Abstract
Purpose
Tumor expression of programmed death-ligand 1 (PD-L1) is associated with evasion of immune response in several types of malignancies and such expression may render patients eligible for PD-L1 inhibitors. The use of immune checkpoint blockade therapy has been recently approved for the treatment of breast cancer. However, PD-L1 expression data are lacking among Jordanian breast cancer patients. In this study, the tumor PD-L1 expression was characterized in breast cancer patients to assess their eligibility for immune checkpoint blockade therapy. The study also aimed to explore the association between tumoral PD-L1 expression and the clinicopathologic characteristics and the prognostic factors in patients with breast cancer.
Patients and Methods
Tissue samples were available from 153 female patients with primary invasive breast cancer. Immunohistochemistry was performed on paraffin-embedded tumor sections that were stained with a PD-L1 antibody. Expression of tumor PD-L1 was correlated with demographics, clinicopathologic characteristics, and prognosis.
Results
The mean age at diagnosis was 54.2±12.8 years (median 52, interquartile range 45–65). The percentage of PD-L1-positive tumors was 26.1%. PD-L1 expression on tumor cells significantly and positively correlated with tumor size (rho=0.174, p=0.032). PD-L1 positivity was significantly associated with the grade of carcinoma (p=0.001), HER2-positivity (p=0.015), and lymphovascular invasion (p=0.036). PD-L1 intensity was significantly associated with tumor stage (p=0.046). No significant associations were observed for the PD-L1 expression status or intensity with patient menopausal status, hormone receptor expression, and molecular subtypes. PD-L1 expression significantly correlated with a worse prognosis of breast cancer patients at the time of diagnosis (rho=0.230, p=0.005).
Conclusion
Tumor PD-L1 expression was associated with advanced clinicopathologic features and worse prognosis in this cohort of Jordanian breast cancer patients. Future studies are needed to better understand the impact of PD-L1 blockade therapy on treatment outcomes in eligible breast cancer patients in Jordan.
Introduction
Cancer cells are characterized by their ability to escape the immune response through multiple tumor-mediated escape mechanisms.Citation1,Citation2 Tumors evade immune surveillance through immunoediting that allows the selection of tumor variants resistant to immune effectors as well as the establishment of an immune-suppressive status within the tumor microenvironment.Citation1,Citation2 The evasion of the immune system by the tumor is mediated by different mechanisms. The utilization of immune checkpoints to suppress the cell-mediated immune response and further establish a status of immune tolerance is currently identified to be a key immune evasion mechanism in cancer cells.Citation2 Immune checkpoints are inhibitory immunoreceptors that act mainly to maintain self-tolerance and avoid overstimulation of immune response.Citation3,Citation4 Several immune checkpoints have been identified in cancers, however, of major importance are the programmed death-1 (PD-1) and its ligand, programmed death-ligand 1 (PD-L1).Citation3,Citation4 The binding of PD-1 to PD-L1 blocks T cell function and activation.Citation2,Citation5 Under normal conditions, the activation of the PD-1/PD-L1 pathway prevents overt immune response and maintains tolerance to self-antigens.Citation6 Nevertheless, tumoral PD-L1 expression is associated with diminished immune response in the tumor microenvironment.Citation6 PD-L1 is overexpressed on tumor cells in various solid cancers including lung cancer,Citation7 renal cell carcinoma,Citation8 glioblastoma,Citation9 melanoma,Citation10 bladder cancer,Citation11 colorectal cancer,Citation12 and breast cancer.Citation13,Citation14 Although several studies have described the expression of PD-1 and PD-L1 in breast cancer patients, substantial discrepancies exist in the expression rates and the prognostic impact. Noda et al reported significantly greater PD-1 mRNA expression in tumor tissues of breast cancer patients compared to normal tissues.Citation15 Besides, mRNA expression of PD-1 in peripheral blood was higher in breast cancer patients compared to healthy individuals. In term of prognostic impact, poor overall survival (OS) was associated with reduced PD-1 mRNA expression in tumor tissue as well as increased PD-1 expression in peripheral blood of breast cancer patients.Citation15 Nevertheless, in another study, no difference was shown for the levels of PD-1-positive T cells in the peripheral blood of breast cancer patients among different disease stages or molecular subtypes compared to the healthy controls.Citation16 The rate of PD-1-positive tumor-infiltrating lymphocytes was reported in 104 out of 660 breast cancer cases (15.8%).Citation17 PD-1 positivity was associated with tumor size, grade, lymph node status, and worse OS in the luminal B and the basal-like subtypes.Citation17 Similarly, Vidula et al revealed that PD-1 expression was higher in human epidermal growth factor receptor 2 (HER2)-positive and triple-negative breast cancer (TNBC) molecular subtypes and was further associated with advanced tumor grade and pathologic complete response.Citation18 Alternatively, a recent study showed that PD-1 protein expression was associated with improved OS in a cohort of 564 patients with early breast cancer.Citation19 The expression of PD-L1 in breast tumors has been largely associated with unfavorable clinicopathologic features. In a systematic analysis of 47 studies by Huang et al, PD-L1 positivity was associated with large tumor size, high grade tumors, high Ki-67, and triple-negative subtype in patients with primary breast cancer.Citation20 Muenst et al showed that PD-L1 was expressed in 152 of the 650 breast cancer tissues investigated (23.4%).Citation21 The expression was significantly associated with the age of patients, tumor size and grade, lymph node status, estrogen receptor (ER)-negative status, and high Ki-67 expression.Citation21 However, the prognostic and/or predictive impact for the expression of PD-L1 in breast cancer lacks consensus.Citation22 PD-L1 expression was a negative prognostic factor associated with a significantly worse OS in patients with luminal B, HER2-positive, and basal-like molecular subtypes.Citation21 However, in other studies, PD-L1 expression was associated with improved therapeutic outcomes and prognosis in patients with breast cancer.Citation23–Citation26
