Abstract
Breast cancer is a major health issue in developed countries. Overexpression of HER2, a member of epidermal growth factor receptor family, occurs in 20%–30% of breast cancers. HER2 drives the cancer cells to develop a more aggressive phenotype, to metastasize to viscera and central nervous system, and to be less sensitive to chemotherapeutic agents. Trastuzumab (Herceptin®) is a monoclonal antibody directed against the extracellular domain of HER2. As single agent or with chemotherapy, trastuzumab improves survival of HER2-positive breast cancers. In the past years, trastuzumab has completely revolutionized the scenario of the treatment of HER2-positive breast cancer, representing one of the most remarkable examples of targeted therapy in oncology. However, issues such as the best chemotherapeutic companion to associate with trastuzumab, cardiac toxicities, and clinical resistance still require tremendous efforts by researchers. Here, we review pharmacology, efficacy studies, and toxicities of trastuzumab in metastatic breast cancer. Moreover, we provide some insights on resistance to therapy. Finally, we briefly discuss trastuzumab’s place in the clinical setting.
Introduction to HER2-positive metastatic breast cancer management
Breast cancer is a major public health issue in developed countries in terms of morbidity, mortality, and costs.Citation1 Integrating multiple strategies such as early diagnosis, surgery, radiotherapy, and chemotherapy has resulted in decreased mortality in previous years.Citation2 A part of the merit should be given to a better understanding of mechanisms underlying breast cancer development. This, in turn, has resulted in the identification of druggable molecular targets in cancer cells.
Epidermal growth factor receptor 2 (ErbB2/HER2) is a ligandless tyrosine kinase receptor, member of the epidermal growth factor receptor (EGFR) family and is overexpressed in 20%–30% of breast cancers.Citation34 Its overexpression distinguishes a subgroup of breast cancers characterized by increased aggressiveness, mortality, and high sensitivity to anthracyclines.Citation5 HER2 overexpression is primarily associated with amplification of the HER2/neu.Citation6
Its activation follows dimerization with other tyrosine kinase receptors belonging to the EGFR family (EGFR, HER3, or HER2 itself) or to other families, such as insulin-like growth factor receptor 1 (IGF-1R).Citation7,Citation8 Dimerization activates downstream signaling cascades, including mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways, which promotes cellular proliferation, survival, migration, invasion, and differentiation.Citation8
Several features render HER2 an optimal target for breast cancer treatment:Citation9
HER2 overexpression strongly correlates with tumor progression.
HER2-overexpressing breast cancer cells become almost totally dependent for their survival on signaling network cascades triggered by HER2.
The level of expression of HER2 in normal adult tissue is much lower than in cancer cells that overexpress the protein.
For these reasons, research has focused on developing HER2 inhibitors as potential anticancer agents. The first of such agents registered for clinical use was trastuzumab (Herceptin®).
HER2 status assessment
Clinical studies have shown that women who most benefit from trastuzumab have high levels of HER2 expression.Citation10 Aspects regarding the best way to assess HER2 status have been largely discussed, and clinical implications have been outlined in recent guidelines.Citation11
Currently, HER2 status is assessed by immunohistochemistry (IHC), fluorescent in situ hybridization (FISH), and chromogenic in situ hybridization (CISH).
IHC identifies HER2 overexpression on the cell membrane. Results are usually expressed using a semiquantitative scoring system ranging from 0+ (no expression) to 3+ (high expression). Tumors that show no (0+) or low levels (1+) of expression are considered HER2-negative; vice-versa tumors that show high levels (3+) of expression should be considered as HER2-positive. This method is economically advantageous and readily available, but suffers from low sensitivity and high interobserver variability.Citation12
FISH detects HER2 gene amplification and is more specific and sensitive than IHC.Citation6,Citation13 FISH offers quantitative results on the number of HER2 gene copies/centromere. Another FDA-approved method to assess HER2 gene amplification is CISH. CISH is very similar to FISH but utilizes conventional peroxidase or alkaline phosphatase reactions visualized under a standard bright-field microscope. Both gene amplification detected by FISH or CISH and protein expression by IHC are commonly used as initial test to assess HER2 status. Equivocal cases, defined as either IHC 2+ or FISH/CISH ratio of 1.8–2.2 or average HER2 gene copy number four to six signals/nucleus for test systems without an internal control probe, undergo further testing with the alternative method.
A recent report by the ASCO/CAP demonstrated that, after a rigorous standardization, concordance between HER2 3+ and gene amplification detection is about 98%–98.5%.Citation14 Phase II and III trials in metastatic disease showed that trastuzumab has relevant clinical activity against HER2-positive metastatic breast cancer. In the next paragraphs, we will summarize pharmacological issues, clinical activity, toxicities, and some biology on resistance to trastuzumab.
Review of mode of action, pharmacology, and pharmacokinetics of trastuzumab in breast cancer
Mechanism of action
Trastuzumab is a humanized IgG1k monoclonal antibody that selectively binds to the extracellular domain (ECD) of the human ErbB2 protein HER2.Citation4,Citation15 In vivo, the most relevant mechanism of action is antibody-dependent cellular cytotoxicity (ADCC). In brief, natural killer (NK) cells are able to bind trastuzumab on HER2-positive cancer cells through an Fc receptor. Upon binding, NK cells are able to induce cancer cell death by releasing lytic enzymes.Citation16 Trastuzumab triggers antibody-dependent cell-mediated cytotoxicity (ADCC) principally by activating Fcγ receptor on NK cells.Citation16,Citation17 Unfortunately, clinical trials failed to show clinical benefit derived from association of trastuzumab with immune-modulating agents, such as IL-2, despite NK cell expansion with enhanced in vitro targeted killing of HER2-expressing cells.Citation18
Musolino et alCitation19 studied a population of 54 patients with HER2-amplified breast cancer who have received taxanes plus trastuzumab for metastatic disease and evaluated genotypes for the FcγRIIIa-158 valine(V)/phenylalanine(F), FcγRIIa-131 histidine(H)/arginine(R), and FcγRIIb-232 isoleucine(I)/threonine(T) polymorphisms. Interestingly, the authors showed that the FcγRIIIa-158 V/V genotype, alone and in combination with the FcγRIIa-131 H/H genotype, was significantly associated with better response rate and progression-free survival (PFS) to trastuzumab compared with other FcγR genotypes. This study supports the hypothesis that FcγR polymorphisms play a role in trastuzumab-mediated ADCC and predict response to trastuzumab.
At ASCO 2009, Tamura et alCitation20 presented preliminary results of a similar study on a population of 19 operable and 36 metastatic patients with HER2-overexpressing breast cancer treated with trastuzumab-containing chemotherapy, showing that FcγRIIa-131 H/H genotype was significantly correlated with pCR (P = 0.0034) and OR (P = 0.037), whereas FcyRIIIa-158V/V genotype had a tendency to be correlated with pCR (P = 0.067) and was significantly correlated with OR (P = 0.037).
Similarly to trastuzumab, it was shown that Fcγ receptor polymorphisms play a role also in differential response to other antibodies, such as cetuximab in colorectal cancer.Citation21,Citation22
Furthermore, impaired T cell and NK function can possibly contribute to trastuzumab resistance.
Several studies showed that a reduced number or impaired function of NK cells is correlated with shortened response to trastuzumab,Citation23,Citation24 and often, this is due to surgical or chemo-and radiotherapyCitation25
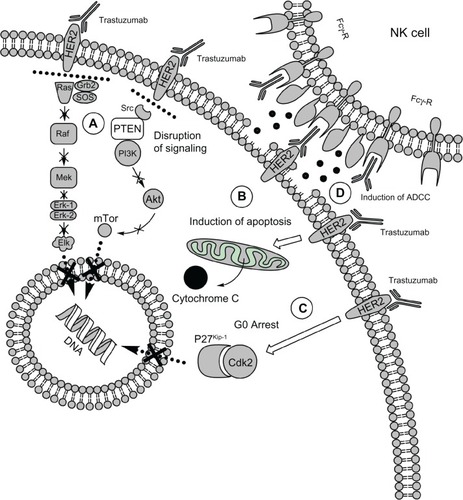
In vitro, trastuzumab induces the following perturbations in cancer cells: HER2 receptor downregulation and degradation and subsequent attenuation of downstream signaling;Citation26 G0 arrest;Citation27 and induction of apoptosis.Citation28 The mechanisms of action of trastuzumab are summarized in .
