Abstract
The gut microbiota is a remarkable asset for human health. As a key element in the development and prevention of specific diseases, its study has yielded a new field of promising biotherapeutics. This review provides comprehensive and updated knowledge of the human gut microbiota, its implications in health and disease, and the potentials and limitations of its modification by currently available biotherapeutics to treat, prevent and/or restore human health, and future directions. Homeostasis of the gut microbiota maintains various functions which are vital to the maintenance of human health. Disruption of the intestinal ecosystem equilibrium (gut dysbiosis) is associated with a plethora of human diseases, including autoimmune and allergic diseases, colorectal cancer, metabolic diseases, and bacterial infections. Relevant underlying mechanisms by which specific intestinal bacteria populations might trigger the development of disease in susceptible hosts are being explored across the globe. Beneficial modulation of the gut microbiota using biotherapeutics, such as prebiotics, probiotics, and antibiotics, may favor health-promoting populations of bacteria and can be exploited in development of biotherapeutics. Other technologies, such as development of human gut models, bacterial screening, and delivery formulations eg, microencapsulated probiotics, may contribute significantly in the near future. Therefore, the human gut microbiota is a legitimate therapeutic target to treat and/or prevent various diseases. Development of a clear understanding of the technologies needed to exploit the gut microbiota is urgently required.
Introduction
The human gastrointestinal tract houses a huge microbial ecosystem, the gut microbiota. This intestinal ecosystem is partially responsible for maintaining human health. However, particular changes in the ecosystem might contribute to the development of certain diseases. With this in mind, there is a need for an exhaustive review on the functions of the gut microbiota, occurrence of gut dysbiosis (alteration of the microbiota), mechanisms by which intestinal bacteria can trigger development of disease, how this ecosystem can be exploited for understanding human health, development of biotherapeutics, expert opinion on current biotherapeutics, and future perspectives. This review presents a descriptive and comprehensive analysis on “the good, the bad, and the ugly” of the gut microbiota, and methods to study these and their modulation of human health.
Composition
The human gut microbiota represents the trillions of microorganisms located in our intestines. Collectively, the number of intestinal microbial cells is 10 times greater than the number of human body cells.Citation1 It was recently demonstrated that the microbiome, which represents the collective genomes of the gut microbiota, is approximately 150 times larger than the human gene complement, with an estimated set of 3.3 million microbial genes.Citation2 Seven bacterial divisions constitute the gut microbiota, ie, Firmicutes, Bacteroides, Proteobacteria, Fusobacteria, Verrucomicrobia, Cyanobacteria, and Actinobacteria, with Firmicutes and Bacteroides being the most abundant species.Citation3 Bacterial communities exhibit quantitative and qualitative variations along the length of the gastrointestinal tract due to host factors (eg, pH, transit time, bile acids, digestive enzymes, and mucus), nonhost factors (eg, nutrients, medication, and environmental factors), and bacterial factors (eg, adhesion capacity, enzymes, and metabolic capacity).Citation4
Acquisition
It is generally accepted that humans are born with a sterile gut. However, new evidence suggests that colonization of the gastrointestinal tract starts before birth, with the fetus ingesting amniotic fluid containing microbes.Citation5 Subsequently, intestinal colonization is acquired during the first months of life, with aerobic and facultative anaerobic colonization, followed by obligate anaerobes and Bifidobacteria.Citation6 Establishment of the gut microbiota is recognized as a complex process influenced by factors at the level of the host and of the microbes themselves.Citation3
Exploration
Study of the composition of human colonic microbiota and metabolism has methodological and ethical limitations. Attempts to circumvent these limitations have led to the development of models. In vitro models are of interest for ecological, fermentation, and metabolic studies.Citation7 These provide reproducible results and controlled mechanistic studies (). Fecal inocula are most often utilized as a representation of the intestinal microbiota.Citation2 In vivo models for the exploration of the gut microbiota encompass various species of laboratory animals. Effects of the indigenous microbiota on the host have been determined by gnotobiology, ie, selective colonization of germ-free animals with defined organisms.Citation8
Table 1 In vitro and in vivo models of the human gut microbiota and their potentials and limitations
Analysis
Until recently, the analysis of bacterial ecosystems was performed by growth on defined media, which has some limitations because this method is labor-intensive and, more importantly, only 80% of stool bacteria can be cultivated.Citation6,Citation8 As a consequence, new molecular techniques have been developed. In terms of qualitative measurements of the microbiota, techniques such as fingerprinting (denaturing gradient gel electrophoresis), terminal restriction fragment length polymorphism, ribosomal intergenic spacer analysis, and 16S ribosomal RNA sequencing are widely used.Citation8–Citation11 Specifically, genome sequencing has provided tremendous information in the microbial world, spearheading technologies such as microarrays.Citation8 New automated parallel sequencing technologies, based on the 16S ribosomal RNA gene present in all prokaryotes, can offer a cost-effective solution for rapid sequencing and identification of bacterial species of the gut. Prominent high-throughput sequencing technologies include 454 Life Sciences’ Genome Sequencer™, Applied Biosystems’ SOLiD™ 3 Plus system, Illumina’s Genome Analyzer IIx, and other technologies developed by Affymetrix, Helicos, Qiagen, and Microchip. For quantitative measurements of the gut microbiota, fluorescence in situ hybridization, catalyzed reporter deposition-fluorescence in situ hybridization, quantitative polymerase chain reaction, and scanning electron microscopy in situ hybridization, can be useful.Citation8,Citation10–Citation12 Fluorescence in situ hybridization allows for the visualization of microorganisms in their natural environment using labeled probes specific for selected bacteria. This method has been used for the determination of changes in the bacterial populations of fecal homogenates and in tissue sections from individuals with certain diseases. Catalyzed reporter deposition-fluorescence in situ hybridization is a modified method of fluorescence in situ hybridization which allows for in situ amplification using horseradish peroxidase, enhancing bacterial cell detection in samples where ribosomal RNA is insufficient for fluorescence in situ hybridization.Citation12 Real-time quantitative polymerase chain reaction is a recent and widely used technique for exploration of the roles of gut microflora in health and disease, based on the presence of specific RNA sequences. There is also scanning electron microscopic in situ hybridization which uses deposition of nanogold particles to enable detectionCitation12 (). Finally, metagenomics is an approach to analyze the genomic content of microbial communities living in a particular niche, such as the gut, and for identifying and quantifying the bacterial species present.Citation8
Table 2 Available techniques for human gut microbiota characterization
The good
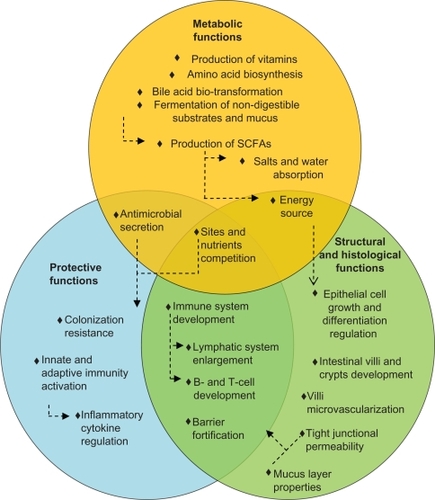
The gut microbiota performs essential functions in maintenance of health, including having protective, structural, and metabolic roles.