Immune checkpoint inhibitors are a breakthrough in cancer therapy.Citation27 Immune checkpoint blockade with anti-PD-1/PD-L1 antibodies has been approved for the treatment of different types of cancers.Citation3 In 2019, the United States Food and Drug Administration (US FDA) approved atezolizumab (a PD-L1 inhibitor) in combination with protein-bound paclitaxel for the treatment of TNBC patients with an unresectable locally advanced or metastatic disease whose cells express PD-L1 as determined by an FDA-approved test.Citation28 Additionally, the PD-1 inhibitor, pembrolizumab, was recently approved for the treatment of patients with locally recurrent unresectable or metastatic TNBC in combination with chemotherapeutic agents such as paclitaxel protein-bound, paclitaxel, or gemcitabine plus carboplatin.Citation29 The introduction of these drugs to the treatment plans of breast cancer requires the evaluation of the expression of the immune checkpoints by cancer cells and the understanding of the immunogenicity of breast cancer in a specific population. Thus, the assessment of the PD-L1 expression status among Jordanian patients would be necessary to understand their eligibility for immune checkpoint inhibitors. Such expression data are lacking among Jordanian patients. In this study, we aimed to investigate the expression of PD-L1 in a cohort of Jordanian breast cancer patients. Also, to explore association between the expression of PD-L1 and the clinicopathologic characteristics and the prognostic factors among this cohort.
Materials and Methods
Patients and Tumor Samples
One hundred and fifty-three adult female patients (n=153) with a histologically confirmed diagnosis of primary invasive breast cancer were obtained from the archives of King Abdullah University Hospital (KAUH) through the period of 2014–2020. Patients who received neoadjuvant therapy were not included in this study. All patients were discussed in a dedicated tumor board before the commencement of treatment. Loco-regional control was achieved either by a modified radical mastectomy or conservation breast surgery with radiotherapy. Further chemotherapy, hormonal treatment, and/or targeted treatment were individualized according to receptor status and tumor board recommendations. The electronic database at KAUH was used to retrieve the demographic and anthropometric data of patients. According to the World Health Organization (WHO), body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters, and patients were classified as underweight, normal, overweight, and obese, based on the WHO classification system.Citation30
Relevant tumor data were obtained from pathology reports issued by the Pathology Department for eligible patients at the time of diagnosis of breast cancer. Pathological data included the size of the tumor, histopathologic type, ipsilateral axillary lymph node status, the status of lymphovascular invasion (LVI), and the expression status of receptors (ER, progesterone receptor (PR), and HER2). The tumor-node-metastasis (TNM) stage was determined according to the American Joint Committee on Cancer,Citation31 and the tumor grade was indicated based on the Nottingham Combined Histologic Grade system.Citation32 Breast tumors were classified into a low grade (grade I), intermediate grade (grade II), and high grade (grade III) carcinomas. For HER2, negative expression was indicated by an immunohistochemistry (IHC) score of 0 or +1 whereas HER2 overexpression was determined by a score of +3. Fluorescence in situ hybridization analysis was used for equivocal results (score of +2 by IHC), and gene amplification was considered positive for HER2 overexpression. Four molecular subtypes of breast cancer were determined based on the expression of receptors,Citation33 these are luminal A (ER+ and/or PR+, HER2–), luminal B (ER+ and/or PR+, HER2+), HER2-enriched (ER–, PR–, HER2+), and triple-negative (ER–, PR–, HER2–).
Prognostic Factors and Prognostic Score
A prognostic score was generated for each breast cancer patient by considering 8 prognostic factors based on the paper by Cianfrocca and Goldstein.Citation34 Patient and tumor characteristics at diagnosis were included such as the age of the patient, primary tumor size, tumor grade, lymph node status, the status of LVI, and the expression status of hormone receptors and HER2.Citation35 Each patient was given a score value for each of the prognostic factors and a final score was generated by summing the scores for all factors (). The prognostic score ranges from 0 to 10, at which higher scores imply a worse prognosis.Citation35
Table 1 Prognostic Score for Breast Cancer Patients
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumor tissues were obtained from the Pathology Department, and IHC was performed on sections that were cut at a thickness of 3 µm. For the detection of PD-L1 (an immune checkpoint), the sections were heated for 1 hr using an oven at 62°C and were let to cool down at room temperature. Afterward, the staining procedure was performed using the Ventana BenchMark ULTRA IHC/ISH fully automated staining system,Citation36 followed by the standard immunohistochemical staining procedures of the Pathology laboratory. The primary PD-L1 antibody was added per manufacturer recommendations and the incubation time was 80 minutes (Clone 22C3, Dako, USA). Positive control slides were composed of tonsil tissue and negative controls were applied by replacing the primary antibody with a buffer.
