Abstract
Hereditary angioedema (HAE) is an autosomal dominant, potentially life-threatening condition, manifesting as recurrent and self-limiting episodes of facial, laryngeal, genital, or peripheral swelling with abdominal pain secondary to intra-abdominal edema. The estimated prevalence of HAE in the general population is one individual per 50,000, with reported ranges from 1:10,000 to 1:150,000, without major sex or ethnic differences. Various treatment options for acute attacks and prophylaxis of HAE are authorized and available in the market, including plasma-derived (Berinert®, Cinryze®, and Cetor®) and recombinant (Rhucin® and Ruconest™) C1 inhibitors, kallikrein inhibitor-ecallantide (Kalbitor®), and bradykinin B2 receptor antagonist-icatibant (Firazyr®). Some of these drugs are used only to treat HAE attacks, whereas others are only approved for prophylactic therapies and all of them have improved disease outcomes due to their different mechanisms of action. Bradykinin and its binding to B2 receptor have been demonstrated to be responsible for most of the symptoms of HAE. Thus icatibant (Firazyr®), a bradykinin B2 receptor antagonist, has proven to be an effective and more targeted treatment option and has been approved for the treatment of acute attacks of HAE. Rapid and stable relief from symptoms of cutaneous, abdominal, or laryngeal HAE attacks has been demonstrated by 30 mg of icatibant in Phase III clinical trials. Self-resolving mild to moderate local site reactions after subcutaneous injection of icatibant were observed. Icatibant is a new, safe, and effective treatment for acute attacks of HAE. HAE has been reported to result in enormous humanistic burden to patients, affecting both physical and mental health, with a negative impact on education, career, and work productivity, and with substantial economic burdens. The timely and proper use of disease-specific treatments could improve patients’ quality of life, reduce the disease-specific morbidity and mortality, and, last but not least, reduce costs associated with hospitalizations and emergency room visits. Therefore, the paradigm of HAE treatment has the potential to evolve significantly, thereby exponentially improving a patient’s quality of life.
Introduction
Angioedema is a transient, intense, and most often disfiguring swelling of a localized body area involving the skin, mucosa, and subcutaneous tissues. The most commonly involved areas include the face, lips, tongue, pharynx, and supraglottic area and, uncommonly, the subglottic area.Citation1 Angioedema may also involve the hands and feet, as well as the gastrointestinal mucous membranes and the genitalia.
Hereditary angioedema (HAE) is a rare autosomal dominant disorder, characterized by recurrent attacks of angioedema resulting from a deficiency of C1 inhibitor enzyme. Historically, two types of HAE have been described.Citation1 Type 1 HAE, which is more common than type 2, is caused by the decreased production of C1-inhibitor (C1-INH), thereby resulting in decreased C1-INH activity both in tissues and in blood; however, in type 2 HAE, normal or supranormal quantities of functionally impaired C1-INH are produced. A variant type of HAE has been recently described,Citation2 in which C1-INH levels and function are normal. All three types are symptomatically indistinguishable.
The primary biological role of C1-INH is to regulate activation of the complement system, the contact system (Hageman factor and plasma kallikrein), and the intrinsic coagulation system. Thus, in HAE, due to decreasing levels of C1-INH, there is unchecked activation of complement and contact system, causing the complement levels to fall and release of increased quantities of bradykinin respectively.Citation1 Bradykinin, in turn, has been shown to be the primary mediator responsible for capillary leakage and the consequent edema in HAE.Citation3
Natural history, epidemiology, pathophysiology, and diagnosis of HAE
History
J L Milton was the first to describe HAE, in 1876,Citation4 and Quincke was the first to assign the name “angioneurotic edema” to the disease, in 1882.Citation5 Mental stress was observed to have an effect on exacerbations of the disease, thus the word “neurotic” was used as part of its name. Sir William Osler, in 1888, was the first to provide a detailed description of HAE over five generations, thus noting the hereditary component of this disease.Citation6 The biochemical basis for hereditary angioneurotic edema – the absence of C1-INH – was discovered several decades later and first published by Donaldson and Evans in 1963.Citation7 Since that study, a plethora of information regarding the genetic basis, pathophysiology, clinical manifestation, and management of HAE has been discovered and published.
Epidemiology
The estimated prevalence of HAE is 1 in 50,000, with reported ranges from 1:10,000 to 1:150,000.Citation1,Citation8,Citation9,Citation19 HAE has been reported in all races and sexes. Type 1 is estimated to occur in 80% to 85% of HAE patients and type 2 in the remaining 15% to 20%. HAE can present as a cutaneous swelling in almost three-fourths of patients and as severe abdominal attacks in approximately one-fourth.Citation1,Citation10 In one series of patients, recurrent abdominal pain and facial/upper airway edema occurred in 52% and 36%, respectively.Citation11 In 39% of these cases, patients could attribute their first episode to an identifiable traumatic event.Citation11
Episodes of laryngeal edema are the least frequent, but are the primary cause of mortality in patients with HAE because they may progress to asphyxiation. Those episodes usually evolve over a period of hours however at times can progress expeditiously.