Essential metabolic functions
Metabolic functions of the gut microbiota include production of vitamin, amino acid synthesis, and bile acid biotransformation. Bile acid biotransformations, performed by microbial enzymes, have implications for cholesterol and glucose metabolism.Citation13 Importantly, the microbiome provides biochemical pathways required for the fermentation of nondigestible substrates and endogenous mucus. Through fermentation, bacterial growth is stimulated, producing short-chain fatty acids and gases.Citation14 The major short-chain fatty acids produced are acetate, butyrate, and propionate. Other bacterial end products include lactate, ethanol, succinate, formate, valerate, caproate, isobutyrate, 2-methyl-butyrate, and isovalerate. Bacterial fermentation is present in the cecum and colon, where the short-chain fatty acids are absorbed, stimulating the absorption of salts and water. One property of short-chain fatty acids is their trophic effect on the intestinal epithelium.Citation14 Butyrate is the preferred energy source for epithelial cells, and is almost entirely cleared by the colonic epithelium. Acetate is the principal short-chain fatty acid in the colon and the primary substrate for cholesterol synthesis. Finally, propionate supplementation in the diet was shown to reduce cholesterol levels in vivo.Citation15,Citation16 Clinical trials have yet to confirm these observations. Therefore, the metabolic activities performed by the gut microbiota are various and essential for host metabolism ().
Ensures protection
Pathogen displacement or “colonization resistance” is an accepted function of the gut microbiota. Commensal organisms prevent pathogenic colonization by competing for attachment sites and nutrients, and also through the production and secretion of antimicrobials. Those mechanisms are relevant for reducing the level of lipopolysaccharides, peptidoglycans, bacterial CpG-DNA motifs, and superantigens, which can all be detrimental to the host.Citation17 The indigenous microbiota is also essential for development of the immune system.Citation18 Germ-free mice display underdeveloped lymphatic systems, with fewer Peyer’s patches and isolated lymphoid follicles.Citation19,Citation20 Moreover, intestinal dendritic cells are fewer in germ-free animals, and there is evidence to support a role for bacterial signals in B cell development.Citation21,Citation22 Furthermore, signals from intestinal bacteria appear important for the development of regulatory T, T helper type 1 and 2 cells, and T helper 17 cells.Citation23–Citation25 The first commensal microorganism molecule shown to influence an immune response beneficially was capsular polysaccharide A, produced by Bacteroides fragilis.Citation26 Short-chain fatty acids, such as butyrate, may also exert potent immunomodulatory effects by suppressing nuclear factor-kB activation and/or by acting on G-coupled receptors, as demonstrated with acetate.Citation27,Citation28 These concepts illustrate a dynamic relationship between the immune system and the microbiota. The intestinal mucosa averts threats by signaling to the innate immune system through pattern recognition receptors, such as toll-like receptors. Pattern recognition receptors recognize and bind to specific microbial macromolecules, referred to as microbial-associated molecular patterns. These include lipopolysaccharide, flagellin, peptidoglycan, and N-formylated peptides. In the intestinal mucosa, activation of pattern recognition receptors initiates nuclear factor-kB pathways, mitogen-activated protein kinase, and caspase-dependent signaling cascades. These lead to the production and release of protective peptides, cytokines, chemokines, and phagocytes. The result can be a protective response to commensal bacteria, an inflammatory response to pathogenic organisms, or a trigger of apoptosis. Therefore, commensal bacteria of the gastrointestinal tract play active roles in the development and homeostasis of the immune system, as shown in .
Structural and histological function
The microbiota ensures intestinal structure and function. Firstly, the mucus layer, which reflects the balance between mucus secretion and bacterial degradation, constitutes an obstacle to the uptake of antigens and proinflammatory molecules.Citation29 There is evidence indicating that butyrate reinforces the colonic defense barrier by inducing the secretion of mucins, trefoil factors, and antimicrobial peptides.Citation30 Secondly, some bacterial communities may strengthen the barrier at the level of the tight junctions, ie, protein clusters that form a barrier between the lumen and the lamina propria. Moreover, the gut microbiota is involved in cell and tissue development. Butyrate regulates cell growth and differentiation, inhibiting transformed cell growth while encouraging reversion of cells from a neoplastic to a non-neoplastic phenotype.Citation31 The cecum villi are longer and wider, while the colonic crypts are shorter and contain fewer cells in germ-free than conventionally reared animals, possibly due to an altered rate of epithelial cell turnover or to anatomical changes arising from a reduction in bacterial count.Citation32 Moreover, indigenous microbes shape the development of the villus microvasculature, as demonstrated in germ-free animals colonized during or after completion of postnatal gut development.Citation33 Therefore, most of the structural and morphological development of the gut contributes to and manages the gut bacterial system ().
The bad
Dysbiosis is a state in which the microbiota becomes altered as a consequence of an alteration in the composition of the microbiota, a change in bacterial metabolic activity, and/or a shift in local distribution of communities. Many factors can alter the gastrointestinal ecosystem, including antibiotics, psychological and physical stresses, radiation, altered peristalsis, and dietary changes.Citation34 At present, the focus is on the description of dysbiosis in a plethora of human disorders.
Autoimmune disease
Autoimmune diseases occur when the body’s immune system attacks and destroys healthy cells and tissues, as is the case in type 1 diabetes mellitus, celiac disease, inflammatory bowel diseases, and allergic asthma. Most often, the immune response is initiated by unknown factors. Alteration of the gut microbiota as a result of modern lifestyles is an attractive hypothesis to explain the rise in prevalence of celiac disease, type 1 diabetes mellitus, and inflammatory bowel diseases.
Celiac disease is an inflammatory disease of the small intestine that is triggered and maintained by the storage proteins of wheat, barley, and rye. Studies have investigated the composition of the microbiota in patients with celiac disease. Fecal samples from patients with celiac disease had reduced the proportions of Bifidobacterium, Clostridium histolyticum, Clostridium lituseburense, Faecalibacterium prausnitzii, and increased proportions of Bacteroides/Prevotella.Citation35 In addition, increased proportions of total and Gram-negative bacteria, with an increase in Bacteroides and Escherichia coli in biopsies of patients with celiac disease in the active as compared with inactive disease state and control individuals, was shown by fluorescence in situ hybridization coupled with flow cytometry.Citation36
Type 1 diabetes mellitus, characterized by insulin deficiency resulting from immune-mediated destruction of pancreatic β cells, is thought to be triggered by environmental factors in genetically susceptible individuals. Given that antibiotics prevented type 1 diabetes mellitus in biobreeding diabetes-prone rats and in nonobese diabetic mice, alteration of the microbiota has been associated with progression of type 1 diabetes mellitus.Citation37,Citation38 Moreover, evidence shows that bacterial communities from biobreeding diabetes-prone and diabetes-resistant rats differ, marked by a higher abundance of Lactobacillus and Bifidobacterium in diabetes-resistant rats.Citation39
Inflammatory bowel diseases include ulcerative colitis and Crohn’s disease. Crohn’s disease is characterized by patchy and transmural inflammation that may affect any part of the gastrointestinal tract, while ulcerative colitis is a chronic episodic inflammatory condition that involves only the large bowel.Citation40 There is evidence that species belonging to the normal gut microbiota are involved in the etiology and/or maintenance of inflammatory processes. Reduced microbial diversity, increased Bacteroidetes and Enterobacteriaceae, and decreased Firmicutes were all observed in patients with inflammatory bowel diseases.Citation41 Another clinical study observed that Eubacterium rectale, B. fragilis, Bacteroides vulgatus, Ruminococcus albus, R. callidus, R. bromii, and F. prausnitzii were 5–10-fold more abundant in healthy subjects than in patients with Crohn’s disease, while Enterococcus spp, C. difficile, E. coli, Shigella flexneri, and Listeria spp were more abundant in the Crohn’s disease group.Citation42 Thus, inflammatory bowel diseases, celiac disease, and type 1 diabetes mellitus are autoimmune diseases marked by an alteration of the gut microbiota. Autoimmune regulation may be linked with the disruption of the intestinal ecosystem.