Evaluation of Immunostaining
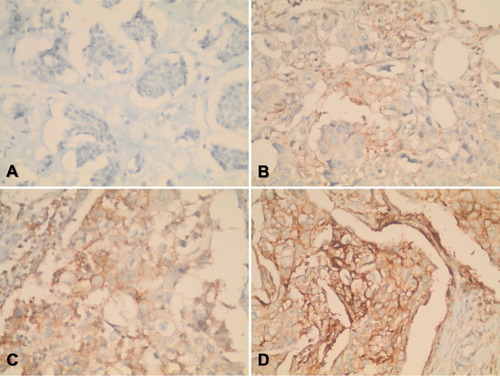
Two independent pathologists (RM and SA) evaluated immunostaining and were blind to patient demographic and pathologic data. Discrepancies were resolved by joint discussion. PD-L1 immunostaining was evaluated by scanning the whole tumor section by the pathologists who provided a percentage of PD-L1 tumor cell positivity along with the intensity of staining. PD-L1 staining intensity was scored as negative, mild, moderate, or strong staining intensity. To assess positive versus negative expression of PD-L1 in the tumor tissue, a cut-off point value of 1% was considered for this analysis based on the percentage of PD-L1 positive cells.Citation29 All evaluations were performed avoiding areas with necrosis, folded tissue, suboptimal preservation, and technical artifacts. Representative images of tumor PD-L1 staining are shown in .
Statistical Analysis
The IBM SPSS statistical package was used to perform data analysis (IBM Corp. Version 26.0. Armonk, NY, USA). Continuous variables are presented as the mean ± standard deviation (SD) or the median and interquartile range (IQR). Categorical variables were displayed as frequency and percentages (n, %). For group comparisons, the Mann–Whitney U-test and the Kruskal–Wallis analysis of variance were applied to compare two and multiple independent groups, respectively. Pearson’s chi-square test of independence was used to assess associations between categorical variables and Spearman correlation test was applied to assess correlations between continuous variables. All p-values were two-sided and were statistically significant at p<0.05.
Dichotomizing of some study variables was considered ahead of performing statistical analysis to avoid a small sample size upon further data analysis.Citation37 Hence, the tumor stage was divided into early (I/II) and advanced (III/IV) and the grade was grouped into grades (I/II) and grade (III) breast cancer. The selection of these categories for variables was based on previously published cut points in the literature.Citation35,Citation37
Results
Demographic and Tumor Characteristics of Breast Cancer Patients
The demographic and tumor characteristics of patients are shown in . The mean age at diagnosis was 54.2±12.8 years, ranging from 29 to 84 (median 52, IQR 45–65). The average BMI at diagnosis was 30.5±5.9 kg/m2, ranging from 16.8 to 46.9 (median 30.1, IQR 26.2–34) and approximately half of patients (53.4%) were obese. A positive family history of breast cancer in first-degree relatives was reported in 25.2% of patients. Sixty-four patients (48.1%) were premenopausal and 69 (51.9%) were postmenopausal.
Table 2 Demographic and Tumor Characteristics of Breast Cancer Patients
The average tumor size was 4.1±2.4 cm ranging from 1 to 20 (median 3.5, IQR 2.6–4.9). The mean number of involved lymph nodes was 5.6±7.8 ranging from 0 to 38 (median 2, IQR 0–7). Stage II disease was indicated in 43.4% of patients and 51.3% of them had grade II carcinoma. Invasive ductal carcinoma was the most commonly reported histopathologic type (73.9%) and luminal A was the most frequent molecular subtype (69.5%). Other demographic and tumor characteristics are shown in .
Expression of PD-L1 in Breast Cancer Tissues
The percentage of PD-L1-positive tumors and the staining intensity are shown in . The percentage of PD-L1-positive tumors was 26.1%. Only five patients (3.3%) had PD-L1 percentage positivity of 40% or greater. PD-L1 staining intensity was mild in 18 patients (11.8%), moderate in 19 patients (12.4%), and strong in 6 patients (3.9%) (). When the data were stratified based on the molecular subtype, the percentage of PD-L1-positive staining was 21.4% in luminal A, 37.9% in luminal B, 57.1% in HER2-enriched, and 14.3% in triple-negative tumors. The percentage of PD-L1-positive tumor cells was not significantly different among the molecular subtypes (p=0.111, Kruskal–Wallis test).
Table 3 PD-L1 Expression in Tumor Tissues of Breast Cancer Patients
The percentage of PD-L1-positive tumor cells significantly and positively correlated with primary tumor size in breast cancer patients (rho=0.174, p=0.032, ). No significant correlation was observed between PD-L1 expression and age of the patient at diagnosis, BMI, and the number of lymph nodes with detectable tumor cells ().