Abdominal attacks, caused by intestinal edema, can be very debilitating. They usually manifest as dramatic abdominal spasms, and almost 80% of patients with HAE will experience a gastrointestinal attack.Citation11
The frequency of attacks in most symptomatic untreated patients ranges from weekly to less than a year. Each attack typically lasts for a few days before spontaneous resolution, thus it is estimated that the average patient with HAE has the potential to be debilitated by their symptoms for 20 to 100 days per year.Citation4
Pathophysiology and clinical manifestations
HAE results from mutations in the C1-INH gene on chromosome 11, inherited in almost three-quarters of HAE patients with autosomal dominant mode; in one-fourth of HAE patients, the mutation appears de novo. Thus the diagnosis of HAE should not be ruled out in the absence of family history.Citation1,Citation12 This mutation leads to either decreased (HAE-1) or dysfunctional (HAE-2) production of C1-INH, which is responsible for inhibition of the first complement system component; inactivation of coagulation factors XIIa, XIIf, and XIa; and direct inhibition of activated kallikrein.Citation13
HAE type 3 is no longer considered to be an X-linked disease, since it has been associated with a gain-of-function mutation in the coagulation factor XII, inherited in an autosomal dominant pattern. Other unidentified mutations are likely to exist that could affect regulation of the kinin–kallikrein system.Citation14 Furthermore, hormone replacement therapy or estrogen-containing oral contraceptives, which may influence the kinin pathway, have also been thought to be associated with this type of HAE.Citation14
Within the complement system, the biological role of C1-INH is to prevent the autoactivation of C1 by dissociating C1q subunit and binding to C1r and C1s. This binding forms an inactive C1r2-Cs2-(C1-INH)2 complex which is unable to cleave and activate complement factors C2 and C4, keeping the Classical pathway dormant.Citation1 Therefore, in HAE, with decreased or absent C1-INH, there is unchecked activation of the early complement cascade (C1, C2, and C4) even before other inhibitors (C4-binding protein and factor I) can abort the pathway, resulting in consumption of the complement factors (C4) and increased formation of anaphylatoxins (C3a, C5a) and chemotaxins (C3b), perpetuating the inflammation, local edema of skin and visceral organs, ascites, and intravascular volume depletion.Citation1
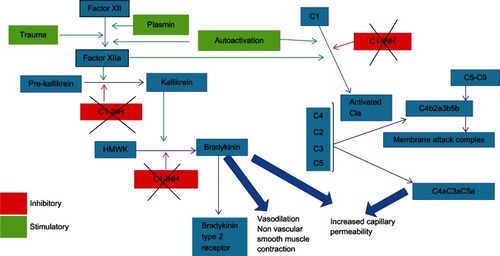
The C1-INH plays a pivotal role in inactivation of coagulation factors XIIa and its metabolite XIIf as well as causes direct inhibition of activated kallikrein. A decrease in CI-INH level and activity allows generation of significantly increased quantities of factors XIIa and XIIf. Factor XIIa activates factor XII, which in turn activates further molecules of factor XIIa. The unopposed enhancement of this positive feedback loop contributes to the significant increase in factor XIIa levels. Factor XIIa also cleaves prekallikrein to the active enzyme kallikrein, which, in turn, cleaves high-molecular-weight plasma kininogens, resulting in excessive release of bradykinin. Moreover, decreased C1-INH activity also results in loss of its direct inhibitory effect on kallikrein activity, which, as stated earlier, cleaves high-molecular-weight plasma kininogens to release bradykinin, thus further promoting bradykinin generation.Citation1 Hence, in the absence of normal production of CI-INH in HAE, there is unchecked generation of the contact system mediator, the bradykinin, which binds to Bradykinin type 2 receptors on endothelial cells, causing increased vascular permeability (edema, swelling, and ascites), vasodilation (congestion, erythema, and hypotension), and contraction of nonvascular smooth muscle (cramps, spasms, and pain) (see ).
Figure 1 In the absence of normal production of C1-INH in HAE, there is unchecked generation of the bradykinin that binds to B2 receptors on endothelial cells, causing increased vascular permeability, vasodilatation, and smooth muscle contraction.
HAE is characterized by episodes of marked, diffuse, and recurrent edema, which usually follow a typical pattern of gradual progressive swelling over the first 24 hours, followed by slow resolution of symptoms over the next 48–72 hours, although there can be a high degree of inter- and intra-individual symptom variability among HAE patients. The swelling involves all skin layers as well as layers of the walls of hollow visceral organs and solid organs. The cutaneous edema is non-pitting, non-urticarial, with ill-defined margins, and most commonly involves areas of the face, extremities, and genitals. Facial swelling occurs in approximately 80% of patients, involving lips, tongue, oropharynx, and periorbital tissues. Extremity swelling is also quite common and can emerge in an asymmetric fashion, progressing over hours or days, to affect large areas of the arms or legs. Patients can experience considerable discomfort if the swelling is moderate-to-severe in sensitive regions such as the face or urogenital areas.Citation15
The gastrointestinal tract is also commonly involved in HAE, causing bowel angioedema which, in turn, can be extremely painful and can mimic an acute abdomen as it can be unaccompanied by cutaneous symptoms. It is usually accompanied by nausea and vomiting and, less frequently, by diarrhea. This myriad of acute abdominal symptoms can be a cause of frequent emergency room visits and, at times, may lead to unnecessary surgical abdominal explorations, cholecystectomies, and appendectomies.
Laryngeal edema, although significantly less common than cutaneous or abdominal involvement, can be a primary cause of mortality in HAE due to the potential for asphyxiation secondary to upper-airway obstruction. In undiagnosed cases, mortality can be as high as 30% to 40%.Citation16,Citation17 The early presenting symptoms can be sensation of tightness or a “lump” in the throat, subtle voice changes, or dysphagia, which can potentially progress to dyspnea due to airway obstruction. Episodes of HAE-associated laryngeal edema usually evolve gradually over a period of several hours; however, more rapid progression from onset to airway obstruction cannot be ruled out.
HAE attacks are usually spontaneous without a clear triggering factor, although local tissue trauma (eg, dental and medical procedures), emotional stress, menstruation, oral contraceptive use, infections, or the use of medications such as ACE inhibitors can trigger attacks.Citation18,Citation19 Furthermore, HAE attacks are highly variable and unpredictable, which can be a cause of significant anxiety and concern among patients and caregivers.
