μM, and (D) 5 μM for 24 hours.
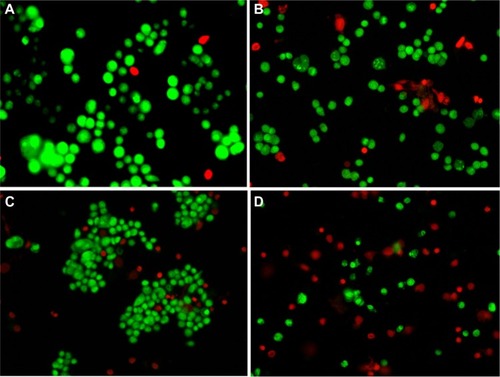
Figure 5 Representative phase-contrast micrographs of qualitative MTT assay. (A) Control cells and those treated with wortmannin (B) 500 nM, (C) 1 μM, (D) 2 μM, and (E) 4 μM, for 24 hours.
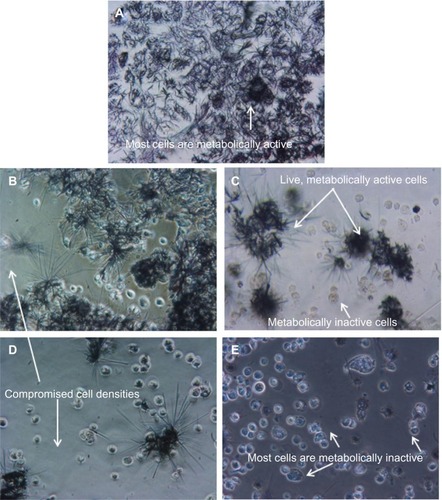
Figure 6 Flow cytometric dot plots showing status of wortmannin-induced apoptosis in MCF-7 cells. Yo-PRO®-1 and propidium iodide (Life Technologies, Carlsbad, CA) were used to stain the cells for FACS analysis. (A) Control cells (without wortmannin), and cells incubated with wortmannin at concentrations of (B) 50 nM, (C) 200 nM, (D) 500 nM, (E) 1 μM, and (F) 2 μM for 24 hours.
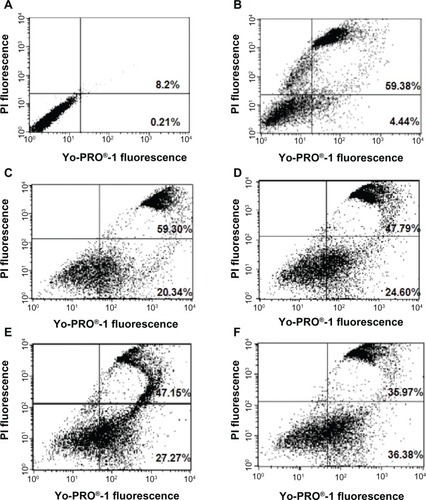
Figure 7 Wortmannin-treated MCF-7 cells demonstrating chromatin condensation effect. Representative nuclear staining of MCF-7 cells with Hoechst 33342. Changes in cellular nuclear morphology examined using fluorescence microscopy and a DAPI filter. (A) Control cells uniformly stained blue without condensed chromatin, with normal, round, and unpunctuated nucleus (blue arrow), recorded as nonapoptotic cells. (B–D) Cells with condensed and fragmented nuclei. Wortmannin-treated cells (B) show nuclear shrinkage, and chromatin and DNA condensation (white arrow), nuclear fragmentation with brighter blue-whitish (red arrow) fluorescent appearance compared with control, and were scored as apoptotic cells. (C) Percentage of chromatin condensation increases with higher concentrations of wortmannin. (D) Nuclear fragmentation in higher magnification.
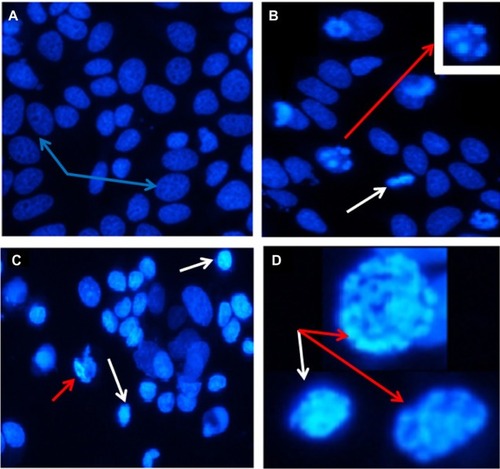
Figure 8 Phase-contrast micrographs of wortmannin-treated MCF-7 cells showing typical apoptotic morphological changes, eg, rounded, shrunken, and suspended cells, with apoptotic blebbing. (A and B) are representative of cells treated with wortmannin for 24 hours.