Table 4 Correlation of PD-L1-Positive Tumor Cells with Demographic and Tumor Characteristics of Breast Cancer Patients
Association of PD-L1 Expression with Demographic and Tumor Characteristics of Breast Cancer Patients
PD-L1 positivity was significantly associated with the grade of carcinoma (p=0.001) (). PD-L1 was more positive in grade III (60.0%) compared to grade I/II tumors (40.0%). Furthermore, PD-L1-positive expression was significantly associated with both HER2-positivity and LVI (p=0.015 and p=0.036, respectively). Additionally, PD-L1 staining intensity was significantly associated with tumor grade and stage (p<0.001 and p=0.046, respectively). In this regard, all patients who had strong PD-L1 staining intensity had grade III tumors and the majority (83.3%) had advanced-stage disease. No significant associations were observed for the PD-L1 expression status and intensity with the menopausal status of patients, the expression status of hormone receptors, and molecular subtypes ().
Table 5 Association of PD-L1 Expression Status and Intensity with Demographic and Clinicopathologic Characteristics of Breast Cancer Patients
Impact of Tumoral PD-L1 Expression on Prognosis of Breast Cancer Patients
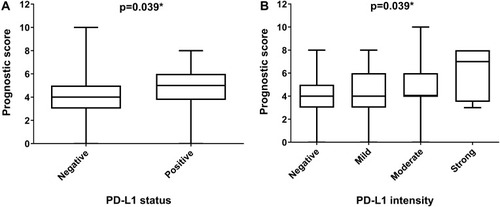
A prognostic score was generated for each patient using 8 prognostic factors. The mean prognostic score for breast cancer patients in this study was 4.1±1.8, ranging from 0 to 10 (median 4, IQR 3–5). A significant positive correlation was found between the prognostic score and the percentage of PD-L1-positive tumor cells (rho=0.230, p=0.005). The median prognostic score was significantly different based on PD-L1 expression status (p=0.039) (). Patients with PD-L1-positive status had a significantly higher median of prognostic scores compared to patients with PD-L1-negative expression. Further, Kruskal–Wallis analysis revealed a significant difference in prognostic score among the PD-L1 staining intensity groups (p=0.039, ). Patients with strong PD-L1 staining intensity had a significantly higher prognostic score median compared to patients with negative staining.
Figure 2 Prognostic scores in breast cancer patients based on PD-L1 expression.
Discussion
Breast cancer is the most common carcinoma among Jordanian females and the incidence of the disease is increasing annually.Citation38 Recently, immune checkpoint inhibitors have been approved for several types of solid cancers and their indications are constantly expanding. Thus, the understanding of the expression landscape of target immune checkpoints is essential to delineate the status of antitumor immunity and further explore the eligibility of breast cancer patients for immunotherapy in Jordan. The fact that Jordanian patients with breast cancer have some different demographic characteristics compared to Western populations adds to the significance of studying such cohort of patients. These demographic characteristics are well demonstrated in our results and include: a younger mean age at diagnosis (54.2±12.8 years), a high percentage of positive family history of breast cancer in first-degree relatives (probably a reflection of the popularity of consanguineous marriage), and a high percentage of premenopausal breast cancer cases (48.1%).
PD-L1 is the main ligand of PD-1 that is constitutively expressed in myeloid, lymphoid, and normal epithelial cells.Citation39 The binding of PD-1 to PD-L1 leads to the phosphorylation of the cytoplasmic region of PD-1 and the subsequent recruitment of phosphatases and downstream proteins such as spleen tyrosine kinase and phosphatidylinositol 3-kinase.Citation6 Collectively, the activation of the PD-1/PD-L1 pathway inhibits cytokine production and negatively regulates the immune function by inhibiting the activation and proliferation of T lymphocytes.Citation6,Citation40 In the tumor microenvironment, the expression of PD-L1 on tumor cells promotes tumor cell immune evasion by delivering inhibitory signals to maintain immune tolerance.Citation6,Citation40 The expression of PD-L1 has been reported in breast cancer but not in normal breast tissue.Citation41 Lou et al indicated that PD-L1 was expressed in samples of invasive ductal breast carcinoma but not the adjacent normal breast tissue.Citation42 In this study, we demonstrated a relatively low frequency of PD-L1 expression in Jordanian female breast cancer patients (26.1%). Other studies showed a similar rate of PD-L1 positivity in patients with primary invasive breast cancer.Citation43,Citation44 Alternatively, Ali et al showed that PD-L1 expression was detectable in 1.7% of breast tumor cells and was mostly expressed in basal-like tumors.Citation45 In another study, only 4.1% of the invasive ductal carcinomas were PD-L1-positive.Citation46 PD-L1 expression was higher in Middle Eastern and Brazilian breast cancer patients with expression rates of 32.8% and 56.6% of cases, respectively.Citation13,Citation47 PD-L1 expression rates vary widely as no standardized method for scoring is available in the meantime. Additionally, the differences in the cut-off values for PD-L1 positivity in clinical studies (1% vs 5%) and the multiple primary antibodies available for staining are potential reasons for the variability in the rates of PD-L1 expression among the different studies.Citation25,Citation48,Citation49 This discrepancy calls for the need to standardize staining and scoring methods to better understand and analyze the PD-L1 expression data among the different populations of breast cancer patients.Citation48 Other potential causes for the discrepancies in the expression of PD-L1 can be explained by the variable populations of breast cancer patients examined, their varied demographics, and clinical characteristics. summarizes findings from selected studies regarding the expression of PD-L1 in tumor tissues of female breast cancer patients.