Diagnosis
It is essential to make an accurate and an early diagnosis of HAE, to avoid encumbrance due to inappropriate medical care and to reduce the mortality associated with undiagnosed cases of HAE, respectively. A detailed history and physical examination is as vital as are the confirmatory laboratory diagnostic tests. In patients with HAE types 1 and 2, laboratory tests indicate markedly decreased C1-INH activity and C4 levels but normal C1q levels, with decreased (type 1), normal, or supranormal but dysfunctional (type 2) levels of C1-INH; however, in type 3, these levels are within normal limits. Some patients with acquired angioedema will also show a marked decrease in C1-INH activity, but with decreased C1q levels.Citation1 All tests should be carried out on a serum sample that is fresh (drawn less than 4 hours before) or freshly frozen. C4 and C1-INH protein antigen are routine laboratory tests assessed by immunochemistry, but C1-INH functional assays are specialized laboratory tests only done in reference laboratories.Citation19,Citation20 Both the chromogenic or C1s-binding enzyme-linked immunosorbent assay (ELISA) may be used for C1-INH functional assays; however, the C1s ELISA assay performance may be poor if the manufacturer’s normal range (>67%) is used instead of the higher cut-off (84%). Thus, it is necessary that the reference laboratory determines the normal range locally with receiver-operating characteristic (ROC) analysis.Citation20,Citation22
Management options
The management of HAE is directed towards treating acute attacks and/or preventing further attacks. Multiple drugs have been available for both approaches since 1970, but, due to the sparsity of controlled studies, consensus guidelines were, until recently, primarily based on observational studies and expert opinions instead of evidence-based recommendations.Citation19,Citation21,Citation22
The management guidelines for HAE have been revitalized enormously in the past decade by the emergence of three new drugs and two plasma-derived C1-INH concentrates in the market, after they underwent controlled trials. An evidence-based consensus guideline was reframed by 58 HAE expert physicians named as HAWK (Hereditary Angioedema International Working Group) in an International conference held in Gargnano del Garda, Italy, in September 2010 and published in February 2012.Citation23 The recommendations, based on high-quality double-blind, randomized, placebo-controlled trials for the treatment of acute attacks “on demand” in all patients (even if asymptomatic) with HAE owing to C1-INH deficiency, indicated that plasma-derived (Berinert®; CSL Behring, King of Prussia, PA, USA; Cinryze®; ViroPharma Incorporated, Exton, PA, USA; Cetor®; San Quin, Amsterdam, Netherlands) and recombinant C1-INH concentrates (Rhucin®in USA, Santarus, Inc, San Diego, CA, USA, /Ruconest™ in Europe; Pharming NV, Leiden, Netherlands), Kallikrein inhibitor ecallantide (Kalbitor®; Dyax Corporation, Cambridge, MA, USA), or bradykinin B2 receptor antagonist icatibant (Firazyr®; Shire Human Genetic Therapies AB, Lund, Sweden) are appropriate and efficacious.Citation24–Citation28,Citation41 Moreover, it is highly desirable that one of these medications should be made accessible to patients with HAE patients.Citation23
The HAWK group also conferredCitation23 that long-term prophylactic (LTP) treatment was appropriate for patients in whom on-demand acute treatment was not adequate; however, due to lack of evidence-based trials, there was no unanimity about the circumstances under which on-demand treatment would be deemed inadequate and LTP treatment would have a net benefit. There was a marginal consensus recommending LTP treatment if the demand treatment was inadequate only if the expert physician deemed it appropriate for that particular patient; however, many others were of the opinion that inadequate treatment be defined based on an objective criteria such as having more than 24 attacks (including mild ones) per year or more than 12 severe acute attacks per year.Citation23 For long term-prophylaxis in HAE, two classes of drugs, namely attenuated androgens and plasma-derived C1-INH concentrates, were recommended based on their proven efficacy in clinical trials and practice, with the caveat, however, that 17-alpha androgens should be given neither to patients under 16 years of age nor to pregnant or lactating women.Citation23 As far as antifibrinolytic therapy for LTP is concerned, they have been traditionally limited to LTP of patients under 16 years of age in whom other prophylactic agents especially androgens could not be used.Citation21–Citation23 Moreover, there have been no trials confirming the efficacy in the general patient population, thus this class of medications was not discussed.Citation23,Citation29
The HAWK group did not recommend any changes made to the already established consensus guidelines regarding short-term prophylaxis in patients with HAE because of the unavailability of further studies.Citation23 Prophylactic administration of fresh frozen plasma, C1-INH concentrate, or oral 17-α alkylated androgens before any major surgical or dental procedure is necessary to prevent an acute episode of HAE.Citation22,Citation30
Severe upper airway compromise from laryngeal edema necessitates intubation and ventilator support to establish an airway until the resolution of an acute attack. Typically, HAE does not respond to administration of antihistamines, glucocorticoids, or epinephrine.
Icatibant and its role in the management of HAE
Bradykinin, the key element responsible for manifestations of HAE, mediates its effects through a bradykinin B2 receptor, which is constitutively expressed by vascular endothelial and smooth muscle cells. The binding of bradykinin to the B2 receptor on the vascular endothelium results in the generation of various inflammatory mediators, including nitric oxide, prostacyclin, and endothelium-derived hyperpolarizing factor.Citation31 It has been shown that local bradykinin concentrations are elevated in patients during an HAE attack.Citation32,Citation33 Icatibant has demonstrated the reversal of increased vascular permeability in C1-INH-knockout mice. It was also established that mice deficient in the bradykinin B2 receptor as well as C1-INH exhibited lesser vascular permeability compared with the mice that were only deficient in C1-INH.Citation34 Therefore, the inhibition of receptor binding of bradykinin represents a feasible approach to treating acute attacks of HAE. This has been accomplished by icatibant, a selective and potent bradykinin B2 receptor antagonist that provides a targeted as well as more specific treatment than other currently available treatment options, including replacement therapy with C1-INH, kallikrein inhibitors, or attenuated androgens.Citation35
Currently, icatibant injection (Firazyr®) is licensed in 37 countries, and approval was also granted for its self-administration in the EU and US in 2011.Citation23,Citation40
Pharmacology, efficacy, and safety/tolerability of icatibant
Pharmacology
Chemistry
Icatibant is a synthetic decapeptide (H-D-Arg-Arg-Pro-Hyp-Gly-Thi-Ser-D-Tic-Oic-Arg-OH) with a similar structure to bradykinin except with five non-proteinogenic amino acids.Citation36 Icatibant has a relatively long shelf life; it retains its stability for up to 6 months at 25°C and up to 24 months at 5°C. Icatibant injections are supplied as 3 mL pre-filled syringes for subcutaneous use.