Allergic disease
The etiology of allergic diseases is ambiguous. They may be initiated and maintained by environmental factors associated with a change in gut microbiota. Correlations between allergic disease and altered fecal microbiota, antibiotic use, and dietary changes have been made.Citation43–Citation45 Studies of the microbiota in allergic patients have shown decreased intestinal Bifidobacteria counts, an increased prevalence of B. fragilis, and higher counts of Staphylococcus aureus and E. coli.Citation46–Citation48 A study reported higher levels of Bifidobacterium adolescentis and Lactobacilli Group I (Lactobacillus rhamnosus, L. paracasei, L. casei) in fecal samples of nonallergic compared with allergic children.Citation43 Hence, it is clear that differing gut microbial communities have been observed in allergic patients. Further clinical studies are required on several age-matched groups of allergic versus control individuals classified with short age intervals.
Irritable bowel syndrome
Irritable bowel syndrome is characterized by abdominal pain, bloating, and changes in bowel habit, in the absence of any overt mucosal abnormality. Observations have directed attention towards the gut microbiota, identifying a postinfectious variant of the syndrome, ie, evidence that antibiotics induced a reduction in the microbiota which may be a risk factor, and the proposal that some patients may have bacterial overgrowth in the small bowel.Citation49 Studies have demonstrated that patients with irritable bowel syndrome have fewer intestinal Bifidobacteria, Collinsella aerofaciens, Coprococcus eutactus, and Clostridium cocleatum, and an increase in Veillonella and Enterobacteriaeae.Citation50–Citation52 Thus, irritable bowel syndrome is associated with abnormal intestinal communities, but their significance in the pathogenesis of irritable bowel syndrome remains unclear.
Metabolic disease
There are genetic and environmental factors that influence obesity, with the impact of the gut microbiota being well documented. Research on the microbiota of obese mice establishes the link between obesity, weight gain, and intestinal dysbiosis. Findings indicate that a high-fat diet modulates the microbiota independently of obesity.Citation53 Mice genetically predisposed to develop obesity (ob/ob) harbor more Firmicutes and fewer Bacteroidetes.Citation54 Studies in human twins concordant for obesity have demonstrated that the fractional representation of Bacteroidetes is directly correlated with leanness.Citation55 Moreover, microbiota transplantation from normal chow-fed ob/ob and Western diet-fed wild-type to germ-free wild-type mice caused an adiposity increase greater than that caused by transplantation from wild-type donors fed standard chow.Citation56,Citation57 This demonstrates a causal effect of intestinal bacteria on development of obesity. Aberrant development of the microbiota might precede obesity, because the childhood representation of Bifidobacteria and S. aureus has been suggested to predict the development of adulthood obesity in an inverse and direct manner.Citation58 In light of these findings, a study was initiated which demonstrated that total short-chain fatty acids were higher in an obese group and individual short-chain fatty acid proportions shifted towards propionate in overweight subjects.Citation59 It is assumed that gut dysbiosis may contribute to the development of obesity.
Type 2 diabetes is characterized by defects in insulin secretion and action. Research has characterized the fecal microbiota composition of adults with type 2 diabetes and showed reduced proportions of Firmicutes and Clostridia.Citation60 The ratios of Bacteroidetes to Firmicutes, as well as Bacteroides/Prevotella to Clostridium coccoides and E. rectale groups correlated positively with plasma glucose concentration. Similarly, class β-Proteobacteria was highly enriched in diabetic individuals and positively correlated with plasma glucose. Other authors have demonstrated that Prevotella was associated with healthy groups, while Bacteroides and Parabacteroides were prevalent in diabetic patients, who also had fewer counts of fecal B. vulgatus and Bifidobacteria.Citation61 The evidence demonstrates that metabolic diseases are associated with a shift in the balance of the microbiota. Confirmation of gut dysbiosis in type 2 diabetes patients requires additional clinical trials.
Alcoholic liver disease
Alcoholic liver disease, arising from excessive ingestion of alcohol, is the primary cause of liver failure in the Western world. It has been demonstrated in one study that daily alcohol consumption affects composition of the colonic microbiota.Citation62 The authors pointed to a specific fingerprint of dysbiotic microbiota which could potentially identify susceptible heavy drinkers at high risk for alcoholic liver disease. Further examination is required to support fully the link between gut dysbiosis and alcoholic liver disease.
Bacterial infection
It is well established that a disruption in the commensal microbiota increases susceptibility to enteric infections. Antibiotic-treated mice are particularly useful for studying colitis induced by Salmonella spp, Shigella spp, and E. coli infections. In addition, in murine Citrobacter rodentium infections, pathogen colonization is associated with a reduced total density and a relative increase in γ-Proteobacteria.Citation63 Furthermore, elderly patients with C. difficile-associated diarrhea demonstrate reduced numbers of Bacteroides, Prevotella, and Bifidobacteria, and a greater diversity of facultative species, ie, Lactobacilli and Clostridia.Citation64 The evidence suggests an association between disruption of the gut microbiota and bacterial infections, further accentuating the dysbiosis.
Colorectal cancer
Colorectal cancer is a disease of the Western world, with the most common type being adenocarcinomas which develop from glandular cells lining the wall of the bowel.Citation65 Genetic and induced models of intestinal neoplasia have shown that, under germ-free conditions, colitis and tumor formation are reduced compared with monoassociated and conventional animals.Citation66–Citation68 In addition, in vivo colonization of enterotoxigenic B. fragilis a human colonic commensal bacteria, has been linked to the development of colorectal cancer.Citation69 Furthermore, colorectal cancer patients demonstrate decreased levels of E. rectale and F. prausnitzii compared with healthy volunteers, and increased populations of E. faecalis.Citation70 Recently, a clinical trial showed that patients with colorectal cancer had significant elevation of the Bacteroides-Prevotella population.Citation71 Therefore, studies that have examined the gut microbiota in colorectal cancer have reported an association between microbiota and pathogenesis of colorectal cancer. The abnormal profile of bacterial communities, activities, and metabolites in human disease is summarized in .
Table 3 Imbalances of human gastrointestinal bacterial communities in human disease
The ugly
Altered composition of the human gastrointestinal ecosystem can lead to physiological changes in the intestinal environment, disrupting the functions of the microbiota and having serious consequences for human health.