Figure 1 Mechanisms of action of trastuzumab. A) In vitro, trastuzumab is able to disrupt signaling through PI3K/Akt and MAPK signaling pathways; causes a disruption of the binding of Src to HER2, allowing PTEN to inhibit Akt B); induces apoptosis of target cells and C) cell cycle arrest in G0-G1 phase, via modulating the cyclin-dependent kinase (CDK) inhibitor 27 Kip1. D) In vivo, trastuzumab binds the Fcγ receptor on NK cells and triggers the antibody-dependent cell-mediated cytotoxicity (ADCC).
Pharmacology
The pharmacokinetics of trastuzumab has been studied in patients with metastatic breast cancer and subsequently in early-stage breast cancer patients in addition to adjuvant chemotherapy. Short-duration intravenous infusions of 10, 50, 100, 250, and 500 mg of trastuzumab once weekly demonstrated dose-dependent pharmacokinetics. The half-life averages 1.1 (using a one-compartment model at 10 mg) and 23 (using a two-compartment model at 500 mg) days at the 10- and 500-mg dose levels, respectively. At the highest weekly dose studied (500 mg), mean peak serum concentration was 377 μg/mL.Citation29
In clinical trials, where a loading dose of 4 mg/kg trastuzumab followed by a subsequent weekly dose of 2 mg/kg was used the mean clearance was 0.225 L/day Between weeks 16 and 32, trastuzumab serum concentrations reached a steady state with a mean through and peak concentrations of approximately 79 μg/mL and 123 μg/mL, respectivelyCitation30
Population pharmacokinetics analysis of data from the initial phase I, II, and III studies suggested a half-life of 28.5 days, which justifies an every 3-week schedule: this long half-life is similar to that of endogenous IgG1 immunoglobulins (23 days).Citation31 Moreover, it was shown that trastuzumab administered every three weeks has pharmacokinetics similar to the weekly regimen;Citation32 the washout period is up to 20 weeks (95% confidence interval, 18–24 weeks); steady state pharmacokinetics should be reached by approximately 20 weeks (95% confidence interval, 18–24 weeks).
Trastuzumab’s volume of distribution is approximately that of serum volume (44 mL/kg).Citation29
The distribution of trastuzumab does not seem to be altered by age or serum creatinine (up to 2.0 mg/dL), although formal interaction studies have not been performed.Citation33
Monoclonal antibodies can face added difficulties due to their high molecular weights, target specificity, kinetics of metabolism and internalization, as well as the patterns of antigen expression in combination with target-binding affinity. The high molecular weight (145.5 KDa) and binding affinity of trastuzumab, in combination with microenvironmental factors, may limit its distribution and efficacy.Citation34,Citation35 Tumor vascular abnormalities contribute to inefficient drug penetration by creating regions of irregular or intermittent blood flow, slowed interstitial fluid velocity, and often elevated interstitial fluid pressure.Citation36,Citation37 Notwithstanding considerable heterogeneity in trastuzumab distribution, the ability of trastuzumab to penetrate through tissues seems to be relatively efficient.Citation35
Trastuzumab and blood–brain barrier
Because of its high molecular weight, trastuzumab is not able to cross an intact blood–brain barrier (BBB). However, the patients with brain metastases (BM) are commonly treated as indicated with at least one of the following treatment modalities: whole brain radiotherapy (WBRT), stereotactic radiotherapy, and metastasectomy.
These treatments for BM could disrupt the BBB and subsequently make it possible to deliver trastuzumab into the central nervous system (CNS).Citation38 In a pilot study, Stemmler et alCitation39 have evaluated the ability of trastuzumab to penetrate the BBB measuring trastuzumab levels in the serum and in cerebrospinal fluid of HER2-positive breast cancer patients with BM. The authors demonstrated that, at different time points, trastuzumab levels are increased in cerebrospinal fluid when BBB is impaired (eg, in the presence of meningeal carcinomatosis or radiotherapy).
Efficacy studies in metastatic breast cancer
Trastuzumab as single agent or in combination with chemotherapy
Phase II trials of single-agent trastuzumab showed overall response rate of 11.6%–15% in women with heavily pretreated HER2-positive metastatic breast cancerCitation30,Citation40 and of 35% in the first-line settingCitation33 and, very interestingly, median duration of response was similar to the one that can be obtained with the association with chemotherapy. The first-line study also showed that dose escalation does not increase overall response rate.
These studies led to the pivotal clinical trial, H0648g, which started in 1995 and enrolled 469 patients with previously untreated, HER2-positive metastatic breast cancer. Patients were randomly assigned to receive chemotherapy (either doxorubicin or epirubicin combined with cyclophosphamide, in patients without previous exposure to adjuvant anthracyclines, or paclitaxel in patients who had previously received adjuvant anthracyclines) with or without trastuzumab. The primary end point of this study was median time to disease progression, which was 4.6 months in patients who received chemotherapy alone and 7.4 months for those who received chemotherapy plus trastuzumab (P < 0.001); trastuzumab was also associated with an increase in objective response rate (50% versus 32%; P < 0.001), longer duration of response (median 9.1 versus 6.1 months; P < 0.001), and longer median survival (25.1 versus 20.3 months; P = 0.046).Citation10 A subsequent randomized phase II trial of docetaxel with trastuzumab or docetaxel alone published in 2005 also showed an improved overall response rate (61% versus 34%; P = 0.0002), better overall survival (median 31.2 versus 22.7 months; P = 0.0325), and longer time to disease progression (median 11.7 versus 6.1 months; P = 0.0001) for the association arm.Citation41 Various non-randomized phase II trials have shown the efficacy and safety of trastuzumab in combination with most other chemotherapies (also including liposomal anthracyclines) for treatment of breast cancer. Currently, it is unclear whether any specific chemotherapeutic drug or class can be particularly effective in combination with trastuzumab ().Citation42–Citation58
Table 1 Results of the main studies of trastuzumab with chemotherapy in metastatic breast cancer
Second-line treatment
Continuation versus discontinuation of trastuzumab after disease progression is controversial among oncologists. Retrospective studies have had conflicting results.Citation59,Citation60 The only available randomized phase III trial which apparently supports continuation of trastuzumab in association with capecitabine in patients who have progressed while receiving trastuzumabCitation61 prematurely closed accrual and, most importantly, was biased by significant unbalances in treatment arms.