Preclinical pharmacological assays
Icatibant has been tested in several in vitro and in vivo pharmacological assays. It was shown to be a highly active bradykinin 2 receptor antagonist by displaying high-affinity binding in crude membrane preparations of guinea pig ileum.Citation36 Compared to other bradykinin antagonists, icatibant was found to be 70 times more potent in smooth muscle preparations.Citation36 The drug further established its selectivity to bradykinin 2 receptors by demonstrating inactivity in isolated rabbit aorta, which contains the bradykinin 1 receptor type.Citation36
Wirth et al tested the potency, duration of action, and tolerability of icatibant in different in vivo models and compared it with the well-known bradykinin antagonist D-Arg-[Hyp2, Thi5,8, D-Phe7] bradykinin.Citation37 It established itself as highly potent and long-acting inhibitor of bradykinin-induced hypotensive responses in rats. After 4 hours of subcutaneous administration of 20 nmol kg−1 of icatibant, significant inhibition was demonstrated by icatibant as compared to D-Arg-[Hyp2, Thi5,8, D-Phe7] bradykinin. Furthermore, it strongly inhibited the bradykinin-induced bronchoconstriction in guinea pigs, which confirmed the findings obtained in the blood pressure experiments in rats.Citation37 At intravenous doses between 0.1 and 1 mg kg−1 of icatibant, carrageenin-induced inflammatory edema of the rat paw was considerably inhibited. Moreover, it demonstrated a very good tolerability in conscious dogs at intravenous doses of 0.01 and 0.1 mg kg−1.37
Pharmacokinetics
Icatibant is a highly selective competitive antagonist of the bradykinin B2 receptor with a similar B2 receptor affinity to that of bradykinin, although 100-fold lower for B1 receptor. At higher concentrations (>3.2 mg/kg), agonist activity has been reported, which could explain the local injection-site reactions.Citation38
Icatibant has an excellent bioavailability profile with low protein binding and a large distribution volume. Absorption from the local injection site is rapid, reaching its peak concentration in 20 to 30 minutes.Citation39 Intravenous and subcutaneous formulations were found to have a similar pharmacokinetic profile; however, the subcutaneous route was considered more convenient. Icatibant has demonstrated a poor distribution in adipose tissue and does not appear to cross the blood–brain barrier; however, it crosses the placenta and is excreted in breast milk in rats.Citation40
Icatibant is metabolized in the liver and the cleavage products (90% metabolites and 10% intact icatibant) are excreted in the urine.Citation39 The elimination half-life is 1.2 to 1.5 hours. Liver and renal dysfunction do not affect the pharmacokinetics, nor do weight and sex;Citation39 however, the clearance of icatibant is reduced in the elderly.Citation40
Pharmacodynamics
In Phase III clinical trials, doses of intravenous 0.4 mg/kg icatibant were given as a single dose and subcutaneous doses of 30–45 mg up to three times/day with 6 hours between the doses, which was based on the pharmacological data from the Phase II studies.Citation40–Citation42 From these data, the optimum calculated subcutaneous dose of 30 mg given up to three times daily was proposed in accordance to the half-life of icatibant and the margin up to the dose levels where agonist reactions may occur.Citation40 Furthermore, if indicated, administration of a second or third 30 mg dose is favored over a higher single dose, because the duration of action of icatibant is not dose dependent.Citation40
At supratherapeutic doses of 3.2 mg/kg or higher, partial bradykinin agonist activity was demonstrated in animal studies, which could explain the local injection-site reactions.Citation40
Side effects
Mild-to-moderate local injection-site reactions to icatibant have been reported by patients in Phase III clinical studies.Citation41,Citation42 Partial agonist activity is implicated as the reason for those local transient injection-site reactions.Citation41,Citation42 Based on all Phase III trials as well as the data collected from the cumulative exposures of icatibant in the post-marketing setting, the majority of the adverse events reported have appeared to be related to the HAE itself.Citation40–Citation43 The adverse event rate was found to be similar in icatibant and the placebo group.Citation41–Citation43 Most commonly reported adverse events were recurrent or worsening angioedema.
Bradykinin is thought to play a cardioprotective role, thus icatibant could potentially impair cardiac function.Citation44 This has been demonstrated in animal studies, therefore it is recommended not to use icatibant in patients with acute cardiac or brain ischemia. Bradykinin antagonism could theoretically increase blood pressure; however, intermittent use of icatibant is not considered to be a long-term risk factor for hypertension because of its short duration of action. The concept of bradykinin antagonism could also translate into a decreased antihypertensive effect of ACE inhibitors.Citation45 It is noteworthy that clinical trials so far have excluded patients on ACE inhibitors.