Altered gut microbiota may trigger serious immune deregulation
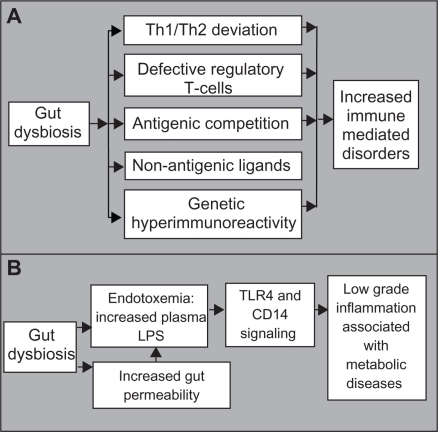
The hygiene hypothesis predicts that increased hygiene, use of antibiotics, and sterile food preparation result in isolation of the immune system from positive microbial exposure, favoring susceptibility to immune-mediated disorders.Citation72 Epidemiological studies have linked a decreasing burden of infection with a rising incidence of immunological disorders.Citation73,Citation74 The presence of environmental viral agents are also associated with the incidence of spontaneous type 1 diabetes.Citation75 Furthermore, the microbiota of nonobese diabetic mice deficient for the myeloid differentiation primary response gene 88 (MyD88) signaling molecule protects mice against developing diabetes.Citation76 Inactivation of MyD88 leads to gut dysbiosis, further inhibiting the autoimmunity occurring in MyD88−/− germ-free nonobese diabetic mice. These findings support a causal inverse relationship between microbial exposure and immunological disorders.
There are several underlying mechanisms of the hygiene hypothesis, ie: lack of microbial burden in childhood, predisposing the host to allergic disorders due to a T helper type 1/type 2 deviation; defective maturation of regulatory T cells as a consequence of modern lifestyles; antigenic competition from infectious agents inhibiting responses to weak antigens; protection from allergic diseases through mechanisms independent of their constitutive antigens, leading to stimulation of nonantigen-specific receptors; and development of an aggressive immune response caused by genetic hyperimmunoreactivity triggered by dysbiosis.Citation72 Therefore, the disruption of cross-talk between the commensal microbiota and the immune system, as a result of modern lifestyles, may lead to development of allergic, autoimmune, and autoimmune-associated diseases ().
Specific gut dysbiosis can engender metabolic endotoxemia
Obesity and type 2 diabetes are associated with chronic low-grade inflammation and endotoxemia. Lipopolysaccharide administration and a high-fat diet lead to an increase in adipose tissue, impaired glucose tolerance, and insulin resistance, while dietary modulation reverses this phenotype.Citation77,Citation78 Chronic inflammation, elicited by endotoxemia, may cause activation of the immune system. The importance of gut-derived endotoxins in alcoholic liver disease has also been supported.Citation79 Circulating endotoxin levels were increased in mice and rats following chronic alcohol ingestion, while antibiotic treatment provided protection from alcoholic liver disease. In addition, plasma endotoxins were augmented in patients with alcoholic liver disease. The evidence also suggests that endotoxins contribute to the pathogenesis of nonalcoholic fatty liver disease, considered to be a hepatic manifestation of metabolic syndrome and obesity. Indeed, plasma endotoxins are higher in patients with nonalcoholic fatty liver disease and are associated with intestinal overgrowth and induction of hepatic toll-like receptor 4.Citation80,Citation81 Gut permeability, which influences the systemic distribution of endotoxins, may further induce metabolic endotoxemia. Mice fed a high-fat diet demonstrate increased gut permeability and metabolic endotoxemia, associated with disruption of tight junction proteins.Citation82 Furthermore, an increase in Bifidobacteria induced by nutritional supplements is correlated with an improved gut barrier, lower portal lipopolysaccharide levels, and lower inflammatory tone in ob/ob mice.Citation83 Taken together, we suggest that gut dysbiosis, characterized by decreased Bifidobacteria and increased lipopolysaccharide release, disrupts the epithelial barrier, resulting in a leaky gut and leading to colonic and systemic inflammation which contributes to alcoholic liver disease, nonalcoholic fatty liver disease, obesity, and type 2 diabetes (). It remains unclear which Gram-negative bacteria are responsible for the increase in lipopolysaccharide.
Bacterial infection might be promoted by gut dysbiosis
Pathogenic bacteria can invade the gastrointestinal tract and infect the body, producing sepsis, shock, multisystem organ failure, and death of the host. The mechanisms by which pathogens overcome obstacles to achieve successful infection are uncertain. Pathogenic infections might be facilitated by disruption of the intestinal ecosystem by environmental factors. A mechanism based on the triggering of the host’s immune defenses was elucidated using models of C. rodentium (mimicking diarrheal pathogen-associated inflammation), Campylobacter jejuni infection, and chemically and genetically induced models of intestinal inflammation are used for altered microbiota investigations.Citation63 An overgrowth of Enterobacteriaceae was observed in all models, indicating that inflammation-induced microbiota changes support colonization by aero-tolerant bacteria. The inflammatory response, triggered by the invading pathogen, may function to enhance its colonization, further facilitating its virulence. Thus, alteration of the gut microbiota, initiated by host and environmental factors, may participate in the initiation of diseases caused by infectious agent ().
Figure 3 Proposed mechanism whereby an altered microbial balance in the gut can A) be driven by foreign pathogenic invasion and further increase the likelihood of future infections, and B) lead to the promotion of carcinogenesis.
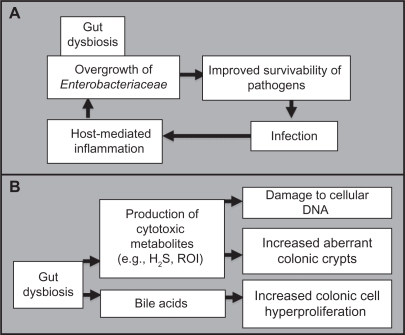
Abnormal bacterial metabolite levels may trigger cancer
Many etiological bacterial mechanisms have been hypothesized to promote carcinogenesis. Amongst those, hydrogen sulfide, a product of bacterial sulfate reduction, appears to be linked to the incidence of chronic disorders, such as ulcerative colitis and colorectal cancer. Because DNA strand breaks are associated with mutation and promotion of carcinogenesis, bacterial hydrogen sulfide may be responsible for the induction of mutations in the development of sporadic colorectal cancer.Citation84 Reactive oxygen intermediates also cause DNA damage, and their numbers are higher in chronic inflammation and colorectal cancer, as observed in the fecal matrix.Citation85 In addition, hydrophobic bile acids have been shown to promote colorectal carcinogenesis by inducing micronuclei formation, mitotic perturbations, and decreases in spindle checkpoint proteins.Citation86 Intestinal bacteria may also function as promoters of carcinogenesis by increasing the progression of chemically induced aberrant crypt foci.Citation87,Citation88 Therefore, despite limited studies on the occurrence of gut dysbiosis and tumorigenesis, we bring to a close the mechanisms that support the implication of detrimental intestinal bacteria in promoting carcinogenesis ().