Lapatinib is an orally bioavailable, small-molecule dual HER1/HER2 tyrosine kinase inhibitor. In patients whose disease has progressed after prior treatment with an anthracycline, a taxane, and trastuzumab, a randomized, controlled phase III study supports the use of the lapatinib in combination with capecitabine.Citation62 The median time to progression for patients who received capecitabine plus lapatinib was 8.4 months compared with 4.4 months in women who received capecitabine monotherapy (HR 0.49; 95% CI 0.34–0.71; P < 0.001), and there was a possibility of improved overall response (22% versus 14%; P = 0.09). Phase II data also show modest activity of lapatinib either alone or in combination with capecitabine for the treatment of brain metastases in patients with HER2-positive breast cancer.Citation63 Trastuzumab and lapatinib without chemotherapy is another treatment option in women with HER2-positive breast cancer who have disease progression while receiving trastuzumab. In a randomized phase III study, 296 patients with metastatic disease who had progression on trastuzumab treatment were randomly assigned to receive lapatinib plus trastuzumab or lapatinib alone. Patients were heavily pretreated and had received a median of six prior anticancer regimens and a median of three prior lines of trastuzumab. In the combination group, compared with lapatinib monotherapy, the median progression free survival (PFS) was 12 weeks versus 8.1 weeks and the overall clinical benefit rate was 24.7% versus 12.4% (P = 0.01).Citation64
Trastuzumab and endocrine therapy
About 40%–50% of HER2-positive tumors coexpress hormone receptors (HR). Coexpression of HER2 in HR-positive tumors is associated with reduced efficacy of endocrine therapy.Citation65,Citation66 One of the most credited mechanism that has been proposed to explain endocrine resistance is cross-talk between the estrogen receptor (ER) and HER family pathways.Citation67,Citation68 Hyperactive HER2 or EGFR can activate the ER directly, in the absence of its natural ligand. This explains not only resistance to selective estrogen receptor downmodulators, but also to aromatase inhibitors. Combined blocking of both pathways has been tested in two randomized clinical trials in patients with previously untreated HER2-/HR-positive advanced breast cancer.Citation69–Citation71
The trastuzumab in dual HER2-positive, ER-positive metastatic breast cancer (TAnDEM) trial randomly assigned women with HER2-positive and hormone-receptor-positive untreated metastatic breast cancer to anastrozole with or without trastuzumab. This study found a significant advantage for both response rate (6.8% versus 20.3%; P = 0.018) and PFS (2.4 versus 4.8 months; P = 0.0016) with the addition of trastuzumab.Citation69
A similar study of the same population of patients randomly assigned women to receive letrozole plus lapatinib or letrozole alone and showed increased PFS (8.2 months versus 3 months; HR 0.71; P = 0.019) and higher overall response rate (28% versus 15%; P = 0.021).Citation70 In conclusion, in postmenopausal women with hormone-receptor positive, HER2-positive, metastatic breast cancer with low disease burden, the combination of anti-HER2-targeted therapy with an aromatase inhibitor is a reasonable option.
Review of the mechanisms of trastuzumab resistance in breast cancer
The outstanding results obtained with the introduction of trastuzumab in the clinical management of HER2-overexpressing breast cancer are limited by primary and acquired resistance to the antibody. Currently, there is no widely accepted definition of resistance. In the clinical setting, resistance is defined as progressive disease.Citation72
Clinical practice shows that initial response to trastuzumab is almost invariably followed by tumor progression.Citation59,Citation73 Moreover, a large percentage of women fail to respond to trastuzumab, showing primary resistance.Citation10,Citation74
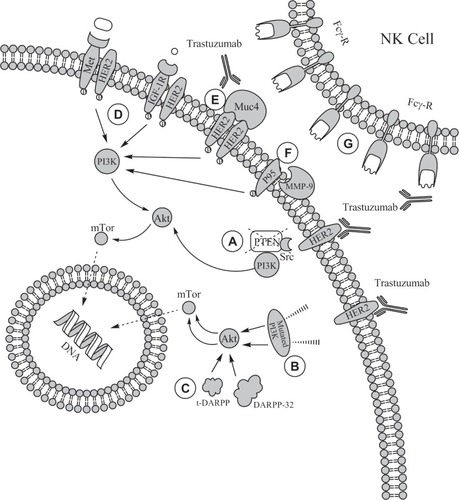
At present, neither the mechanism of action nor the mechanisms of resistance to trastuzumab are completely understood. Nevertheless, several hypotheses have been proposed ().
Figure 2 Mechanisms of resistance to trastuzumab. A) Loss of PTEN protein can impair efficacy of trastuzumab; B) mutation of PI3KCA gene sequence C) Overexpression of DARPP-32 and its truncated form t-DARPP, leading to increased phosphorylation of Akt; D) HER2 can dimerize with other tyrosine kinase receptors, such as Met or IGF-1R, activating alternative signaling pathways; E) binding of HER2 with trastuzumab can be prevented by other cellular surface proteins, such as Muc4, F) or by the shedding of the extracellular domain of HER2 mediated by metalloproteinases; G) Fcγ-receptor polymorphisms can impair antibody-dependent cell-mediated cytotoxicity (ADCC) in vivo.
HER2 downstream signaling adaptability
HER2 plays a key role as positive regulator of a high complexity signaling network, consisting of distinct semiautonomous functional units that show strong internal connections, high adaptability, and very complex regulation.
This complexity and, at the same time, the flexibility and modularity of the signaling transduction pathway explain why the network can maintain proper function in the face of efficient inhibition of individual component, such as HER2.Citation75
An increasing body of evidence sustains the central role of adaptive modulation of PI3K/Akt/mTor signaling pathway in resistance to anti-HER2-targeted agents.
Sergina et alCitation76 have provided evidence that a chronic exposure to HER2 inhibitors may result in enhanced positive feedback mechanism controlled by Akt, whereby Akt inhibition leads to HER3 redistribution to the cell membrane and to sustained HER3 phosphorylation, resulting in cellular adaptation with the potential of reducing the efficacy of these drugs.
Nagata et alCitation77 suggested an important role of decreased expression of the PTEN (phosphatase and tensin homolog) protein in development of resistance to trastuzumab. In preclinical models, they demonstrated that trastuzumab causes a disruption of the binding of Src to HER2, allowing PTEN to inhibit Akt therefore inducing growth arrest. When PTEN levels are low, however, Akt remains active and trastuzumab efficacy is impaired. A correlation between PTEN loss and resistance to trastuzumab was also shown in a retrospective analysis on HER2 positive BC patients.
Moreover, mutations in PI3KCA gene sequence can impair the ability of PTEN to inhibit Akt, also in presence of normal levels of PTEN, therefore contributing to trastuzumab resistance.Citation78
More recently, Belkhiri et alCitation79 described a possible mechanism of resistance involving both DARPP-32, a neuronally characterized protein that is centrally involved in dopamine-induced signaling pathways in the brain and is best known as a potent inhibitor of phosphatase I in neurosignaling and its truncated form, known as t-DARPP. Overexpression of both DARPP32 and t-DARPP led to increased phosphorylation of Akt and increased BCL2 protein levels in trastuzumab-resistant cell linesCitation79 and in vivo.Citation80 Moreover, t-DARPP contributes to trastuzumab resistance by blocking the antibody’s effect on HER2 and maintaining its high levels in these cells via HSP90-mediated stabilization.Citation79
Furthermore, a role in developing resistance to trastuzumab is probably played by dysregulation of mechanisms controlling survival and apoptosis.Citation81 Recently, it has been shown that upregulation of survivin leads to the development of resistance to lapatinib, a dual inhibitor of EGFR and HER2.Citation82,Citation83
Moreover, although downregulation of survivin seems to be essential for trastuzumab-mediated induction of apoptosis,Citation84 a failure in its downregulation could contribute to resistance.Citation85
Activation of alternative signaling pathways
HER2 is the preferred dimerization partner of the other ErbB family receptors and acts as an ‘amplifier’ for the signal transduction coming outside the cell.Citation75 However, the activation of downstream signaling components can also occur via the formation of complexes that do not contain HER2, such as EGFR homodimers or EGFR/HER3 heterodimers.Citation5 Signaling through these alternate complexes might provide a mechanism for bypassing the requirement for HER2-mediated signaling and thereby circumvent HER2-targeted inhibitors.Citation86 Moreover, overexpression of EGF family ligands, such as TGF-α, EGF, heregulin, betacellulin, and others, can contribute to trastuzumab resistance.Citation87–Citation90
IGF-1R plays a role in resistance. It was shown that cells overexpressing both HER2 and IGF-1R are insensitive to trastuzumab inhibition.Citation91 Nahta et alCitation92 demonstrated that IGF-1R can heterodimerize with HER2 and can induce its phosphorylation in trastuzumab-resistant cells, resulting in activation of a downstream cascade that involves phosphorylation of PI3K/Akt.