Special patient populations
To the authors’ knowledge, there are, as yet, no available studies on icatibant use in pregnant or lactating mothers; however, a study involving a pediatric patient population is in progress, which will be investigating the pharmacokinetics, tolerability, and safety of icatibant in children with HAE.Citation46
Icatibant has demonstrated a satisfactory tolerance in a trial for resistant ascites in liver cirrhosis, wherein patients with severe liver and renal impairment were included.Citation40
Efficacy, safety, and tolerability
Based on data from recently conducted high-quality studies.Citation35,Citation41,Citation42 icatibant has been deemed clinically efficacious for the treatment of acute HAE in the latest international consensus guidelines.Citation23 In a Phase II, open-label, uncontrolled pilot study, icatibant was administered either as a single subcutaneous (30 or 45 mg) or intravenous (0.4 mg/kg over a period of 30 minutes or 2 hours or 0.8 mg/kg over a period of 30 minutes) injection to 15 patients who had experienced 20 attacks.Citation35 The median time to onset of symptom relief was 1.50, 1.42, and 1.13 hours in the intravenous groups and 0.58 and 0.45 hours in the subcutaneous groups, respectively. In contrast to the untreated attacks, icatibant treatment reduced the mean time to onset of symptom relief by 97%. There were no differences between the 30 mg and 45 mg doses. Median bradykinin concentration was elevated to 48.5 pmol/L (seven times normal) during acute attacks, which, after 4 hours of icatibant administration, decreased to 18.0 pmol/L. This is the first report validating the clinical efficacy of icatibant for cutaneous and abdominal attacks in HAE.Citation35
Three Phase III controlled, double-blind, randomized multicenter studies (For Angioedema Subcutaneous Treatment [FAST] trials) have been done to investigate the clinical efficacy and safety of icatibant in the treatment of acute HAE attacks.Citation41,Citation42 In all three similarly designed studies (FAST-1, -2 and -3), the inclusion criteria were adults above the age of 18 who had a documented diagnosis of HAE type 1 or type 2. The exclusion criteria included a diagnosis other than HAE type 1 or type 2, concomitant serious illness, pregnancy, and lactating mothers. The FAST-3 trial included additional exclusion criteria, including treatment with angiotensin-converting enzyme inhibitors or any pain medications and previous treatment with icatibant. Furthermore, in both the FAST-1 and -2 trials, patients with potentially life- threatening laryngeal angioedema were treated with open-label icatibant. Additionally, any patient who had a subsequent severe attack in the course of the study, necessitating treatment was included in an open-label extension phase. In FAST-3, patients with mild laryngeal attack were included, but patients with severe laryngeal symptoms were treated in an open-label study. In all three trials, the treatment was administered no later than 6 hours after an acute attack became moderate in severity, or mild for laryngeal attacks in FAST-3. Moreover, in all three trials, response to therapy was measured by visual analog scale as well as the patient/physician-reported symptoms.Citation41,Citation42
In the FAST-1 trialCitation41, 56 patients were randomized to receive either icatibant (n = 27) or placebo (n = 29), whereas in the FAST-2 trial, 74 patients were randomized to receive either icatibant (n = 36) or tranexamic acid (n = 38). A total of eleven patients had laryngeal symptoms and received open-label icatibant in the FAST-1 and -2 trials. Rescue therapy was permitted, but withheld until 8 or 9 hours after study-drug administration.Citation41
The primary endpoint was the time to onset of significant relief of index symptoms (cutaneous swelling, cutaneous pain, and abdominal pain) defined by sustained improvement of at least 30% on the visual analog scale for three consecutive measurements. Secondary endpoints included response rate at 4 hours after injection, time to onset of improvement of the index symptom according to the patient and the investigator, time to relief of all symptoms, global assessment and overall patient improvement, and a patient satisfaction questionnaire at week 24.Citation41
The primary endpoint was reached in 2.5 hours with icatibant as compared with 4.6 hours with the placebo (P = 0.14) and in 2 hours as compared with 12 hours with tranexamic acid in the FAST-2 study (P < 0.001). Thus, a statistically significant benefit of icatibant, with regard to the primary endpoint as compared to tranexamic acid, was found in the FAST-2 trial, which was not seen in the placebo-controlled FAST-1 trial. For the secondary endpoints, improvement was significant in FAST-2, whereas a nonsignificant trend to improvement was seen in FAST-1. After post hoc analysis, early use of rescue medications was identified as a reason for obscuring the benefit of icatibant in FAST-1. There were no reports of icatibant-associated serious adverse events.Citation41
In the FAST-3 trial,Citation42 subjects with moderate-to-severe cutaneous or abdominal symptoms received icatibant (n = 43) or placebo (n = 45). Five subjects with laryngeal (mild-to-moderate) first attacks received icatibant (n = 3) or placebo (n = 2) and five subjects with severe laryngeal first attacks received open-label icatibant. For cutaneous or abdominal attacks, icatibant, in comparison to placebo, significantly reduced time to 50% reduction in symptom severity (2.0 vs 19.8 hours; P < 0.001, primary endpoint), onset of primary symptom relief (1.5 vs 18.5 hours; P < 0.001, key secondary endpoint), or almost-complete symptom relief (8.0 vs 36.0 hours; P = 0.012) and demonstrated a shorter time to initial symptom relief (0.8 vs 3.5 hours; P < 0.001). For laryngeal attacks, median time to 50% or more reduction in symptom severity was 2.5 hours with icatibant versus 3.2 hours with placebo. No icatibant-treated subject required rescue medication before symptom relief occurred. The incidence of adverse events was similar in icatibant- and placebo-treated subjects (41% and 52%, respectively). All icatibant-treated subjects experienced injection-site reactions, but none reported clinically relevant changes in safety parameters or serious adverse events.Citation42
This study achieved its primary endpoint (statistically significant shorter time to symptom relief) as well as the secondary endpoint (statistically significant shorter time to onset of primary symptom relief). The efficacy results were similar for laryngeal and cutaneous/abdominal attacks. The incidence of adverse events was similar in both the treatment and placebo group. There were no reports of serious adverse events or any clinically significant alterations in safety parameters, except transient local injection-site reactions with icatibant. Overall, this study reaffirmed the efficacy of subcutaneously administered icatibant in the treatment of acute attacks of type 1 and type 2 HAE. Furthermore, the rescue medications were not required before the onset of symptoms in the treatment group, thus establishing the statistical significance that could not be achieved in the FAST-1 trial.Citation42
Laryngeal attacks
Eight patients in FAST-1 and three patients in FAST-2 received open-label icatibant for laryngeal attacks and the time to first symptom improvement according to the patient was 0.6 hours and 1.0 hour, respectively. At 4 hours after icatibant administration, nine of the eleven patients were symptom free and the remaining two had mild symptoms.Citation41