Gut microbiota alters energy and lipid metabolism
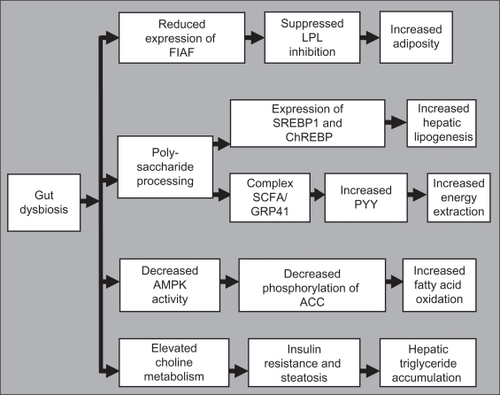
Gordon et al investigated the influence of microbiota on fat and lipid metabolism, demonstrating that reared mice have more body and gonadal fat than germ-free mice, despite reduced chow consumption. The increase in fat was accompanied with increased fasting glucose and insulin levels and an insulin-resistant state.Citation89 Transplantation of the microbiota from ob/ob mice to germ-free mice resulted in a greater increase in body fat than from lean donors.Citation56 Two mechanisms are suggested. First, colonization of the gut may suppress expression of fasting-induced adipose factor, increasing the activity of lipoprotein lipase, leading to an increased uptake of fatty acids and triglyceride storage. The second mechanism is based on the processing of dietary polysaccharides by bacteria that may increase hepatic lipogenesis through expression of sterol response element binding protein 1 and carbohydrate response element binding protein. Other authors suggest that the complex formed by short-chain fatty acids and G protein-coupled receptor 41 may increase circulating levels of peptide YY, which may further increase energy extraction from the diet.Citation90 It was also demonstrated that in the absence of gut microbiota, AMP-activated protein kinase activity is constitutively higher in muscle, leading to higher phosphorylation of its specific target, acetyl CoA carboxylase, promoting mitochondrial fatty acid oxidation.Citation91 Finally, the microbiota may affect insulin resistance and steatosis by regulating choline metabolism.Citation92 Thus, alteration of the gut microbiota causes defective energy and lipid metabolism leading to the development of metabolic disease ().
Figure 4 Proposedmechanisms by which an altered balance of the gut microbiota can lead to dysfunctional energy and lipid metabolism.
Alter the gut microbiota to favor human health
The relationship between health and the gastrointestinal system is established. Due to the inherent plasticity of microbiota, one can consider exploiting it to develop biotherapeutics.
Probiotics
Probiotics are “live microorganisms, which, when administered in adequate amounts, confer a health benefit on the host”.Citation93 Probiotics have been shown to have effects on irritable bowel diseases, metabolic syndromes, enterocolitis, immunomodulation, pathogen defense, and urogenital infections.Citation94–Citation96 Mechanisms of probiotics include remodeling of microbial communities and suppression of pathogens, suppression of proinflammatory factors, effects on epithelial cell differentiation, and proliferation and promotion of the intestinal barrier.Citation97
Microencapsulation and other methods for targeted delivery
Microencapsulation, which provides living cells with a physical protection barrier, is primarily used to improve probiotic activity during gastrointestinal transit, while allowing the diffusion of metabolites and substrates into and out of the capsule. Secondly, it offers a bacterial delivery system targeted to a specific part of the gastrointestinal tract. Thirdly, microencapsulation ensures immunomodulation by preventing interaction between the host immune system and entrapped probiotic bacterial cells.Citation98,Citation99 In addition, there are other excellent formulations available for delivery of probiotic cells to achieve optimal clinical benefits.
Prebiotics
Prebiotics are “nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or the activity of one or a limited number of bacteria in the colon, and thus improves host health”.Citation100 A prebiotic should not be hydrolyzed by human intestinal enzymes, but selectively fermented by bacteria, benefiting the host. Effects of prebiotic administration include reduced triglyceride levels, improved postprandial glucose levels, and reduced intestinal permeability and inflammation.Citation100–Citation102 In addition to prebiotics, known foods and drugs are also being developed to enhance the growth of health-promoting bacterial cell populations in the gastrointestinal tract, with the aim of preventing or treating a number of diseases.
Symbiotic association of probiotics and prebiotics
It has been hypothesized that in combining probiotics and prebiotics, one would not only achieve the combined effects of the two components, but also a synergistic effect. This is the principle on which symbiotics are based.Citation103
Antibiotics combined with probiotics
The evidence suggests that probiotic bacteria suppress gastrointestinal pathogens and potentiate antibiotic efficacy by production of antibacterial factors, including bacteriocins.Citation97 With the use of antibiotic drugs, bacterial overgrowth can be controlled, and translocation in specific conditions of increased risk can be prevented.Citation83 In brief, strong evidence is available that probiotics, prebiotics, and antibiotics can successfully exploit the natural microbial composition of the gut to treat and/or prevent diseases for improving human health and well being.
Future directions
Advances in exploring and modeling the microbiota are generating a wealth of knowledge about human health and disease, and contributing to the development of new biotherapeutics. Significant advances have been made in the selection and characterization of specific probiotic cultures and/or prebiotics and subsequent substantiation of health claims relating to their consumption. However, there is still much skepticism in the medical community with respect to the effects demonstrated. Firstly, the disease model used affects the results significantly. Secondly, there is a lack of large, controlled, randomized human trials supporting the beneficial claims. Thirdly, the harsh conditions of the gastrointestinal tract can impede the delivery of probiotic bacterial cells. One significant advantage of using prebiotics as opposed to probiotics is that they overcome the viability issue. Prebiotics are, by definition, not hydrolyzed by enzymes during gastrointestinal transit and, therefore end up being available to stimulate selective growth of gut microbiota. Nevertheless, it is hardly possible to demonstrate the selectivity criterion of prebiotics. On the contrary, microencapsulation of probiotic bacteria is a delivery method that overcomes the viability issue and is also selective.
Therefore, it is easy to envisage that microencapsulated probiotics may soon be available on the market, and research in the long term may focus on developing formulations combining microencapsulated probiotics, prebiotics and/or antibiotics. The main challenge is developing suitable models to characterize and understand the microbiota, and developing effective treatment formulations, such as targeted delivery of probiotics within the gastrointestinal tract.
Towards new models of human gut microbiota
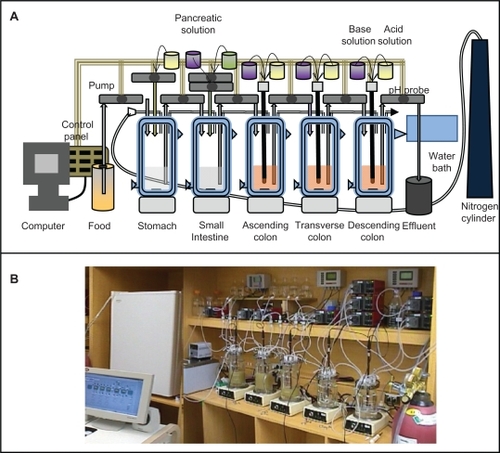
In vitro models of the human microbiota are essential to screen, characterize, develop, and perform mechanistic studies under controlled parameters. To test the therapies being developed effectively, special attention has to be directed to the development and optimization of in vitro models of the microbiota, given that these are rare. Models to mimic the whole gastrointestinal tract and specific compartments are essential. The challenge is to create a fermentation system featuring microbial diversity similar to that in the human gastrointestinal tract. Due to sampling complications, the microbiota of a fecal inoculum is assumed to be representative of the intestinal microbiota.Citation104 The computer-controlled dynamic human gastrointestinal model consists of a succession of five vessels: the stomach, the small intestine, and the ascending, transverse, and descending colon ( and ).Citation105,Citation106 Temperature, pH, and anaerobic parameters are all controlled using Labview® software. The system is equipped with portholes for the addition of medium, the removal of spent culture, and the administration of therapeutics. Attention is also needed to simulate the bacterial microhabitat of the intestines, because the metabolism and functioning of the mucosal-associated microbiota differ from those free-living in the lumen.Citation106 Efforts should be made to understand the effect of gastrointestinal tract adhesion of bacterial cells on bacterial metabolism by designing a model to investigate adhesion of probiotics for gut microbiota modulation.