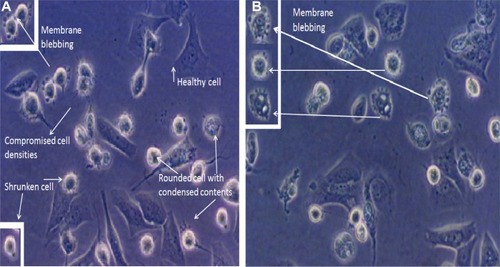
Figure 9 Intracellular accumulation of reactive oxygen species in MCF-7 cell lines. The x axis shows the fluorescent intensity of DCF-DA oxidation, and the y axis indicates cell numbers. (D) Histograms showing representative results of wortmannin-treated cells compared with untreated control cells (A and B). (C) H2O2 positive control. (E) Bar graph showing the percentage of cells from both groups (treated and not treated with wortmannin) with mean fluorescence intensity.
References
- YangHLChenCSChangWHGrowth inhibition and induction of apoptosis in MCF-7 breast cancer cells by Antrodia camphorataCancer Lett200623121522716399223
- MousaviSHTavakkol-AfshariJBrookAJafari-AnarkooliIDirect toxicity of Rose Bengal in MCF-7 cell line: role of apoptosisFood Chem Toxicol2009485585919271285
- ParkinDMBrayFFerlayJPisaniPEstimating the world cancer burden: Globocan 2000Int J Cancer20019415315611668491
- GoldsteinLJCianfroccaMVon MehrenMBreast Cancer Research. Fox Chase Cancer Center Scientific ReportPhiladelphia, PAFox Chase Cancer Center2000
- RussoRYangXHuYFBiological and molecular basis of human breast cancerFront Biosci1993944960
- HeadJJohnstonSRNew targets for therapy in breast cancer: farnesyl transferase inhibitorsBreast Cancer Res2004626226815535857
- ChopraRThe Indian sceneJ Clin Oncol20011918Suppl 1106111
- SanliogluADDiriceEAydinCErinNKoksoySSanliogluSSurface TRAIL decoy receptor-4 expression is correlated with TRAIL resistance in MCF7 breast cancer cellsBMC Cancer200555415916713
- WelmBBehbodFGoodellMARosenJMIsolation and characterization of functional mammary gland stem cellsCell Prolif200336173214521513
- HossainMZKleveMGNickel nanowires induced and reactive oxygen species mediated apoptosis in human pancreatic adenocarcinoma cellsInt J Nanomedicine201161475148521845039
- WuQChenYGuohui CuiChengYWortmannin inhibits K562 leukemic cells by regulating PI3k/Akt channel in vitroJ Huazhong Univ Sci Technolog Med Sci20092945145619662361
- PriullaMCalastrettiABrunoPPreferential chemosensitization of PTEN-mutated prostate cells by silencing the Akt kinaseProstate20076778278917373720
- MartelliAMM NyåkernMTabelliniGPhosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemiaLeukemia20062091192816642045
- TangJMHeQYGuoRXChangXJPhosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosisLung Cancer20065118119116324768
- GeorgePBaliPAnnavarapuSCombination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3Blood20051051768177615514006
- ZhuTGuJYuKPegylated wortmannin and 17-hydroxywortmannin conjugates as phosphoinositide 3-kinase inhibitors active in human tumor xenograft modelsJ Med Chem200691373137816480272
- WangXWuQZhangLWuYShuYWortmannin induced apoptosis of leukemia cells by reducing PI3K/AktThe Chinese-German Journal of Clinical Oncology20109734738
- KnuefermannCLuYLiuBHER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cellsOncogene2003223205321212761490
- AhmadSSinghNGlazerRIRole of AKT1 in 17beta-estradiol-and insulin-like growth factor I (IGF-I)-dependent proliferation and prevention of apoptosis in MCF-7 breast carcinoma cellsBiochem Pharmacol19995842543010424760
- LiangKJinWKnuefermannCTargeting the phosphatidylinositol 3-kinase/Akt pathway for enhancing breast cancer cells to radiotherapyMol Cancer Ther2003235336012700279
- ZhouYEppenberger-CastoriSEppenbergerUBenzCCThe NFkB pathway and endocrine-resistant breast cancerEndocr Relat Cancer200512S37S4616113098
- BachmanKEArganiPSamuelsYThe PIK3CA gene is mutated with high frequency in human breast cancersCancer Biol Ther2004377277515254419
- JänickeRUMCF-7 breast carcinoma cells do not express caspase-3Breast Cancer Res Treat200911721922118853248
- KurokawaHNishioKFukumotoHAlteration of caspase-3 (CPP32/Yama/apopain) in wild-type MCF-7 breast cancer cellsOncol Rep1999633379864397
- SyamsudinPSimanjuntakRDjamilHeffenWLApoptosis of human breast cancer cells induced by ethylacetate extracts of propolisAm J Biochem Biotechnol201068488
- RaviDHarish MuniyappaHDasKCEndogenous thioredoxin is required for redox cycling of anthracyclines and p53-dependent apoptosis in cancer cellsJ Biol Chem2005280400844009616159878
- YuanHLuoJWeisslederRCantleyLJosephsonLWortmannin-C20 conjugates generate WortmanninJ Med Chem20064974074716420059
- ShimHYParkJHPaikHDAcacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activationMol Cells2007249510417846503
- HuangLZhuCSunYPhospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: a redox/polyamine-dependent effectCarcinogenesis2010311982199020627873
- KusmartsevSNefedovaYYoderDGabrilovichDIAntigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen speciesJ Immunol200417298999914707072
- WangQZhengXLYangLReactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cellsJ Exp Clin Cancer Res20102915921143894
- RoyAGangulyABoseDasguptaSMitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovaniMol Pharm20087412921307
- SunLChenTWangXChenYWeiXBufalin induces reactive oxygen species dependent bax translocation and apoptosis in ASTC-a-1 cellsEvid Based Complement Alternat Med6 182009 [Epub ahead of print.]