In the FAST-3 trial, after post hoc analysis, a total of 21 subjects with 21 first laryngeal attacks had received icatibant, including three in the laryngeal attack arm randomized to the double-blind treatment with icatibant and 18 subjects who received open-label icatibant (two in the laryngeal attack arm initially randomized to placebo but who eventually received icatibant, five with severe laryngeal attacks, and eleven from the abdominal and/or cutaneous attack arm treated with open-label extension phase). The median time to 50% or more reduction in symptom severity was 2.5 hours with icatibant versus 3.2 hours with placebo. Time to first symptom improvement was similar for laryngeal attacks in the icatibant population and the icatibant group for the non-laryngeal population in the FAST-1, -2, and -3 trials.Citation42
Rescue medication
A larger proportion of the placebo patients in FAST-1 needed rescue medications than in FAST-3, which was suggestive of inferior symptom control in the comparator arm, explaining the lack of significance in the FAST-1 trial and supporting the clinical efficacy of icatibant in FAST-3. In FAST-1, post hoc analysis demonstrated that the primary endpoint adjusted for rescue medication was 2.5 hours with icatibant and 9 hours with the placebo (P = 0.02).Citation41,Citation42
Self-administration
Since acute attacks of HAE are unpredictable and can be potentially life-threatening if appropriate treatment is not timely, self-administration of medications at home at the onset of the attack could reduce the mortality associated with this disease. This was recommended at the consensus report of the HAWK Group at the international conference,Citation23 but with a low level of evidence because it is based on observational studies. Nevertheless, a larger prospective, open-label Phase III-b (Evaluation of the Safety of Self-administration with Icatibant [EASSI]) study has recently been conducted to evaluate the safety of self-administration injections. In this study, 56 patients were evaluated for safety and efficacy of self-administering icatibant during an acute attack. If needed, health care professionals administered second and/or third doses. Overall, the study demonstrated that self-administration of icatibant for an acute attack of HAE was generally well tolerated and safe. The times to symptom relief were consistent with the Phase III studies and were similar whether self-administered or administered by a health care professional.Citation43
Patient-focused perspectives such as quality of life, patient satisfaction, and acceptability
Quality of life
The debilitating nature of HAE has a life-altering impact on health-related quality of life. However, little is known about the humanistic and economic burden of HAE on patients, caregivers, and health care systems because of the low incidence of the disease.Citation47 Lumry et al published the first comprehensive study to look at the quality of life of patients with HAE and assess its humanistic burden.Citation48 It is a web-based survey of patients that entailed collecting information on attack characterization, treatment, side effects, pain, and functional and emotional burden of disease management. A significantly poorer health-related quality of life versus population norms was reported by the patients. HAE patients also demonstrated a higher incidence of depression. A 34% overall work impairment was seen, thus impacting productivity. Workers lost a mean of 3.3 days and students a mean of 1.9 days due to their most recent HAE attack. Thus, it was concluded that HAE results in enormous humanistic burden to patients, affecting both physical and mental health; negative impact on education, career, and work productivity; and substantial economic burdens.Citation48
Both acute attacks and the chronic nature of the disease contribute to the substantial economic costs associated with this disease. Before the approval of C1-INH therapy and icatibant in the US, Wilson et al conducted a survey that demonstrated the economic burden associated with HAE.Citation49 They estimated the total annual per-patient costs at US$42,000 for the average HAE patient. Furthermore, costs increased considerably with increasing attack severity: US$14,000 for patients with mild attacks and up to US$96,000 for patients with severe attacks. High rates of work absence, lower productivity, and lower income were also reported, which in turn contributed to indirect annual costs of US$16,000 for the average patient.Citation49
Patient satisfaction and acceptability
Patient satisfaction with health care professional-administered icatibant and their level of interest in self-administration was assessed as part of the open-label extension phases of the FAST-1 and -2 clinical trials in adults by use of a short questionnaire completed by the patients at the 24-week follow-up visit. A total of 94 patients (74.6%; 53 patients in FAST-1 and 41 patients in FAST-2) responded to the questionnaire.Citation50
Overall, favorable experiences following treatment with health care professional-administered icatibant were reported by subjects in the FAST-1 and FAST-2 open-label extension phases who responded to the questionnaire. In comparison with their usual therapies for HAE attacks, most patients were more or much more satisfied with the way icatibant relieved their symptoms and were more likely to continue using icatibant upon completion of the study. Furthermore, self-administration of icatibant at home was preferred by the majority of patients.Citation50
Conclusion and place in therapy
HAE can be a disfiguring and debilitating illness, thus accurate and timely diagnosis is crucial to decrease the morbidity and mortality associated with it. Over the last few years, there has been a renaissance in the pharmacologic treatment options for HAE, prompted by an improved understanding of pathophysiologic processes. This advent of innovative treatment options has made a positive impact on physical, mental, and social health domains. According to the recently revised international HAWK group consensus guidelinesCitation23 for HAE treatment, C1-INH concentrates (plasma-derived [Berinert®, Cinryze®, and Cetor®] and recombinant (Rhucin® and Ruconest™)), kallikrein inhibitor ecallantide (Kalbitor®), and bradykinin B2 receptor antagonist icatibant (Firazyr®) have been deemed suitable for the acute treatment of HAE; however, no head-to-head trials between the five licensed therapies have been conducted to date. Attenuated androgens and plasma-derived C1-INH concentrates were conferred as options for prophylaxis for HAE attacks.
Multiple double-blind Phase III studies and repeated open-label administrations have demonstrated the efficacy and safety of icatibant for the treatment of acute attacks of HAE with considerable reproducibility and consistency, thereby establishing robust evidence for the utility of icatibant in the treatment of acute HAE attacks in adults. The international HAWK group consensus guidelinesCitation23 recommend icatibant as one of the prime options for the acute treatment of HAE. Furthermore, it was emphasized that all patients should carry two doses of a specific treatment and that all patients should be trained for self-administration since the attacks should be treated as soon as they are recognized.