More recently, it was also shown that other tyrosine kinase receptors such as Met receptor (hepatocite growth factor receptor) can interact with HER2 and IGF-1R and can contribute to resistance through the activation of the PI3K/Akt signaling cascade. Met receptor is overexpressed with HER2 in a subset of aggressive breast cancersCitation93 and trastuzumab treatment induces upregulation of Met.Citation94 Moreover, Shattuck et alCitation94 have provided evidence that in trastuzumab-resistant cell lines, Met receptor activation protects cells against trastuzumab inhibition; conversely, loss of Met function, either through RNA-interference-mediated depletion or small-molecule-mediated inhibition, restores sensitivity to the antibody.
The nuclear factor NF-kB, which is a downstream mediator of growth signaling, has been shown to be frequently overexpressed and deregulated in HER2-positive breast cancers.Citation95,Citation96 It has been shown by Cardoso et al that the proteasome inhibitor bortezomib, which specifically inhibits the activity of NF-kB, is able to synergize with trastuzumab in HER2-overexpressing breast cancer cell lines.Citation97
Inaccessibility of epitope to trastuzumab
Trastuzumab exerts its effects by binding an epitope on the juxtamembrane extracellular domain (ECD) of HER2.Citation98 Steric interference by extracellular molecules can prevent the binding of the antibody to its target and can therefore contribute to resistance.
Overexpression of MUC4, a membrane-associated sialomucin, was associated by Nagy et alCitation99 with resistance to trastuzumab in a HER2-overexpressing cancer cell line (JIMT-1) established from a patient showing resistance to the antibody. The authors observed that the expression of MUC4 was higher in the resistant clone (JIMT-1) than in trastuzumab-sensitive lines, and its level was inversely correlated with the trastuzumab binding capacity of single cells. Knockdown of MUC4 expression by RNA interference increased the binding of trastuzumab.
Furthermore, overexpression of hyaluronan receptor CD44 and MUC1 (a membrane mucin) truncated forms were shown to have a similar role in trastuzumab-resistant cancer cells.Citation100,Citation101
On the basis of the observation that trastuzumab binds a HER2 epitope which is different from that employed by IHC methods, Bussolati et alCitation102 retrospectively tested a biotinylated trastuzumab (BiotHER) in HER2-amplified (FISH positive) breast cancer specimens. Positivity to BiotHER seems to predict more accurately than IHC and FISH the efficacy of trastuzumab-based therapy. These results indicate that lack of accessibility of the epitope to trastuzumab may limit the activity of this antibody in vivo, although further validation is needed.Citation103
The association between trastuzumab and HER2 can also be prevented by shedding the ECD of the receptor and producing a truncated form of protein named p95-ErbB2. This truncated form of protein has constitutive kinase activity, but cannot bind trastuzumab; furthermore, its overexpression contributes to resistance.Citation98,Citation104
The major responsible factors for the proteolytic cleavage of HER2 are metalloproteinases,Citation105 and inhibition of their proteolytic activity can circumvent trastuzumab resistance.Citation106
Moreover, monitoring serum HER2 levels is a fascinating and relatively cheap strategy to predict response to trastuzumab treatment. Although several studies have shown a potential utility of the approach,Citation107–Citation109 others have failed to demonstrate a clear correlation between serum ECD and response to trastuzumab.Citation110
Safety and tolerability
Although the focus of our critical review is metastatic breast cancer, we will discuss also the available data on cardiac toxicity derived from adjuvant trials.
Cardiac toxicity in metastatic breast cancer
Cardiac toxicity was an unexpected finding during the clinical development of trastuzumab; therefore, early clinical trials in the metastatic setting did not perform baseline or prospective cardiac monitoring, and did not exclude patients with underlying cardiac disease. The first signal of trastuzumab-associated cardiotoxicity was seen in the pivotal phase III trial by Slamon et al, which randomized 469 patients with HER2-overexpressing breast cancer to standard chemotherapy (either an anthracycline-containing regimen or paclitaxel) alone or standard chemotherapy plus concurrent weekly trastuzumab.Citation10 This led to the formation of an independent Cardiac Review and Evaluation Committee (CREC) to retrospectively review the cardiac toxicity data from seven phase II and III studies in 1219 patients who received trastuzumab as monotherapy or in combination with chemotherapy.Citation111 The CREC defined cardiac dysfunction as cardiomyopathy characterized by a decrease in cardiac left-ventricular ejection fraction (LVEF) that was either global or more severe in the septum, by symptoms or associated signs of congestive heart failure (CHF), or a by decline in LVEF of ≥5% (with symptoms) or 10% (no symptoms) with an absolute LVEF of ≤55%.
In the phase III trial, data from the CREC analysis demonstrated an unacceptable rate of cardiac toxicity in the group receiving concurrent anthracycline and trastuzumab.Citation10 Cardiotoxicity (both symptomatic and asymptomatic) was reported in 27% of patients who received trastuzumab plus adriamycin and cyclophosphamide (AC) compared with 8% who received AC without trastuzumab. In contrast, 13% of patients in the paclitaxel plus trastuzumab arm developed cardiotoxicity, while only 1% of patients receiving paclitaxel alone were affected. Among patients with cardiac toxicity, the incidence of New York Heart Association (NYHA) class III or IV CHF was highest among patients who had received an anthracycline-based chemotherapy with trastuzumab (16%) when compared to patients who received an anthracycline-based regimen without trastuzumab (3%), paclitaxel-based chemotherapy with trastuzumab (2%), and paclitaxel only (1%). Furthermore, the recovery of patients who developed cardiac dysfunction differed between the treatment groups. Of the 34 patients who developed cardiac dysfunction while receiving AC plus trastuzumab, 7 had persistent NYHA class III/IV symptoms after treatment for CHF, compared with none of the 10 patients in the paclitaxel and trastuzumab arm.Citation40 Recently, an interesting randomized phase II trial in HER2-positive metastatic breast cancer demonstrated that the association of the less-cardiotoxic anthracycline epirubicin at a low dose (60 mg/sqm) with trastuzumab is able to induce a significant amount of durable responses (57%) and low cardiac toxicity (only 1.7% of dose-limiting cardiac toxicity).Citation112 Moreover, most of the trials associating liposomal anthracyclines with trastuzumab in metastatic disease revealed low incidence of symptomatic cardiac events.Citation50,Citation53–Citation57
The CREC also reviewed a number of trastuzumab monotherapy trials and found a 3%–7% range of cardiac dysfunction. Patients enrolled in these trials were largely unselected and many of the patients who developed cardiac dysfunction had underlying cardiac disease or had received a cumulative anthracycline dose >400 mg/m.Citation2,Citation33,Citation40,Citation113 Guarneri et al evaluated the cardiac safety of long-term trastuzumab therapy in patients with advanced disease at a single institution. Cardiac events in this study were defined as an asymptomatic decrease of LVEF < 50%, an absolute 20% LVEF drop from baseline, or signs or symptoms of heart failure. Of 173 evaluable patients who received ≥1 year of trastuzumab-based therapy (median length of treatment was 21.3 months), 49 patients (28%) experienced a cardiac event, 19 of whom (10.9%) suffered grade 3 cardiac toxicity. All but 3 of these patients had improvement in LVEF and/or symptoms with the discontinuation of trastuzumab and activation of appropriate medical therapy.Citation114
Cardiac toxicity in the adjuvant setting
The studies with trastuzumab in the adjuvant setting were designed considering, among other factors, the modest cardiotoxicity of this compound. The cardiac exclusion criteria were very strict in some trials and took into account the fact that no information of the long-term cardiac safety of trastuzumab was available at the time of study planning. In all studies, the baseline LVEF measured either by ultrasonography or multiple-gated acquisition scan (MUGA) had to be above the institutional lower normal limit, which is usually 50%–55%. Furthermore, LVEF was monitored at regular intervals during treatment (every 3 months in most of the trials) in asymptomatic patients. Symptomatic cardiac toxicity occurred at acceptably low rates in all trials. It was slightly more frequent in trials where trastuzumab was administered concomitantly with taxanes, after exposure to anthracyclines, and less frequent in trials adopting the ‘sequential’ strategy. A relevant finding is that, in the two North American trialsCitation115 considering patients with symptomatic cardiotoxicity, those unable to start trastuzumab because of LVEF drop after AC and those developing asymptomatic cardiac toxicity, a total of 20% of patients did not receive the planned treatment with trastuzumab. This is much higher than what registered in the HERA trial,Citation116 where only 5.2% of the patients discontinued trastuzumab because of cardiac toxicity.