Table 6 A Selected List for Studies Describing PD-L1 Expression in Tumor Tissues of Female Breast Cancer Patients
An increasing number of clinical studies demonstrated the association of PD-L1 tumor expression with poor prognostic features and high-risk clinicopathological parameters in breast cancer patients. In this study, PD-L1 expression on tumor cells positively correlated with tumor size. Additionally, PD-L1-positivity was associated with grade III tumors, HER2-positivity, and LVI. These findings agree with several previous studies that have reported correlations between PD-L1 and unfavorable clinicopathological features, including larger tumor size, advanced grade, positive lymph nodes, LVI, and hormone receptor negativity.Citation14,Citation20,Citation26,Citation44,Citation50 In a study by Hou et al, tumoral PD-L1 expression was reported in 17% of HER2-positive breast cancers analyzed and was positively associated with high tumor grade.Citation51 In another study, an analysis of 126 patients with HER2-positive breast cancer indicated that 17.5% of patients were PD-L1 positive.Citation23 PD-L1 expression was associated with response in patients who received neoadjuvant chemotherapy with the anti-HER2 drug trastuzumab. In agreement, Hou et al indicated that PD-L1 expression correlated with better outcomes in patients with invasive HER2-positive tumors who are treated with a combination of chemotherapy and HER2-targeted therapy.Citation24 Other studies showed that intratumoral PD-L1 expression was significantly associated with the expression of hormone receptors and Ki-67.Citation43,Citation52 In a study by Qin et al, factors that were more likely associated with high PD-L1 expression in Chinese breast cancer patients included age younger than 35 years, advanced stage, larger tumor size, and LVI.Citation44 In line with this, Parvathareddy et al showed that PD-L1 expression was associated with younger age, advanced grade, hormone receptors negativity, and triple-negative subtype in Middle Eastern breast cancer patients.Citation47
Considering the heterogeneous nature of breast cancer, PD-L1 expression may vary among the different molecular subtypes. Both HER2-enriched and triple-negative molecular subtypes are known for their high mutational burden and are considered more immunogenic than luminal tumors.Citation53 Previous studies demonstrated higher PD-L1 expression in HER2-enriched and TNBC compared to the luminal subtype.Citation26,Citation52,Citation54 In our study, 90.1% of the tumors were of the luminal subtype which could explain the low rate of PD-L1 expression in the entire sample examined. However, the rate of PD-L1 positivity was highest in HER2-enriched tumors compared to other subtypes in our study. In contrast to these findings, Tsang et al showed that the expression rate of PD-L1 was highest in the luminal A subtype (34.1%) and lowest in TNBC (8.3%) among 1091 primary invasive breast cancers, and these differences were statistically significant.Citation55 Furthermore, our results revealed a lack of association between PD-L1 expression and the molecular subtype of breast cancer. Such finding could be due to the relatively small sample size and reduced prevalence of non-luminal breast tumors in our study.
In this study, PD-L1 expression was adversely associated with prognostic features of breast cancer patients. Nevertheless, the impact of tumor PD-L1 expression on prognosis and survival of breast cancer patients generated conflicting results in the literature. While some studies described an association between PD-L1 expression and worse survival, others observed improved survival in PD-L1 positive cases. In a meta-analysis involving 2061 patients, Guo et al reported that positive PD-L1 expression was a negative predictor for breast cancer as indicated by increased mortality risk and adverse clinicopathologic features.Citation56 Moreover, PD-L1 positivity was associated with poor disease-free survival (DFS) and OS compared with PD-L1-negative expression.Citation20,Citation44 Alternatively, other studies indicated improved survival and outcomes in patients with basal-like and TNBC with positive PD-L1 expression.Citation25,Citation26 Recently, Kim et al showed that intratumoral PD-L1 expression was associated with better DFS and favorable outcomes in TNBC patients.Citation54 Besides, PD-L1 positivity was associated with longer DFS and OS compared with PD-L1-negative expression in a cohort of Chinese TNBC patients.Citation57 Other studies, however, did not demonstrate a correlation between tumor PD-L1 expression and survival of breast cancer patients.Citation49,Citation58
The PD-1/PD-L1 pathway had emerged as a promising target for cancer therapy at which high-affinity anti-PD-1 or anti-PD-L1 monoclonal antibodies can reverse the immune tolerance and restore the activation and proliferation of the T cell response.Citation5 The expression of PD-L1 on tumor cells has been used clinically to identify patients who are potential candidates for immunotherapy. In this regard, US FDA had approved atezolizumab, a PD-L1 inhibitor, in combination with chemotherapy for patients with metastatic TNBC who express PD-L1 on their tumor cells (≥1%).Citation59 Nevertheless, increasing evidence indicates that clinical benefit can be also achieved in cancer patients with negative tumor PD-L1 expression. In a meta-analysis by Shen and Zhao, data from 4174 patients with advanced or metastatic cancers from eight randomized controlled trials revealed that both PD-L1-positive and PD-L1-negative patients responded to PD-L1 inhibitors and had significantly prolonged OS compared to conventional therapy.Citation60 Similarly, a recent analysis by Liu et al demonstrated the efficacy of PD-1/PD-L1 inhibitors in both PD-L1-positive and PD-L1-negative breast cancer patients in terms of improving OS compared to controls.Citation61 Collectively, these findings brought into question the value of PD-L1 expression status as the sole determinant for patients who are eligible to PD-L1 blockade therapy.Citation60
This study has some limitations. First, the patient data was collected retrospectively. Second, the low number of breast tumors that correspond with the HER2-enriched and triple-negative tumors had hindered further analysis based on molecular subtype along with the potential to reveal associations with PD-L1 expression, if any. Third, the lack of recurrence and survival data was another limitation in this study. However, the major strengths of this study are the homogeneity of the population studied and the IHC assessment of whole tumor sections. Taking into consideration the substantial intratumoral heterogeneity in PD-L1 expression,Citation14,Citation48 we used entire tumor sections from patients’ archival blocks to examine the expression of PD-L1 in the whole tumor area to overcome the potential bias in scoring core biopsies.