Icatibant is the foremost and the only subcutaneous treatment option for all acute HAE attacks that is licensed for self-administration, therefore, it offers increased patient independence and early effective treatment. It has the advantage of being readily available, offering timely access to effective treatment and thus positively impacting the quality of life of patients affected by HAE and will reduce the need for repeated attendances in emergency departments.Citation22,Citation23,Citation51 The other drug that is approved for patient self-administration in the US (to treat acute facial and abdominal attacks) and EU (to treat all acute attacks) is Berinert®. The route of administration, however, is only by intravenous infusion after proper training by a health care professional which can delay the initiation of treatment and requires practice and drive on the patient’s part. Ecallantide, on the other hand, is only approved for administration in a supervised setting due to the risk of hypersensitivity reactions.Citation52 Moreover, icatibant is less expensive than ecallantide and has an equivalent cost profile to that of the C1-INH Berinert®.Citation53 It is important to conduct head-to-head comparisons of recently approved alternatives for treatment of acute attacks, and more cost-effectiveness studies are also required. Of note, Blankart et al analyzed the availability of and access to orphan drugs, which included icatibant, ecallantide, and two complement C1-INHs, and showed substantial copayments can represent important barriers to patient access to treatment.Citation54 Pricing and reimbursement of orphan drugs have been issues of significant concern. Simoens conducted a literature review to gain more understanding of those issues and established that there is a need for a transparent and evidence-based approach towards orphan drug pricing and reimbursement.Citation55
Recently, a study has been published demonstrating the beneficial effect of early on-demand icatibant treatment of HAE attacks.Citation56 This study could very well strengthen the earlier weak recommendation of the HAWK consensus to treat HAE type 1 and 2 attacks early, regardless of the location and ideally before visible or disabling symptoms occur.Citation23
Other than HAE type 1 and 2, there are other types of bradykinin-mediated angioedema, including HAE type 3, acquired angioedema associated with C1-INH deficiency, and angioedema associated with angiotensin-converting enzyme inhibitors,Citation57,Citation58 wherein icatibant has therapeutic potential given its mechanism of action.Citation59 A number of case reports have been published.Citation60–Citation65 The efficacy of icatibant with acute angiotensin-converting enzyme inhibitor angioedema has been reported by Bas et al in a case series of eight patients.Citation66 After icatibant injection, first symptom improvement was reported at a mean time of 50.6 ± 21 minutes and complete relief of symptoms at 4.4 ± 0.8 hours.Citation66 A randomized study to evaluate the effectiveness of icatibant in patients with angiotensin-converting enzyme inhibitor-associated angioedema is in progress in the US.Citation67 Prospective studies using icatibant in patients with other types of angioedema are much needed.
Disclosure
The authors report no conflicts of interest in this work.
References
- NzeakoUCFrigasETremaineWJHereditary angioedema: a broad review for cliniciansArch Intern Med2001161202417242911700154
- BorkKBarnstedtSEKochPHereditary angioedema with normal C1-inhibitor activity in womenLancet200035621321710963200
- DavisAE3rdThe pathophysiology of hereditary angioedemaClin Immunol200511413915596403
- CicardiMAgostoniAHereditary angioedemaN Engl J Med1996334166616678628365
- QuinckeHConcerning the acute localized edema of the skinMonatsh Prakt Derm18821129131 German
- OslerWHereditary angioneurotic edemaAm J Med Sci1888954362367
- DonaldsonVHEvansRRA biochemical abnormality in hereditary angioneurotic edema: absence of serum inhibitor of C’ 1-esteraseAm J Med196335374414046003
- DreskinSUrticaria and angioedemaGoldmanLSchaferAICecil Medicine24th edPhiladelphia, PASaunders Elsevier2011 chap 260
- TalaveraALarraonaJLRamosJLHereditary angioedema: an infrequent cause of abdominal pain with ascitesAm J Gastroenterol1995904714747872288
- AgostoniAAygören-PürsünEBinkleyKEHereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyondJ Allergy Clin Immunol2004114Suppl 3S51S13115356535
- FrankMMGelfandJAAtkinsonJPHereditary angioedema: the clinical syndrome and its managementAnn Intern Med1976845805931275365
- PappalardoECicardiMDuponchelCFrequent de novo mutations and exon deletions in the C1 inhibitor gene of patients with angioedemaJ Allergy Clin Immunol200010661147115411112899
- DavisAE3rdC1 inhibitor and hereditary angioneurotic edemaAnnu Rev Immunol198865956283289579
- BorkKHereditary angioedema with normal C1 inhibitor activity including hereditary angioedema with coagulation factor XII gene mutationsImmunol Allergy Clin North Am20062670972417085286
- BorkKMengGStaubachPHardtJHereditary angioedema: new findings concerning symptoms, affected organs and courseAm J Med200611926727416490473
- SofaSCasaliABolondiLSonographic findings in abdominal hereditary angioedemaJ Clin Ultrasound19992753754010525217
- BorkKSiedleckiKBoschSSchopfREKreuzWAsphyxiation by laryngeal edema in patients with hereditary angioedemaMayo Clin Proc20007534935410761488
- BorumMLHowardDEHereditary angioedema: complex symptoms can make diagnosis difficultPostgrad Med1998103251255256
- GompelsMMLockRJAbinunMC1 inhibitor deficiency: consensus documentClin Exp Immunol2005139337939415730382
- Wagenaar-BosIGDrouetCAygören-PursunEFunctional C1-Inhibitor diagnostics in hereditary angioedema: Assay evaluation and recommendationsJ Immunol Methods2008338142018655790
- BowenTCicardiMFarkasHCanadian 2003 International Consensus Algorithm For the Diagnosis, Therapy, and Management of Hereditary AngioedemaJ Allergy Clin Immunol2004114362963715356569
- BowenTCicardiMFarkasH2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedemaAllergy Asthma Clin Immunol2010612420667127
- CicardiMBorkKCaballeroTHAWK (Hereditary Angioedema International Working Group)Evidence-based recommendations for the therapeutic management of angioedema owing to the hereditary C1 inhibitor deficiency: consensus report of an International Working GroupAllergy201267214715722126399