- MerlinNJParthasarathyVSanthoshkumarTRInduction of apoptosis in human breast cancer cell line MCF-7 by phytochemicals from Gmelina asiaticaAfr J Biotechnol2010944514456
- ZhangXDGillespieSKHerseyPStaurosporine induces apoptosis of melanoma by both caspase-dependent and -independent apoptotic pathwaysMol Cancer Ther2004318714985459
- ShiYQBlattmannHCromptonNEAWortmannin selectively enhances radiation-induced apoptosis in proliferative but not quiescent cellsInt J Radiat Oncol Biol Phys20014942142511173136
- ChernikovaSBWellsRLElkindMMWortmannin sensitizes mammalian cells to radiation by inhibiting the DNA-dependent protein kinase-mediated rejoining of double-strand breaksRadiat Res19991511591669952300
- SongMMSongWJBiHCytotoxicity and cellular uptake of iron nanowiresBiomaterials2010311509151719945156
- WymannMPBulgarelli-LevaGZvelebilMJWortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reactionMol Cell Biol199616172217338657148
- SchultzRMMerrimanRLAndisSLIn vitro and in vivo antitumor activity of the phosphatidylinositol-3-kinase inhibitor, wortmanninAnticancer Res199515113511397653991
- DavolPABizunehAFrackeltonARJrWortmannin, a phosphoinositide 3-kinase inhibitor, selectively enhances cytotoxicity of receptor-directed-toxin chimeras in vitro and in vivoAnticancer Res1999191705171310470104
- PriceBDYoumellMBThe phosphatidylinositol 3-kinase inhibitor wortmannin sensitizes murine fibroblasts and human tumor cells to radiation and blocks induction of p53 following DNA damageCancer Res1996562462508542574
- LemkeLEPaine-MurrietaGDTaylorCWPowisGWortmannin inhibits the growth of mammary tumors despite the existence of a novel wortmannin-insensitive phosphatidylinositol-3-kinaseCancer Chemother Pharmacol19994449149710550570
- D’AutréauxBToledanoMBROS as signalling molecules: mechanisms that generate specificity in ROS homeostasisNat Rev Mol Cell Biol2007881382417848967
- Lopez-LazaroMDual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapyCancer Lett20072521817150302
- CircuMLAwTYReactive oxygen species, cellular redox systems, and apoptosisFree Radic Biol Med20104874976220045723
- RosatoRRAlmenaraJAGrantSThe histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1Cancer Res2003633637364512839953
- ValenciaAMoránJReactive oxygen species induce different cell death mechanisms in cultured neuronsFree Radic Biol Med2004361112112515082065
- Wallach-DayanSBIzbickiGPazitYCGerstl-GolanRFineABreuerRBleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathwayAm J Physiol Lung Cell Mol Physiol2006290790796
- SchumackerPTReactive oxygen species in cancer cells: live by the sword, die by the swordCancer Cell20061017517616959608
- FruehaufJPMeyskensFLReactive oxygen species: a breath of life or death?Clin Cancer Res20071378979417289868
- KangKSWangPYamabeNFukuiMJayTZhuBTDocosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activationPLoS One20105e1029620421971
- BoehleAKurdowRBoenickeLWortmannin inhibits growth of human non-small-cell lung cancer in vitro and in vivoLangenbecks Arch Surg200238723423912410360
- SeolJWLeeYJKangHSWortmannin elevates tumor necrosis factor-related apoptosis-inducing ligand sensitivity in LNCaP cells through down-regulation of IAP-2 proteinExp Oncol2005712012415995629
- QiongZRuofanHXiaohuaLXinliZJingweiJZhaohuiCRole of dephosphorylation of FOXO1 on apoptosis induced by wortmannin for non-Hodgkin’s lymphoma cellsMol Biol Rep2010372397240219757185