The cardiac safety data of the BCIRG 006 study confirmed that the omission of anthracyclines resulted in reduced cardiac toxicity in the TCH arm.Citation117
Cardiac safety results in the FinHER suggested that a short-term treatment with trastuzumab before anthracycline exposure might minimize the incidence of symptomatic CHF, which occurred in 0.9% and 1.7% of patients receiving chemotherapy alone or with trastuzumab, respectively.Citation118
The cardiac safety findings of the randomized trials have several practical implications that merit being addressed. The cumulative incidence of cardiac events increased gradually during the scheduled trastuzumab treatment period.Citation119–Citation121 However, it remained approximately constant during follow-up after the completion of treatment. Therefore, although regular cardiac monitoring, (every 3 months), by LVEF assessment is generally advised in patients on treatment, its role during patient follow-up needs to be defined.
Another important issue to be addressed is whether the apparently low rates of cardiac events, either symptomatic or asymptomatic, are reproducible in the clinical practice. For example, some reports in patients receiving sequential trastuzumab, including our own, describe an incidence of trastuzumab discontinuation because of either overt cardiotoxicity or of asymptomatic LVEF drops outside clinical trials that is higher than that reported in the HERA trial.Citation122,Citation123 Although clinical cardiotoxicity occurs at a rate that is clinically acceptable, as many as 12%–18% of the patients may need trastuzumab discontinuation even if the sequential strategy is employed. The application of algorithms of trastuzumab discontinuation or prosecution in asymptomatic patients with LFEV dropCitation124 and the establishment of trastuzumab-based regimens that minimize the risk of cardiac toxicity are two possible areas of intervention. One example is the TCH regimen used in the BCIRG 006 study, which several healthcare systems have approved for use in patients with contraindications to anthracyclines.
Despite having been reported as reversible by trastuzumab discontinuation and prompt administration of cardiac medications, trastuzumab-related cardiac toxicity may not recover in a significant proportion of patients.Citation125 Obviously, this calls for the involvement of the cardiologist in the management of patients who are candidates to trastuzumab to evaluate their cardiac risk profile. At the same time, markers that could help predict patients more likely to develop reversible or irreversible cardiotoxicity are eagerly awaited. Recently, for example, an elevation of troponin I during trastuzumab has been shown to correlate with irreversible trastuzumab-related cardiac toxicity.Citation126
The early use of beta-blockers and angiotensin-converting enzyme (ACE) inhibitors to prevent trastuzumab-related cardiotoxicity is another promising approach.Citation127 These drugs can act on myocardial remodeling and have a well-established role in patients with trastuzumab-related cardiac toxicity. Preliminary results of a prospective trial have been recently reported by Munoz et al at the 2010 ASCO meeting.Citation128 The use of beta-blockers and ACE inhibitors concomitantly with trastuzumab was associated with a smaller decrease in mean LVEF (4.7 versus 10.3 percentage points P < 0.001) when compared to untreated patients.
Interestingly, smaller studies in the neoadjuvant setting have shown that the concurrent administration of conventional anthracyclines is associated with a low incidence of cardiac events.Citation129,Citation130 At the present time, it is impossible to ascertain whether these low rates of cardiac toxicity are due to the particular clinical setting or just due to accurate selection of patients. Therefore, outside clinical trials, the concomitant administration of trastuzumab and anthracyclines should be avoided.
Conclusions, place in therapy
Trastuzumab has improved survival of HER2-positive advanced breast cancer patients. On the basis of phase II and III trials, trastuzumab in association with chemotherapy is the standard treatment for HER2-overexpressing metastatic breast cancers. However, the optimal chemotherapeutic companion for trastuzumab is not defined. Probably, the best evidence-based combination is with a taxane (docetaxel or paclitaxel).
Although it is difficult to define an optimal strategy for second-line treatment of HER2-positive breast cancers, the most reasonable approach is the all-oral combination capecitabine-lapatinib. Continuation of trastuzumab beyond disease progression is sustained only by a few retrospective trialsCitation60,Citation131–Citation134 and the weak and biased randomized phase III trial by von Minckwitz et al,Citation61 which compared capecitabine with capecitabine and trastuzumab after failure of a trastuzumab-containing frontline treatment. The only concern about trastuzumab treatment in metastatic disease is cardiac toxicity. However, clinical trials clearly indicate that, if the antibody is not used in association with anthracyclines, the rate of cardiac events is low and most of them are reversible. Moreover, not only associations with taxanes, vinorelbine, gemcitabine, capecitabine are safe and active but also with liposomal anthracyclines. However, in the absence of randomized phase III trials, the last category of drugs cannot be currently recommended in association with trastuzumab.
Future perspectives: novel drugs under investigation
At the moment, a number of novel anti-HER2-targeted agents are under evaluation. Because a comprehensive discussion of all these agents is beyond the scope of the review, in the following section, only the most promising drugs will be briefly considered.
Neratinib (HKI-272), an orally administered small molecule that acts as an irreversible inhibitor of the tyrosine kinase domain of EGFR, HER2, and HER4 (pan-HER inhibitor),Citation135 showed impressive results in a multinational, multicenter, open-label, phase II trial conducted by Burstein et al, on locally advanced or metastatic breast cancer patients.Citation136 Patients enrolled were included in two cohorts according to whether or not they had been previously exposed to trastuzumab. Patients in the latter cohort had to have progressed after at least 6 weeks of trastuzumab given in the metastatic or locally advanced setting, or during or after adjuvant trastuzumab. Treatment with neratinib yielded a response rate of 26% in trastuzumab-treated patients and 55% in trastuzumab-naive patients. About 59% and 78% of trastuzumab exposed and unexposed patients, respectively, were alive and free from disease progression at 16 weeks from study entry (primary end point). Due to these impressive activity data, neratinib is now being actively investigated in combination with cytostatic agents such as paclitaxel, vinorelbine, and capecitabine.Citation137–Citation139
Pertuzumab is a fully humanized monoclonal antibody based on the human IgG1(κ) framework sequences, directed against the ECD of HER2.Citation140 Pertuzumab differs from trastuzumab in the epitope binding regions of the light chain (12 amino acid differences).Citation141 Upon epitope binding, pertuzumab neutralizes the ability of HER2 to dimerize with other HER2 molecules or with other members of the EGFR family.
As single agent, pertuzumab showed disappointing activity in a phase II study.Citation142 Baselga et al conducted a phase II study with trastuzumab and pertuzumab in HER2-positive advanced breast cancer.Citation143 Patients were eligible if they had received ≤3 chemotherapy regimens and had developed progression during trastuzumab-based therapy. In 66 enrolled patients, the authors reported a response rate, clinical benefit rate (CBR), and median PFS of 24.4%, 50%, and 5.5 months, respectively.
Trastuzumab-DM1 is a conjugated antibody that uses trastuzumab to deliver the maytansinoid agent DM1 to HER2-positive cells.Citation144 Once internalized, DM1 is released and binds to tubulin, thereby disrupting microtubule assembly/disassembly dynamics and inhibiting cell division and proliferation of cancer cells that overexpress HER2.Citation144 In a phase I study in trastuzumab-refractory, HER2-positive advanced breast cancer patients, this agent showed a favorable profile of toxicity at the dose of 3.6 mg/kg administered intravenously every 3 weeks.Citation145 The authors also reported a response rate and CBR of 21% and 73%, respectively, which make this agent a promising therapeutic opportunity.