Conclusion
The use of PD-1/PD-L1 checkpoint blockade therapy requires the understanding of the tumor microenvironment to identify patients who would potentially benefit from such therapy. To the best of our knowledge, this is the first study to characterize PD-L1 expression in female breast cancer patients in Jordan, which can be a model for the Arabian Middle East countries, since these countries share specific demographic characteristics. In this observational study, PD-L1 positivity correlated with unfavorable pathologic features and inferior prognosis in this sample of Jordanian breast cancer patients. Nevertheless, the expression of tumoral PD-L1 expression was not different among the molecular subtypes, in part, because of the under presentation of the HER2-enriched and basal-like tumors in this cohort. Collectively, our findings call for future studies on a larger number of breast cancer patients to better assess the value of immunotherapeutic agents in treatment regimens for Jordanian breast cancer patients and to evaluate the impact of PD-1/PD-L1 inhibitors on treatment outcomes in eligible patients.
Data Sharing Statement
The data in this study are available from the corresponding author on reasonable request.
Ethics Approval
The Institutional Review Board (IRB) committee of the Jordan University of Science and Technology (JUST) and KAUH approved the study (Research number 14/126/2019). The study was conducted in concordance with the ethical principles of the Declaration of Helsinki. A consent form was not required by the IRB committee because of the retrospective and observational nature of the study that involved the use of archival tumor tissues. Data confidentiality was strictly maintained throughout the entire study, data were anonymized, and no patient identifiers were applied.
Disclosure
The authors declare that they have no conflicts of interest.
References
- VinayDS, RyanEP, PawelecG, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185–S198.25818339
- AyoubNM, Al-ShamiKM, YaghanRJ. Immunotherapy for HER2-positive breast cancer: recent advances and combination therapeutic approaches. Breast Cancer (Dove Med Press). 2019;11:53–69.30697064
- HeX, XuC. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–669. doi:10.1038/s41422-020-0343-432467592
- PardollDM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc323922437870
- Xu-MonetteZY, ZhangM, LiJ, YoungKH. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response?Front Immunol. 2017;8:1597.29255458
- SetordziP, ChangX, LiuZ, WuY, ZuoD. The recent advances of PD-1 and PD-L1 checkpoint signaling inhibition for breast cancer immunotherapy. Eur J Pharmacol. 2021;895:173867. doi:10.1016/j.ejphar.2021.17386733460617
- YuH, BoyleTA, ZhouC, RimmDL, HirschFR. PD-L1 expression in lung cancer. J Thorac Oncol. 2016;11(7):964–975. doi:10.1016/j.jtho.2016.04.01427117833
- ChoueiriTK, FayAP, GrayKP, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol. 2014;25(11):2178–2184. doi:10.1093/annonc/mdu44525193987
- NduomEK, WeiJ, YaghiNK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18(2):195–205. doi:10.1093/neuonc/nov17226323609
- HinoR, KabashimaK, KatoY, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–1766. doi:10.1002/cncr.2489920143437
- HuangY, ZhangSD, McCruddenC, ChanKW, LinY, KwokHF. The prognostic significance of PD-L1 in bladder cancer. Oncol Rep. 2015;33(6):3075–3084. doi:10.3892/or.2015.393325963805
- LiY, LiangL, DaiW, et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55. doi:10.1186/s12943-016-0539-x27552968
- BaptistaMZ, SarianLO, DerchainSF, PintoGA, VassalloJ. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47(1):78–84. doi:10.1016/j.humpath.2015.09.00626541326