Figure 5 Computer-controlled dynamic human gastrointestinal (GI) model used for studies on the human gut microbiota. A) Schematic representation, B) photograph. vessels in series representing stomach, small intestine, ascending colon, transverse colon, and descending colon. All vessels can be continuously magnetically stirred; temperature can be controlled by the flow of hot water in the double jacketed vessel. Food can be given at a time interval and samples can be collected from any GI part at any time (eg, spent removal of the spent culture at defined intervals). This also allows for the administration of biotherapeutics, control of pH, enzyme, anerobic atmosphere and other GI parameters effecting gut microbiota.
Microencapsulation to boost efficacy of probiotic treatment
There are many methods for microencapsulation of bacterial cells available, and these can be effectively used in boosting probiotic oral delivery and clinical efficacy. Our research interest is mainly focused on the customization of a myriad of microencapsulated probiotic formulations using polyelectrolyte complexation, a widely used technique based on the interaction of oppositely charged polymers that form a physical membrane around the probiotic.Citation99,Citation105–Citation111 The physicochemical properties of the capsules are engineered to guarantee survival of the bacteria, appropriate diffusion of metabolites and substrates, and targeted delivery. In gastrointestinal tract simulators, the enzymatic activity, stability, and viability of microencapsulated probiotics have been demonstrated.Citation105–Citation109 Results confirm the potential of orally delivered microencapsulated bacteria to manage hypercholesterolemia, hypertriglyceridemia, and colon cancer.Citation108,Citation111 For microencapsulated probiotics to be marketable, research must be directed at developing structural and functional models of the microbiota to test the therapeutic formulations. Finally, in vivo models should evaluate the efficacy of microencapsulated probiotic formulations while characterizing preclinical cellular and tissue responses. The potential of microencapsulated Lactobacillus fermentum to lower cholesterol and triglycerides in Bio F(1)B hamsters fed a hypercholesterolemic diet was investigated.Citation108 Treatment with the bacterial formulation reduced total cholesterol, low-density lipoprotein cholesterol, and triglycerides, and also reduced the progression of atherosclerotic lesions. The antitumorigenic properties of microencapsulated probiotics in multiple intestinal neoplasia were also investigated in mice carrying a germline APC mutation.Citation111 Oral administration of microencapsulated Lactobacillus acidophilus resulted in suppression of colon tumor incidence, multiplicity, and size. Preclinical trials confirm the potential of orally delivered microencapsulated probiotics for managing hypercholesterolemia, hypertriglyceridemia, and colon cancer.
Conclusion
This is an extensive and timely review of the gut microbiota and its role in human health and disease. First described are the key players among the microbiota, how they develop into a network in the gastrointestinal tract, their roles in various gastrointestinal and other diseases, methods to study human gut microbiota, and associated health benefits and limitations. Interest in the microbiota arose after the realization that an altered balance in the gut could lead to disease. By using probiotics, prebiotics, and antibiotics, one can tune the composition of the gut to improve the health of the host. Optimization of methods to modulate and characterize the microbiota and probiotics still remains to be done. The microbiota itself can allow for the analysis of health, and biomarkers of a given microbiota can be indicative of disease. Although preliminary, two animal models have shown that microbial metabolism is correlated with specific patterns of metabolites excreted in urine.Citation112 This could potentially be a significant breakthrough in the new realm of personalized medicine.
Gut microbiota is an area of research with potential for pure scientific exploration with significant biotherapeutic applications. It remains largely unexplored. This is due to the complexity of the microbiota and the difficulties in collection and analysis of data. In addition, lack of understanding of the role of bacterial cells in human health and lack of well defined clinical trials on known beneficial probiotics, other bacterial cells, and associated systems, such as delivery systems, are seriously hampering this very important area of biological therapy. Development of cutting-edge technologies for understanding human gut microbiota, their manipulations, and screening and development of cutting edge formulations for probiotic delivery, such as micro-encapsulation, are strongly needed, along with accurate in vitro models.
Highlights
The human gut microbiota is an asset for human homeostasis.
The human gut microbiota, which resides within the gastrointestinal tract system, is composed of trillions of microorganisms with tremendous diversity and complexity
The gut microbiota should be considered as a vital organ, carrying essential metabolic, protective, and structural/histological functions in maintaining body homeostasis, human health and other manifestations
An altered composition of the intestinal ecosystem, a state called gut dysbiosis, can lead to physiological changes in the intestinal environment, disrupting the functions of the gut microbiota, autoimmune, allergic, metabolic and alcoholic liver diseases, irritable bowel syndrome, colorectal cancer, and bacterial infections have all been linked to gut dysbiosis
Bacterial imbalances can be responsible for immunological deregulation, breakdown of colonization resistance, induction of systemic endotoxemia, production of carcinogenic compounds and alteration of energy and lipid metabolism
Due to the inherent plasticity of the gut microbiota, therapeutics such as probiotics, prebiotics, and antibiotics are used to modulate the human intestinal ecosystem to obtain better human health
Major efforts should be directed towards development of in vitro and in vivo models to understand the human gut microbiota and explore development of biotherapeutic possibilities and methods, such as microencapsulation, for suitable delivery of beneficial bacterial cells to the gastrointestinal tract and other systems for human health.
Acknowledgements
This work is supported by the Canadian Institutes of Health Research, research operating grant MOP-64308 (to SP), the Natural Sciences and Engineering Research Council of Canada (to CTD), the Fonds Québécois de la Recherche sur la Nature et les Technologies (to MCC), and Micropharma Limited (to SP).
Disclosure
The authors report no conflicts of interest in this work.