Similarly, a phase Ib/II study associating trastuzumab-DM1 and pertuzumab in patients with trastuzumab resistant, HER2-positive advanced breast cancer was presented at the 2010 ASCO meeting.Citation146 The study included a dose-escalation phase followed by an expansion phase consisting of a formal phase II study in 60 patients. The expansion phase showed that, in the subset of patients with relapsed stage IV disease who could be evaluated for tumor response, this combination yielded a 35.7% ORR, which is an encouraging achievement. More recently, at ESMO 2010 a randomized phase III trial showed that trastuzumab DM1 has similar activity and a significantly lower toxicity in comparison with the association docetaxel and trastuzumab as frontline treatment of HER2 positive metastatic breast cancers.Citation147
In conclusion, the treatment of HER2-positive metastatic breast cancer is in continuous evolution, and many different targeted therapies are in advanced stages of development. The future challenge for oncologists will be to optimally integrate all the available treatments to improve the outcome of HER2-positive metastatic breast cancers.
Notes
Financial and competing interests disclosure
All the authors of this article do not have relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- BroekxSHondEDTorfsRThe costs of breast cancer prior to and following diagnosisEur J Health Econ Epub2010320
- KatanodaKYako-SuketomoHComparison of time trends in breast cancer mortality (1990–2006) in the world, from the WHO mortality database [abstract]Jpn J Clin Oncol20104018220118169
- SlamonDJClarkGMWongSGLevinWJUllrichAMcGuireWLHuman breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogeneScience19872351771823798106
- SlamonDJGodolphinWJonesLAStudies of the HER-2/neu proto-oncogene in human breast and ovarian cancerScience19892447077122470152
- HarariDYardenYMolecular mechanisms underlying ErbB2/HER2 action in breast cancerOncogene2000196102611411156523
- OwensMHortenBDa SilvaMHER2 Amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissuesClin Breast Cancer20045636915140287
- BenderLMNahtaRHer2 cross talk and therapeutic resistance in breast cancerFront Biosci2008133906391218508484
- YardenYSliwkowskiMXUntangling the ErbB signalling networkNat Rev Mol Cell Biol2001212713711252954
- ValabregaGMontemurroFAgliettaMTrastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancerAnn Oncol20071897798417229773
- SlamonDJLeyland-JonesBShakSUse of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2N Engl J Med200134478379211248153
- SauterGLeeJBartlettJMSlamonDJPressMFGuidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerationsJ Clin Oncol2009271323133319204209
- GancbergDJarvinenTdiLAEvaluation of HER-2/NEU protein expression in breast cancer by immunohistochemistry: an interlaboratory study assessing the reproducibility of HER-2/NEU testingBreast Cancer Res Treat20027411312012186371
- PressMFSauterGBernsteinLDiagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trialsClin Cancer Res2005116598660716166438
- MiddletonLPPriceKMPuigPImplementation of American Society of Clinical Oncology/College of American Pathologists HER2 Guideline Recommendations in a tertiary care facility increases HER2 immunohistochemistry and fluorescence in situ hybridization concordance and decreases the number of inconclusive casesArch Pathol Lab Med200913377578019415952
- CoussensLYang-FengTLLiaoYCTyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogeneScience1985230113211392999974
- ClynesRATowersTLPrestaLGRavetchJVInhibitory Fc receptors modulate in vivo cytoxicity against tumor targetsNat Med2000644344610742152
- GennariRMenardSFagnoniFPilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2Clin Cancer Res2004105650565515355889
- RepkaTChioreanEGGayJTrastuzumab and interleukin-2 in HER2-positive metastatic breast cancer: a pilot studyClin Cancer Res200392440244612855616
- MusolinoANaldiNBortesiBImmunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancerJ Clin Oncol2008261789179618347005
- TamuraKShimizuCKoizumiFCorrelation of Fc{gamma} R IIa-H131R and IIIa-V158F polymorphisms and clinical outcome of trastuzumab in both neoadjuvant and metastatic setting in patients with HER-2 positive breast cancer [abstract]J Clin Oncol (Meeting Abstracts)2009271100
- BibeauFLopez-CrapezEDiFFImpact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecanJ Clin Oncol2009271122112919164213
- Lopez-AlbaiteroALeeSCMorganSRole of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cellsCancer Immunol Immunother2009581853186419319529
- BeanoASignorinoEEvangelistaACorrelation between NK function and response to trastuzumab in metastatic breast cancer patientsJ Transl Med200862518485193
- VarchettaSGibelliNOlivieroBElements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2Cancer Res200767119911199918089830
- MozaffariFLindemalmCChoudhuryANK-cell and T-cell functions in patients with breast cancer: effects of surgery and adjuvant chemo- and radiotherapyBr J Cancer20079710511117551492
- LewisGDFigariIFendlyBDifferential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodiesCancer Immunol Immunother1993372552638102322
- LeXFClaretFXLammayotAThe role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibitionJ Biol Chem2003278234412345012700233
- HudziakRMLewisGDWingetMFendlyBMShepardHMUllrichAp185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factorMol Cell Biol19899116511722566907
- BrunoRWashingtonCBLuJFLiebermanGBankenLKleinPPopulation pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancerCancer Chemother Pharmacol20055636136915868146
- BaselgaJTripathyDMendelsohnJPhase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancerJ Clin Oncol1996147377448622019
- BaselgaJCarbonellXCastaneda-SotoNJPhase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly scheduleJ Clin Oncol2005232162217115800309
- GhahramaniPBartonCLeyland-JonesBPharmacokinetics of herceptin administered three-weekly compared to weekly: a simulation based on data from the clinical studies [abstract]The Breast200312S40
- VogelCLCobleighMATripathyDEfficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancerJ Clin Oncol20022071972611821453
- HolligerPHudsonPJEngineered antibody fragments and the rise of single domainsNat Biotechnol2005231126113616151406
- BakerJHLindquistKEHuxhamLAKyleAHSyJTMinchintonAIDirect visualization of heterogeneous extravascular distribution of trastuzumab in human epidermal growth factor receptor type 2 overexpressing xenograftsClin Cancer Res2008142171217918381959
- JainRKBaxterLTMechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressureCancer Res198848702270323191477
- JainRKPhysiological barriers to delivery of monoclonal antibodies and other macromolecules in tumorsCancer Res199050814s819s2404582
- ParkIHRoJLeeKSNamBHKwonYShinKHTrastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancerAnn Oncol200920566218664558
- StemmlerHJSchmittMWillemsABernhardHHarbeckNHeinemannVRatio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrierAnticancer Drugs200718232817159499
- CobleighMAVogelCLTripathyDMultinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic diseaseJ Clin Oncol1999172639264810561337
- MartyMCognettiFMaraninchiDRandomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study groupJ Clin Oncol2005234265427415911866
- PegramMDSlamonDJCombination therapy with trastuzumab (Herceptin) and cisplatin for chemoresistant metastatic breast cancer: evidence for receptor-enhanced chemosensitivitySemin Oncol199926899510482199
- EstevaFJValeroVBooserDPhase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancerJ Clin Oncol2002201800180811919237
- JahanzebMMortimerJEYunusFPhase II trial of weekly vinorelbine and trastuzumab as first-line therapy in patients with HER2(+) metastatic breast cancerOncologist2002741041712401903
- BursteinHJHarrisLNMarcomPKTrastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithmJ Clin Oncol2003212889289512885806
- SledgeGW JrGemcitabine, paclitaxel, and trastuzumab in metastatic breast cancerOncology (Williston Park)200317333514768403