- DillEA, GruAA, AtkinsKA, et al. PD-L1 expression and intratumoral heterogeneity across breast cancer subtypes and stages: an assessment of 245 primary and 40 metastatic tumors. Am J Surg Pathol. 2017;41(3):334–342. doi:10.1097/PAS.000000000000078028195880
- NodaM, MasudaT, ItoS, et al. Circulating PD-1 mRNA in peripheral blood is a potential biomarker for predicting survival of breast cancer patients. Ann Surg Oncol. 2020;27(10):4035–4043. doi:10.1245/s10434-020-08375-z32206951
- DuH, YiZ, WangL, LiZ, NiuB, RenG. The co-expression characteristics of LAG3 and PD-1 on the T cells of patients with breast cancer reveal a new therapeutic strategy. Int Immunopharmacol. 2020;78:106113. doi:10.1016/j.intimp.2019.10611331841754
- MuenstS, SoysalSD, GaoF, ObermannEC, OertliD, GillandersWE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667–676. doi:10.1007/s10549-013-2581-323756627
- VidulaN, YauC, RugoHS. Programmed cell death 1 (PD-1) receptor and programmed death ligand 1 (PD-L1) gene expression in primary breast cancer. Breast Cancer Res Treat. 2021;187(2):387–395. doi:10.1007/s10549-021-06234-333913053
- MatikasA, ZerdesI, LovrotJ, et al. PD-1 protein and gene expression as prognostic factors in early breast cancer. ESMO Open. 2020;5(6):e001032. doi:10.1136/esmoopen-2020-00103233172959
- HuangW, RanR, ShaoB, LiH. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019;178(1):17–33. doi:10.1007/s10549-019-05371-031359214
- MuenstS, SchaerliAR, GaoF, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146(1):15–24. doi:10.1007/s10549-014-2988-524842267
- MatikasA, ZerdesI, LovrotJ, et al. Prognostic implications of PD-L1 expression in breast cancer: systematic review and meta-analysis of immunohistochemistry and pooled analysis of transcriptomic data. Clin Cancer Res. 2019;25(18):5717–5726. doi:10.1158/1078-0432.CCR-19-113131227501
- KurozumiS, InoueK, MatsumotoH, et al. Clinicopathological values of PD-L1 expression in HER2-positive breast cancer. Sci Rep. 2019;9(1):16662. doi:10.1038/s41598-019-52944-631723167
- HouY, NittaH, WeiL, et al. PD-L1 expression and CD8-positive T cells are associated with favorable survival in HER2-positive invasive breast cancer. Breast J. 2018;24(6):911–919. doi:10.1111/tbj.1311230230111
- BarrettMT, LenkiewiczE, MalasiS, et al. The association of genomic lesions and PD-1/PD-L1 expression in resected triple-negative breast cancers. Breast Cancer Res. 2018;20(1):71. doi:10.1186/s13058-018-1004-029996881
- SabatierR, FinettiP, MamessierE, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015;6(7):5449–5464. doi:10.18632/oncotarget.321625669979
- AzourySC, StraughanDM, ShuklaV. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr Cancer Drug Targets. 2015;15(6):452–462. doi:10.2174/15680096150615080514512026282545
- NarayanP, WahbyS, GaoJJ, et al. FDA approval summary: atezolizumab plus paclitaxel protein-bound for the treatment of patients with advanced or metastatic TNBC whose tumors express PD-L1. Clin Cancer Res. 2020;26(10):2284–2289. doi:10.1158/1078-0432.CCR-19-354532001481
- FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. Available from:https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple. Accessed July5, 2021.
- WHO Consultation on Obesity (1999: Geneva, Switzerland) & World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. World Health Organization; 2000. Available from:https://apps.who.int/iris/handle/10665/42330. Accessed October30, 2021.
- EdgeSB, ComptonDR, FritzCC, GreeneAG, TrottiFA. AJCC Cancer Staging Handbook from the AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010.