References
- SavageDCMicrobial ecology of the gastrointestinal tractAnnu Rev Microbiol197731107133334036
- QinJLiRRaesJA human gut microbial gene catalogue established by metagenomic sequencingNature2010464596520203603
- BackhedFLeyRESonnenburgJLPetersonDAGordonJIHost-bacterial mutualism in the human intestineScience20053071915192015790844
- McConnellELFaddaHMBasitAWGut instincts: Explorations in intestinal physiology and drug deliveryInt J Pharm200836421322618602774
- MshvildadzeMNeuJThe infant intestinal microbiome: Friend or foe?Early Hum Dev201086Suppl 1677120116944
- AdlerberthIWoldAEEstablishment of the gut microbiota in Western infantsActa Paediatr20099822923819143664
- RumneyCJRowlandIRIn vivo and in vitro models of the human colonic floraCrit Rev Food Sci Nutr1992312993311581008
- SekirovIRussellSLAntunesLCFinlayBBGut microbiota in health and diseasePhysiol Rev20109085990420664075
- DethlefsenLEckburgPBBikEMRelmanDAAssembly of the human intestinal microbiotaTrends Ecol Evol20062151752316820245
- BlautMCollinsMDWellingGWDoreJVanLJde VosWMolecular biological methods for studying the gut microbiota: The EU human gut flora projectBr J Nutr200287Suppl 2S20321112088520
- McCartneyALApplication of molecular biological methods for studying probiotics and the gut floraBr J Nutr200288Suppl 1S29S3712215179
- PeterHSommarugaRAn evaluation of methods to study the gut bacterial community composition of freshwater zooplanktonJ Plankton Res2008309971006
- LefebvrePCariouBLienFKuipersFStaelsBRole of bile acids and bile acid receptors in metabolic regulationPhysiol Rev20098914719119126757
- WongJMdeSRKendallCWEmamAJenkinsDJColonic health: Fermentation and short chain fatty acidsJ Clin Gastroenterol20064023524316633129
- ChenWJAndersonJWJenningsDPropionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed ratsProc Soc Exp Biol Med19841752152186320209
- BerggrenAMNymanEMLundquistIBjorckIMInfluence of orally and rectally administered propionate on cholesterol and glucose metabolism in obese ratsBr J Nutr1996762872948813902
- Tlaskalova-HogenovaHStepankovaRHudcovicTCommensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseasesImmunol Lett2004939710815158604
- RoundJLMazmanianSKThe gut microbiota shapes intestinal immune responses during health and diseaseNat Rev Immunol2009931332319343057
- CebraJJPeriwalSBLeeGLeeFShroffKEDevelopment and maintenance of the gut-associated lymphoid tissue (GALT): The roles of enteric bacteria and virusesDev Immunol1998613189716901
- BouskraDBrezillonCBerardMLymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasisNature200845650751018987631
- HaversonKRehakovaZSinkoraJSverLBaileyMImmune development in jejunal mucosa after colonization with selected commensal gut bacteria: A study in germ-free pigsVet Immunol Immunopathol200711924325317643495
- HapfelmeierSLawsonMASlackEReversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responsesScience20103281705170920576892
- AtarashiKTanoueTShimaTInduction of colonic regulatory T cells by indigenous Clostridium speciesScience201133133734121205640
- MazmanianSKLiuCHTzianabosAOKasperDLAn immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune systemCell200512210711816009137
- IvanovIIAtarashiKManelNInduction of intestinal Th17 cells by segmented filamentous bacteriaCell200913948549819836068
- MazmanianSKRoundJLKasperDLA microbial symbiosis factor prevents intestinal inflammatory diseaseNature200845362062518509436
- LuhrsHGerkeTMullerJGButyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitisScand J Gastroenterol20023745846611989838
- MaslowskiKMVieiraATNgARegulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43Nature20094611282128619865172
- KleessenBBlautMModulation of gut mucosal biofilmsBr J Nutr200593Suppl 1S35S4015877893
- HamerHMJonkersDVenemaKVanhoutvinSTroostFJBrummerRJReview article: The role of butyrate on colonic functionAliment Pharmacol Ther20082710411917973645
- SalminenSBouleyCBoutron-RuaultMCFunctional food science and gastrointestinal physiology and functionBr J Nutr199880Suppl 1S147S1719849357
- HillDAArtisDIntestinal bacteria and the regulation of immune cell homeostasisAnnu Rev Immunol20102862366720192812
- StappenbeckTSHooperLVGordonJIDevelopmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cellsProc Natl Acad Sci U S A200299154511545512432102
- HawrelakJAMyersSPThe causes of intestinal dysbiosis: A reviewAltern Med Rev2004918019715253677
- DePGNadalIMedinaMIntestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in childrenBMC Microbiol2010106320181275
- NadalIDonatERibes-KoninckxCCalabuigMSanzYImbalance in the composition of the duodenal microbiota of children with coeliac diseaseJ Med Microbiol2007561669167418033837
- BrugmanSKlatterFAVisserJTAntibiotic treatment partially protects against type 1 diabetes in the bio-breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes?Diabetologia2006492105210816816951
- SchwartzRFNeuJSchatzDAtkinsonMAWasserfallCAntibiotic treatment partially protects against type 1 diabetes in the bio-breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes?Diabetologia20075022022117119915
- RoeschLFLorcaGLCasellaGCulture-independent identification of gut bacteria correlated with the onset of diabetes in a rat modelISME J2009353654819225551
- MacFarlaneSSteedHMacFarlaneGTIntestinal bacteria and inflammatory bowel diseaseCrit Rev Clin Lab Sci200946255419107650
- WalkerAWSandersonJDChurcherCHigh-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel diseaseBMC Microbiol201111721219646
- KangSDenmanSEMorrisonMDysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarrayInflamm Bowel Dis2010162034204220848492
- SjogrenYMJenmalmMCBottcherMFBjorkstenBSverremark-EkstromEAltered early infant gut microbiota in children developing allergy up to 5 years of ageClin Exp Allergy20093951852619220322
- WatanabeJFujiwaraRSasajimaNItoSSonoyamaKAdministration of antibiotics during infancy promoted the development of atopic dermatitis-like skin lesions in NC/Nga miceBiosci Biotechnol Biochem20107435836320139606
- GruberCvan StuijvenbergMMoscaFReduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infantsJ Allergy Clin Immunol201012679179720832848
- PendersJStobberinghEEThijsCMolecular fingerprinting of the intestinal microbiota of infants in whom atopic eczema was or was not developingClin Exp Allergy2006361602160817177684
- VaelCNelenVVerhulstSLGoossensHDesagerKNEarly intestinal Bacteroides fragilis colonisation and development of asthmaBMC Pulm Med200881918822123
- WatanabeSNarisawaYAraseSDifferences in fecal microflora between patients with atopic dermatitis and healthy control subjectsJ Allergy Clin Immunol200311158759112642841
- QuigleyEMBacterial flora in irritable bowel syndrome: Role in pathophysiology, implications for managementJ Dig Dis200782717261128
- SiJMYuYCFanYJChenSJIntestinal microecology and quality of life in irritable bowel syndrome patientsWorld J Gastroenterol2004101802180515188510
- KassinenAKrogius-KurikkaLMakivuokkoHThe fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjectsGastroenterology2007133243317631127
- TanaCUmesakiYImaokaAHandaTKanazawaMFukudoSAltered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndromeNeurogastroenterol Motil20102251251519903265
- HildebrandtMAHoffmannCSherrill-MixSAHigh-fat diet determines the composition of the murine gut microbiome independently of obesityGastroenterology20091371716172419706296
- LeyREBackhedFTurnbaughPLozuponeCAKnightRDGordonJIObesity alters gut microbial ecologyProc Natl Acad Sci U S A2005102110701107516033867
- TurnbaughPJHamadyMYatsunenkoTA core gut microbiome in obese and lean twinsNature200945748048419043404
- TurnbaughPJLeyREMahowaldMAMagriniVMardisERGordonJIAn obesity-associated gut microbiome with increased capacity for energy harvestNature20064441027103117183312
- TurnbaughPJBackhedFFultonLGordonJIDiet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiomeCell Host Microbe2008321322318407065
- KalliomakiMColladoMCSalminenSIsolauriEEarly differences in fecal microbiota composition in children may predict overweightAm J Clin Nutr20088753453818326589