- BianchiGAlbanellJEiermannWPilot trial of trastuzumab starting with or after the doxorubicin component of a doxorubicin plus paclitaxel regimen for women with HER2-positive advanced breast cancerClin Cancer Res200395944595114676119
- PegramMDPienkowskiTNorthfeltDWResults of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancerJ Natl Cancer Inst20049675976915150304
- O’ShaughnessyJAVukeljaSMarslandTKimmelGRatnamSPippenJEPhase II study of trastuzumab plus gemcitabine in chemotherapy-pretreated patients with metastatic breast cancerClin Breast Cancer2004514214715245619
- ChiaSClemonsMMartinLAPegylated liposomal doxorubicin and trastuzumab in HER-2 overexpressing metastatic breast cancer: a multicenter phase II trialJ Clin Oncol2006242773277816682726
- RobertNLeyland-JonesBAsmarLRandomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancerJ Clin Oncol2006242786279216782917
- SchallerGFuchsIGonschTPhase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanesJ Clin Oncol2007253246325017577021
- AndreopoulouEGaiottiDKimEFeasibility and cardiac safety of pegylated liposomal doxorubicin plus trastuzumab in heavily pretreated patients with recurrent HER2-overexpressing metastatic breast cancerClin Breast Cancer2007769069617919349
- CortesJDiCSClimentMANonpegylated liposomal doxorubicin (TLC-D99), paclitaxel, and trastuzumab in HER-2-overexpressing breast cancer: a multicenter phase I/II studyClin Cancer Res20091530731419118059
- ChristodoulouCKostopoulosIKalofonosHPTrastuzumab combined with pegylated liposomal doxorubicin in patients with metastatic breast cancer. Phase II Study of the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluationOncology20097627528519262067
- StickelerEKlarMWatermannDPegylated liposomal doxorubicin and trastuzumab as 1st and 2nd line therapy in her2/neu positive metastatic breast cancer: a multicenter phase II trialBreast Cancer Res Treat200911759159819156515
- VenturiniMBighinCPuglisiFA multicentre Phase II study of non-pegylated liposomal doxorubicin in combination with trastuzumab and docetaxel as first-line therapy in metastatic breast cancerBreast201019533333820185313
- MontemurroFChoaGFaggiuoloRSafety and activity of docetaxel and trastuzumab in HER2 overexpressing metastatic breast cancer: a pilot phase II studyAm J Clin Oncol200326959712576933
- MontemurroFRedanaSVialeGRetrospective evaluation of clinical outcomes in patients with HER2-positive advanced breast cancer progressing on trastuzumab-based therapy in the pre-lapatinib eraClin Breast Cancer2008843644218952558
- GelmonKAMackeyJVermaSUse of trastuzumab beyond disease progression: observations from a retrospective review of case historiesClin Breast Cancer20045525815140285
- vonMGduBASchmidtMTrastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 studyJ Clin Oncol2009271999200619289619
- GeyerCEForsterJLindquistDLapatinib plus capecitabine for HER2-positive advanced breast cancerN Engl J Med20063552733274317192538
- LinNUDierasVPaulDMulticenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancerClin Cancer Res2009151452145919228746
- BlackwellKLBursteinHJStornioloAMRandomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancerJ Clin Oncol2010281124113020124187
- DowsettMAllredCKnoxJRelationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trialJ Clin Oncol2008261059106518227529
- RasmussenBBReganMMLykkesfeldtAEAdjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1–98 randomised trialLancet Oncol20089232818083065
- DowsettMHarper-WynneCBoeddinghausIHER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancerCancer Res2001618452845811731427
- ArpinoGWeissHLeeAVEstrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistanceJ Natl Cancer Inst2005971254126116145046
- KaufmanBMackeyJRClemensMRTrastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM studyJ Clin Oncol2009275529553719786670
- JohnstonSPippenJ JrPivotXLapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancerJ Clin Oncol2009275538554619786658
- SchwartzbergLSFrancoSXFloranceAO’RourkeLMaltzmanJJohnstonSLapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor-positive metastatic breast cancerOncologist20101512212920156908
- SpectorNLBlackwellKLUnderstanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancerJ Clin Oncol2009275838584719884552
- StemmlerHJKahlertSSiekieraWUntchMHeinrichBHeinemannVProlonged survival of patients receiving trastuzumab beyond disease progression for HER2 overexpressing metastatic breast cancer (MBC)Onkologie20052858258616249644
- NahtaREstevaFJTrastuzumab: triumphs and tribulationsOncogene2007263637364317530017
- CitriAYardenYEGF-ERBB signalling: towards the systems levelNat Rev Mol Cell Biol2006750551616829981
- SerginaNVRauschMWangDEscape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3Nature200744543744117206155
- NagataYLanKHZhouXPTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patientsCancer Cell2004611712715324695
- BernsKHorlingsHMHennessyBTA functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancerCancer Cell20071239540217936563
- BelkhiriADarAAPengDFExpression of t-DARPP mediates trastuzumab resistance in breast cancer cellsClin Cancer Res2008144564457118579663
- HamelSBouchardAFerrarioCBoth t-Darpp and DARPP-32 can cause resistance to trastuzumab in breast cancer cells and are frequently expressed in primary breast cancersBreast Cancer Res Treat2010120475719301121
- ChenFLXiaWSpectorNLAcquired resistance to small molecule ErbB2 tyrosine kinase inhibitorsClin Cancer Res2008146730673418980964
- XiaWBacusSHegdePA model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancerProc Natl Acad Sci U S A20061037795780016682622
- XiaWBisiJStrumJRegulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancersCancer Res2006661640164716452223
- AsanumaHTorigoeTKamiguchiKSurvivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cellsCancer Res200565110181102516322251
- OzbayTDurdenDLLiuTO’ReganRMNahtaRIn vitro evaluation of pan-PI3-kinase inhibitor SF1126 in trastuzumab-sensitive and trastuzumab-resistant HER2-over-expressing breast cancer cellsCancer Chemother Pharmacol20106569770619636556
- NahtaRYuDHungMCHortobagyiGNEstevaFJMechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancerNat Clin Pract Oncol2006326928016683005
- MotoyamaABHynesNELaneHAThe efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptidesCancer Res2002623151315812036928
- DiermeierSHorvathGKnuechel-ClarkeRHofstaedterFSzollosiJBrockhoffGEpidermal growth factor receptor coexpression modulates susceptibility to Herceptin in HER2/neu overexpressing breast cancer cells via specific erbB-receptor interaction and activationExp Cell Res200530460461915748904
- ValabregaGMontemurroFSarottoITGFalpha expression impairs Trastuzumab-induced HER2 downregulationOncogene2005243002301015735715
- RitterCAPerez-TorresMRinehartCHuman breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor networkClin Cancer Res2007134909491917699871
- LuYZiXZhaoYMascarenhasDPollakMInsulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin)J Natl Cancer Inst2001931852185711752009
- NahtaRYuanLXZhangBKobayashiREstevaFJInsulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cellsCancer Res200565111181112816322262
- LindemannKResauJNahrigJDifferential expression of c-Met, its ligand HGF/SF and HER2/neu in DCIS and adjacent normal breast tissueHistopathology200751546217593080
- ShattuckDLMillerJKCarrawayKL IIISweeneyCMet receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cellsCancer Res2008681471147718316611
- NakshatriHBhat-NakshatriPMartinDAGouletRJ JrSledgeGW JrConstitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growthMol Cell Biol199717362936399199297
- NakshatriHGouletRJ JrNF-kappaB and breast cancerCurr Probl Cancer20022628230912429950
- CardosoFDurbecqVLaesJFBortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER-2-positive breast cancer cells in a synergistic mannerMol Cancer Ther200653042305117148762
- MolinaMACodony-ServatJAlbanellJRojoFArribasJBaselgaJTrastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cellsCancer Res2001614744474911406546
- NagyPFriedlanderETannerMDecreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell lineCancer Res20056547348215695389
- FesslerSPWotkowiczMTMahantaSKBamdadCMUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cellsBreast Cancer Res Treat200911811312419415485
- Palyi-KrekkZBarokMIsolaJTammiMSzollosiJNagyPHyaluronan-induced masking of ErbB2 and CD44-enhanced trastuzumab internalisation in trastuzumab resistant breast cancerEur J Cancer2007432423243317911008
- BussolatiGMontemurroFRighiLDonadioMAgliettaMSapinoAA modified Trastuzumab antibody for the immunohistochemical detection of HER-2 overexpression in breast cancerBr J Cancer2005921261126715812476
- SapinoAMontemurroFMarchioCPatients with advanced stage breast carcinoma immunoreactive to biotinylated Herceptin are most likely to benefit from trastuzumab-based therapy: an hypothesis-generating studyAnn Oncol2007181963196817785760
- HudelistGKostlerWJAttemsJHer-2/neu-triggered intracellular tyrosine kinase activation: in vivo relevance of ligand-independent activation mechanisms and impact upon the efficacy of trastuzumab-based treatmentBr J Cancer20038998399112966413
- LiuPCLiuXLiYIdentification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cellsCancer Biol Ther2006565766416627989
- LiuXFridmanJSWangQSelective inhibition of ADAM metalloproteases blocks HER-2 extracellular domain (ECD) cleavage and potentiates the anti-tumor effects of trastuzumabCancer Biol Ther2006564865616627988
- KostlerWJSchwabBSingerCFMonitoring of serum Her-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancerClin Cancer Res2004101618162415014012
- ColomerRLlombart-CussacALluchABiweekly paclitaxel plus gemcitabine in advanced breast cancer: phase II trial and predictive value of HER2 extracellular domainAnn Oncol20041520120614760109
- FornierMNSeidmanADSchwartzMKSerum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rateAnn Oncol20051623423915668276
- LennonSBartonCBankenLUtility of serum HER2 extracellular domain assessment in clinical decision making: pooled analysis of four trials of trastuzumab in metastatic breast cancerJ Clin Oncol2009271685169319255335
- SeidmanAHudisCPierriMKCardiac dysfunction in the trastuzumab clinical trials experienceJ Clin Oncol2002201215122111870163
- UntchMMuschollMTjulandinSFirst-line trastuzumab plus epirubicin and cyclophosphamide therapy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: cardiac safety and efficacy data from the Herceptin, Cyclophosphamide, and Epirubicin (HERCULES) trialJ Clin Oncol2010281473148020177030
- SeidmanADFornierMNEstevaFJWeekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplificationJ Clin Oncol2001192587259511352950
- GuarneriVLenihanDJValeroVLong-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experienceJ Clin Oncol2006244107411516908934
- RomondEHPerezEABryantJTrastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancerN Engl J Med20053531673168416236738
- Piccart-GebhartMJProcterMLeyland-JonesBTrastuzumab after adjuvant chemotherapy in HER2-positive breast cancerN Engl J Med20053531659167216236737
- SlamonDIermannWRobertNPhase III Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Docetaxel (AC≥T) with Doxorubicin and Cyclophosphamide followed by Docetaxel and Trastuzumab (AC≥TH) with Docetaxel, Carboplatin and Trastuzumab (TCH) in HER2neu Positive Early Breast Cancer Ppatients: BCIRG006 Study [abstract]Cancer Res200969
- JoensuuHBonoPKatajaVFluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer TrialJ Clin Oncol2009275685569219884557
- Tan-ChiuEYothersGRomondEAssessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31J Clin Oncol2005237811781916258083
- PerezEASumanVJDavidsonNECardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trialJ Clin Oncol2008261231123818250349
- ProcterMSuterTMdeAELonger-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trialJ Clin Oncol2010283422342820530280
- McArthurHLChiaSCardiotoxicity of trastuzumab in clinical practiceN Engl J Med2007357949517611218
- MontemurroFRedanaSValabregaGMartinelloRAgliettaMPalmieroRTrastuzumab-related cardiotoxicity in the herceptin adjuvant trialJ Clin Oncol2008262052205318421060
- SuterTMProcterMvan VeldhuisenDJTrastuzumab-associated cardiac adverse effects in the herceptin adjuvant trialJ Clin Oncol2007253859386517646669
- TelliMLHuntSACarlsonRWGuardinoAETrastuzumab-related cardiotoxicity: calling into question the concept of reversibilityJ Clin Oncol2007253525353317687157
- CardinaleDColomboATorrisiRTrastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluationJ Clin Oncol2010283910391620679614
- EwerMSVooletichMTDurandJBReversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatmentJ Clin Oncol2005237820782616258084
- MunozJSheqwaraJArangoBAliHYLoutfiRWeaverDWThe role of beta-blockers and ACE inhibitors in the prevention of trastuzumab-related cardiotoxicity [abstract]J Clin Oncol (Meeting Abstracts)201028555
- BuzdarAUIbrahimNKFrancisDSignificantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancerJ Clin Oncol2005233676368515738535
- GianniLEiermannWSemiglazovVNeoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohortLancet201037537738420113825
- TanchiuEKaufmanPAPaikSregistHER: a prospective, longitudinal cohort study of women with HER2 positive metastatic breast cancer [abstract]J Clin Oncol (Meeting Abstracts)200523670
- TripathyDKaufmanPBrufskyAregistHER: treatment outcomes in patients with HER2-positive (HER2+), hormone receptor-positive (HR+) metastatic breast cancer (MBC) [abstract]J Clin Oncol (Meeting Abstracts)2009271057
- TripathyDSlamonDJCobleighMSafety of treatment of metastatic breast cancer with trastuzumab beyond disease progressionJ Clin Oncol2004221063107015020607
- FountzilasGRazisETsavdaridisDContinuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: a retrospective analysis of 80 cases by the hellenic cooperative oncology groupClin Breast Cancer2003412012512864940
- RabindranSKDiscafaniCMRosfjordECAntitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinaseCancer Res2004643958396515173008
- BursteinHJSunYDirixLYNeratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancerJ Clin Oncol2010281301130720142587
- AwadaADirixLBeckJSafety and efficacy of Neratinib (HKI-272) in combination with Vinorelbine in ErbB2+ metastatic breast cancer [abstract]Cancer Res2009695095
- ChowLGuptaSHershmanDSafety and efficacy of Neratinib (HKI-272) in combination with Paclitaxel in ErbB2+ metastatic breast cancer [abstract]Cancer Res2009695081
- SauraCMartinMMorooseRSafety of Neratinib (HKI-272) in combination with Capecitabine in patients with solid tumors: a Phase 1/2 study [abstract]Cancer Res200969510819509222
- AdamsCWAllisonDEFlagellaKHumanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumabCancer Immunol Immunother20065571772716151804
- FranklinMCCareyKDVajdosFFLeahyDJde VosAMSliwkowskiMXInsights into ErbB signaling from the structure of the ErbB2-pertuzumab complexCancer Cell2004531732815093539
- CortesJBaselgaJPetrellaTPertuzumab monotherapy following trastuzumab-based treatment: activity and tolerability in patients with advanced HER2-positive breast cancer [abstract]J Clin Oncol (Meeting Abstracts)2009271022
- BaselgaJGelmonKAVermaSPhase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapyJ Clin Oncol2010281138114420124182
- Lewis PhillipsGDLiGDuggerDLTargeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugateCancer Res2008689280929019010901
- KropIEBeeramMModiSPhase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancerJ Clin Oncol2010282698270420421541
- MillerKGianniLAndreFA phase Ib/II trial of trastuzumab-DM1 (T-DM1) with pertuzumab (P) for women with HER2-positive, locally advanced or metastatic breast cancer (BC) who were previously treated with trastuzumab (T) [abstract]J Clin Oncol (Meeting Abstracts)2010281012
- PerezEDirixLKocsisJEfficacy and safety of Trastuzumab-DM1 versus trastuzumab plus docetaxel in HER2-positive metastatic breast cancer patients with no prior chemotherapy for metastatic disease: preliminary results of a randomized multicenter, open label phase 2 study (TDM4450G) [abstract]Ann Oncol (meeting Abstracts)201021viii2