- RakhaEA, Reis-FilhoJS, BaehnerF, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12(4):207. doi:10.1186/bcr260720804570
- OnitiloAA, EngelJM, GreenleeRT, MukeshBN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13. doi:10.3121/cmr.2008.82519574486
- CianfroccaM, GoldsteinLJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9(6):606–616. doi:10.1634/theoncologist.9-6-60615561805
- AyoubNM, YaghanRJ, AbdoNM, MatalkaII, Akhu-ZaheyaLM, Al-MohtasebAH. Impact of obesity on clinicopathologic characteristics and disease prognosis in pre- and postmenopausal breast cancer patients: a retrospective institutional study. J Obes. 2019;2019:3820759. doi:10.1155/2019/382075931019808
- AlthammerS, TanTH, SpitzmullerA, et al. Automated image analysis of NSCLC biopsies to predict response to anti-PD-L1 therapy. J Immunother Cancer. 2019;7(1):121. doi:10.1186/s40425-019-0589-x31060602
- StarkA, StahlMS, KirchnerHL, KrumS, PrichardJ, EvansJ. Body mass index at the time of diagnosis and the risk of advanced stages and poorly differentiated cancers of the breast: findings from a case-series study. Int J Obes (Lond). 2010;34(9):1381–1386. doi:10.1038/ijo.2010.6920351736
- Abdel-RazeqH, MansourA, JaddanD. Breast cancer care in Jordan. JCO Glob Oncol. 2020;6:260–268. doi:10.1200/JGO.19.0027932083950
- KythreotouA, SiddiqueA, MauriFA, BowerM, PinatoDJ. Pd-L1. J Clin Pathol. 2018;71(3):189–194. doi:10.1136/jclinpath-2017-20485329097600
- JiaL, ZhangQ, ZhangR. PD-1/PD-L1 pathway blockade works as an effective and practical therapy for cancer immunotherapy. Cancer Biol Med. 2018;15(2):116–123. doi:10.20892/j.issn.2095-3941.2017.008629951336
- LiS, ChenL, JiangJ. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer: a systematic review and meta-analysis. Medicine. 2019;98(16):e15201. doi:10.1097/MD.000000000001520131008945
- LouJ, ZhouY, HuangJ, QianX. Relationship between PD-L1 expression and clinical characteristics in patients with breast invasive ductal carcinoma. Open Med (Wars). 2017;12:288–292. doi:10.1515/med-2017-004228894845
- EvangelouZ, Papoudou-BaiA, KarpathiouG, et al. PD-L1 expression and tumor-infiltrating lymphocytes in breast cancer: clinicopathological analysis in women younger than 40 years old. In Vivo. 2020;34(2):639–647. doi:10.21873/invivo.1181832111764
- QinT, ZengYD, QinG, et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6(32):33972–33981. doi:10.18632/oncotarget.558326378017
- AliHR, GlontSE, BlowsFM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26(7):1488–1493. doi:10.1093/annonc/mdv19225897014
- KassardjianA, ShintakuPI, MoatamedNA. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), in female breast carcinomas. PLoS One. 2018;13(4):e0195958. doi:10.1371/journal.pone.019595829672601
- ParvathareddySK, SirajAK, AhmedSO, et al. PD-L1 protein expression in middle eastern breast cancer predicts favorable outcome in triple-negative breast cancer. Cells. 2021;10:2. doi:10.3390/cells10020229
- MansonQF, Ter HoeveND, BuergerH, MoelansCB, van DiestPJ. PD-1 and PD-L1 expression in male breast cancer in comparison with female breast cancer. Target Oncol. 2018;13(6):769–777. doi:10.1007/s11523-018-0610-130519815
- MoriH, KuboM, YamaguchiR, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8(9):15584–15592. doi:10.18632/oncotarget.1469828107186
- TawfikO, KimlerBF, KarnikT, ShehataP. Clinicopathological correlation of PD-L1 expression in primary and metastatic breast cancer and infiltrating immune cells. Hum Pathol. 2018;80:170–178. doi:10.1016/j.humpath.2018.06.00829936058
- HouY, NittaH, WeiL, BanksPM, ParwaniAV, LiZ. Evaluation of immune reaction and PD-L1 expression using multiplex immunohistochemistry in HER2-positive breast cancer: the association with response to anti-HER2 neoadjuvant therapy. Clin Breast Cancer. 2018;18(2):e237–e244. doi:10.1016/j.clbc.2017.11.00129198959
- ZhouT, XuD, TangB, et al. Expression of programmed death ligand-1 and programmed death-1 in samples of invasive ductal carcinoma of the breast and its correlation with prognosis. Anticancer Drugs. 2018;29(9):904–910. doi:10.1097/CAD.000000000000068330085937
- CriscitielloC, CuriglianoG. Immunotherapy of Breast Cancer. Prog Tumor Res. 2015;42:30–43.26377084
- KimHS, DoSI, KimDH, AppleS. Clinicopathological and prognostic significance of programmed death Ligand 1 expression in Korean patients with triple-negative breast carcinoma. Anticancer Res. 2020;40(3):1487–1494. doi:10.21873/anticanres.1409332132048
- TsangJY, AuWL, LoKY, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat. 2017;162(1):19–30. doi:10.1007/s10549-016-4095-228058578
- GuoY, YuP, LiuZ, et al. Prognostic and clinicopathological value of programmed death Ligand-1 in breast cancer: a meta-analysis. PLoS One. 2016;11(5):e0156323. doi:10.1371/journal.pone.015632327227453
- AiErkenN, ShiHJ, ZhouY, et al. High PD-L1 expression is closely associated with tumor-infiltrating lymphocytes and leads to good clinical outcomes in Chinese triple negative breast cancer patients. Int J Biol Sci. 2017;13(9):1172–1179. doi:10.7150/ijbs.2086829104508
- BrockhoffG, SeitzS, WeberF, et al. The presence of PD-1 positive tumor infiltrating lymphocytes in triple negative breast cancers is associated with a favorable outcome of disease. Oncotarget. 2018;9(5):6201–6212. doi:10.18632/oncotarget.2371729464065
- SoareGR, SoareCA. Immunotherapy for breast cancer: first FDA approved regimen. Discoveries (Craiova). 2019;7(1):e91. doi:10.15190/d.2019.432309609
- ShenX, ZhaoB. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. doi:10.1136/bmj.k352930201790
- LiuX, GuoCY, TouFF, et al. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: a systematic review and meta-analysis. Int J Cancer. 2020;147(1):116–127. doi:10.1002/ijc.3274431633798