- SchwiertzATarasDSchaferKMicrobiota and short-chain fatty acid in lean and overweight healthy subjectsObesity (Silver Spring)20101819019519498350
- LarsenNVogensenFKvan den BergFWGut microbiota in human adults with type 2 diabetes differs from non-diabetic adultsPLoS One20105e908520140211
- WuXMaCHanLMolecular characterisation of the faecal microbiota in patients with type II diabetesCurr Microbiol2010611697820087741
- MutluEKeshavarzianAEngenPForsythCBSikaroodiMGillevetPIntestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in ratsAlcohol Clin Exp Res2009331836184619645728
- LuppCRobertsonMLWickhamMEHost-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of EnterobacteriaceaeCell Host Microbe2007211912918005726
- HopkinsMJMacfarlaneGTChanges in predominant bacterial populations in human faeces with age and with Clostridium difficile infectionJ Med Microbiol20025144845411990498
- SnoverDCUpdate on the serrated pathway to colorectal carcinomaHum Pathol20114211020869746
- VannucciLStepankovaRKozakovaHFiserovaARossmannPTlaskalova-HogenovaHColorectal carcinogenesis in germ-free and conventionally reared rats: Different intestinal environments affect the systemic immunityInt J Oncol20083260961718292938
- KadoSUchidaKFunabashiHIntestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout miceCancer Res2001612395239811289103
- UronisJMMuhlbauerMHerfarthHHRubinasTCJonesGSJobinCModulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibilityPLoS One20094e602619551144
- WuSRheeKJAlbesianoEA human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responsesNat Med2009151016102219701202
- BalamuruganRRajendiranEGeorgeSSamuelGVRamakrishnaBSReal-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancerJ Gastroenterol Hepatol2008231298130318624900
- SobhaniITapJRoudot-ThoravalFMicrobial dysbiosis in colorectal cancer (CRC) patientsPLoS One20116e1639321297998
- OkadaHKuhnCFeilletHBachJFThe ‘hygiene hypothesis’ for autoimmune and allergic diseases: An updateClin Exp Immunol20101601920415844
- RoduitCWohlgensingerJFreiRPrenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitisJ Allergy Clin Immunol201112717918521112617
- PhipatanakulWCeledonJCRabyBAEndotoxin exposure and eczema in the first year of lifePediatrics2004114131815231902
- LikeAAGuberskiDLButlerLInfluence of environmental viral agents on frequency and tempo of diabetes mellitus in BB/Wor ratsDiabetes1991402592621899407
- WenLLeyREVolchkovPYInnate immunity and intestinal microbiota in the development of Type 1 diabetesNature20084551109111318806780
- CaniPDAmarJIglesiasMAMetabolic endotoxemia initiates obesity and insulin resistanceDiabetes2007561761177217456850
- CaniPDNeyrinckAMFavaFSelective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemiaDiabetologia2007502374238317823788
- SzaboGBalaSAlcoholic liver disease and the gut-liver axisWorld J Gastroenterol2010161321132920238398
- ThuySLadurnerRVolynetsVNonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intakeJ Nutr20081381452145518641190
- SabateJMJouetPHarnoisFHigh prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: A contributor to severe hepatic steatosisObes Surg20081837137718286348
- CaniPDBibiloniRKnaufCChanges in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in miceDiabetes2008571470148118305141
- CaniPDPossemiersSVan de WieleTChanges in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeabilityGut2009581091110319240062
- Attene-RamosMSNavaGMMuellnerMGWagnerEDPlewaMJGaskinsHRDNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cellsEnviron Mol Mutagen20105130431420120018
- HopeMEHoldGLKainREl-OmarEMSporadic colorectal cancer – role of the commensal microbiotaFEMS Microbiol Lett20052441715727814
- PayneCMCrowley-SkillicornCBernsteinCHolubecHMoyerMPBernsteinHHydrophobic bile acid-induced micronuclei formation, mitotic perturbations, and decreases in spindle checkpoint proteins: Relevance to genomic instability in colon carcinogenesisNutr Cancer20106282584020661832
- OnoueMKadoSSakaitaniYUchidaKMorotomiMSpecific species of intestinal bacteria influence the induction of aberrant crypt foci by 1,2-dimethylhydrazine in ratsCancer Lett19971131791869065820
- EllmerichSSchollerMDurantonBPromotion of intestinal carcinogenesis by Streptococcus bovisCarcinogenesis20002175375610753212
- BackhedFDingHWangTThe gut microbiota as an environmental factor that regulates fat storageProc Natl Acad Sci U S A2004101157181572315505215
- SamuelBSShaitoAMotoikeTEffects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41Proc Natl Acad Sci U S A2008105167671677218931303
- BackhedFManchesterJKSemenkovichCFGordonJIMechanisms underlying the resistance to diet-induced obesity in germ-free miceProc Natl Acad Sci U S A200710497998417210919
- DumasMEBartonRHToyeAMetabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant miceProc Natl Acad Sci U S A2006103125111251616895997
- Food and Agriculture Organization/World Health OrganizationHealth and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteriaBasel, SwitzerlandWorld Health Organization2001
- FalagasMEBetsiGIAthanasiouSProbiotics for prevention of recurrent vulvovaginal candidiasis: A reviewJ Antimicrob Chemother20065826627216790461
- MshvildadzeMNeuJProbiotics and prevention of necrotizing enterocolitisEarly Hum Dev200985S71S7419781874
- ReiffCKellyDInflammatory bowel disease, gut bacteria and probiotic therapyInt J Med Microbiol2010300253319800289
- PreidisGAVersalovicJTargeting the human microbiome with antibiotics, probiotics, and prebiotics: Gastroenterology enters the metagenomics eraGastroenterology20091362015203119462507
- PrakashSJonesMLArtificial cell therapy: New strategies for the therapeutic delivery of live bacteriaJ Biomed Biotechnol2005456
- PrakashSMartoniCToward a new generation of therapeutics: Artificial cell targeted delivery of live cells for therapyAppl Biochem Biotechnol200612812216415477
- GibsonGRRoberfroidMBDietary modulation of the human colonic microbiota: Introducing the concept of prebioticsJ Nutr1995125140114127782892
- CaniPDLecourtEDewulfEMGut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a mealAm J Clin Nutr2009901236124319776140
- StrowskiMZWiedenmannBProbiotic carbohydrates reduce intestinal permeability and inflammation in metabolic diseasesGut2009581044104519592687
- RafterJBennettMCaderniGDietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patientsAm J Clin Nutr20078548849617284748
- GillSRPopMDeboyRTMetagenomic analysis of the human distal gut microbiomeScience20063121355135916741115
- MartoniCBhathenaJJonesMLUrbanskaAMChenHPrakashSInvestigation of microencapsulated BSH active lactobacillus in the simulated human GI tractJ Biomed Biotechnol200713684
- MartoniCBhathenaJUrbanskaAMPrakashSMicroencapsulated bile salt hydrolase producing Lactobacillus reuteri for oral targeted delivery in the gastrointestinal tractAppl Microbiol Biotechnol20088122523318719901
- BhathenaJKulamarvaAUrbanskaAMMartoniCPrakashSMicroencapsulated bacterial cells can be used to produce the enzyme feruloyl esterase: Preparation and in-vitro analysisAppl Microbiol Biotechnol2007751023102917483939
- BhathenaJMartoniCKulamarvaAUrbanskaAMMalhotraMPrakashSOrally delivered microencapsulated live probiotic formulation lowers serum lipids in hypercholesterolemic hamstersJ Med Food20091231031919459731
- ChenHOuyangWJonesMHaqueTLawuyiBPrakashSIn-vitro analysis of APA microcapsules for oral delivery of live bacterial cellsJ Microencapsul20052253954716361197
- OuyangWChenHJonesMLNovel multi-layer APPPA microcapsules for oral delivery: Preparation condition, stability and permeabilityIndian J Biochem Biophys20094649149720361712
- UrbanskaAMBhathenaJMartoniCPrakashSEstimation of the potential antitumor activity of microencapsulated Lactobacillus acidophilus yogurt formulation in the attenuation of tumorigenesis in Apc(Min/+) miceDig Dis Sci20095426427318633708
- LiMWangBZhangMSymbiotic gut microbes modulate human metabolic phenotypesProc Natl Acad Sci U S A20081052117212218252821