- ZurawBLBussePJWhiteMNanofiltered C1 inhibitor concentrate for treatment of hereditary angioedemaN Engl J Med2010363651352220818886
- ZurawBCicardiMLevyRJRecombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedemaJ Allergy Clin Immunol20101264821827e1420920772
- CicardiMLevyRJMcNeilDLEcallantide for the treatment of acute attacks in hereditary angioedemaN Engl J Med201036352353120818887
- LevyRJLumryWRMcNeilDLEDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedemaAnn Allergy Asthma Immunol2010104652352920568386
- CraigTJLevyRJWassermanRLEfficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacksJ Allergy Clin Immunol2009124480180819767078
- AgostoniACicardiMHereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patientsMedicine (Baltimore)19927142062151518394
- LonghurstHJFarkasHCraigTHAE international home therapy consensus documentAllergy Asthma Clin Immunol2010612220667125
- CugnoMZanichelliAFoieniFCacciaSCicardiMC1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progressTrends Mol Med200915697819162547
- CugnoMNussbergerJCicardiMAgostoniABradykinin and the pathophysiology of angioedemaInt Immunopharmacol20033331131712639808
- NussbergerJCugnoMCicardiMBradykinin-mediated angioedemaN Engl J Med2002347862162212192030
- HanEDMacFarlaneRCMulliganANScafidiJDavisAE3rdIncreased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptorJ Clin Invest200210981057106311956243
- BorkKFrankJGrundtBSchlattmannPNussbergerJKreuzWTreatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (Icatibant)J Allergy Clin Immunol200711961497150317418383
- HockFJWirthKAlbusUHoe 140 a new potent and long acting bradykinin-antagonist: in vitro studiesBr J Pharmacol199110237697731364851
- WirthKHockFJAlbusUHoe 140 a new potent and long acting bradykinin-antagonist: in vivo studiesBr J Pharmacol199110237747771364852
- FélétouMMartinCAMolimardMIn vitro effects of HOE 140 in human bronchial and vascular tissueEur J Pharmacol19952741–357647768281
- DeeksEDIcatibantDrugs2010701738120030426
- Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Evaluation Agency CHMP assessment report for Firazyr®11172008 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Public_assessment_report/human/000899/WC500022970.pdfAccessed April 5, 2013
- CicardiMBanerjiABrachoFIcatibant, a new Bradykinin-receptor antagonist, in hereditary angioedemaN Eng J Med2010363532541
- LumryWRLiHHLevyRJRandomized placebo-controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trialAnn Allergy Asthma Immunol2011107652953722123383
- Advisory Committee Meeting Briefing Package. Firazyr® (Icatibant)Cambridge, MAShire Human Genetic Therapies, Inc2011 Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugs/AdvisoryCommittee/UCM260022.pdfAccessed April 4, 2013
- SharmaJNCardiovascular activities of the bradykinin systemScientificWorldJournal2008838439318454246
- SharmaJNHypertension and the bradykinin systemCurr Hypertens Rep200911317818119442326
- Shire Human Genetic Therapies, IncA Pharmacokinetic, Tolerability and Safety Study of Icatibant in Children and Adolescents With Hereditary Angioedema Clinical Trials gov. Last verified : February 2013. Available from http://clinicaltrials.gov/show/NCT01386658. NLM identifier: NCT01386658Accessed April 9, 2013
- ToscaniMRiedlMMeeting the challenges and burdens associated with hereditary angioedemaManag Care2011209445121991862
- LumryWRCastaldoAJVernonMKBlausteinMBWilsonDAHornPTThe humanistic burden of hereditary angioedema: Impact on health-related quality of life, productivity, and depressionAllergy Asthma Proc201031540741420929608
- WilsonDABorkKSheaEPRentzAMBlausteinMBPullmanWEEconomic costs associated with acute attacks and long-term management of hereditary angioedemaAnn Allergy Asthma Immunol2010104431432020408341
- AlejandroMalbránWernerAbererWilliamLumryPatient Satisfaction with Icatibant During Open-Label Treatment of Hereditary Angioedema Attacks in the FAST-1 and FAST-2 Phase III TrialsPoster presented at EAACI 2011Istanbul, Turkey Available from: http://www.postersessiononline.com/173580348_eu/congresos/30eaaci/aula/poster_42561.pdfAccessed April 9, 2013
- BowenTHereditary angioedema: beyond international consensus – circa December 2010. The Canadian Society of Allergy and Clinical Immunology Dr David McCourtie LectureAllergy Asthma Clin Immunol201171121310025
- FloccardBHautinEBouilletLCoppereBAllaouchicheBAn evidence-based review of the potential role of icatibant in the treatment of acute attacks in hereditary angioedema type I and IICore Evid2012710511423055948
- Aygören-PürsünEMartinez-SaguerIRusickeEKlingebielTKreuzWOn demand treatment and home therapy of hereditary angioedema in Germany – the Frankfurt experienceAllergy Asthma Clin Immunol2010612120667124
- BlankartCRStargardtTSchreyoggJAvailability of and access to orphan drugs: an international comparison of pharmaceutical treatments for pulmonary arterial hypertension, Fabry disease, hereditary angioedema and chronic myeloid leukemiaPharmacoeconomics2011291638221073206
- SimoensSPricing and reimbursement of orphan drugs: the need for more transparencyOrphanet J Rare Dis201164221682893
- MaurerMAbererWBouilletLHereditary angioedema attacks resolve faster and are shorter after early icatibant treatmentPLoS One201382e5377323390491
- ZurawBLClinical practice. Hereditary angioedemaN Engl J Med2008359101027103618768946
- LonghurstHCicardiMHereditary angioedemaLancet2012379981447448122305226
- BouilletLIcatibant in hereditary angioedema: news and challengesExpert Rev Clin Immunol20117326727221595592
- BrightPDempsterJLonghurstHSuccessful treatment of acquired C1 inhibitor deficiency with icatibantClin Exp Dermatol201035555355420337653
- BouilletLHereditary angioedema in womenAllergy Asthma Clin Immunol2010611720667120
- BouilletLBoccon-GibodIPonardDBradykinin receptor 2 antagonist (icatibant) for hereditary angioedema type III attacksAnn Allergy Asthma Immunol2009103544819927548
- WellerKMagerlMMaurerMSuccessful treatment of an acute attack of acquired angioedema with the bradykinin-B2-receptor antagonist icatibantJ Eur Acad Dermatol Venereol201125111912020477917
- GallitelliMAlzettaMIcatibant: a novel approach to the treatment of angioedema related to the use of angiotensin-converting enzyme inhibitorsAm J Emerg Med20123081664e1e222100478
- GuichonCFloccardBCoppéréBOne hypovolaemic shock… two kinin pathway abnormalitiesIntensive Care Med20113771227122821484080
- BasMGreveJStelterKTherapeutic efficacy of icatibant in angioedema induced by angiotensin-converting enzyme inhibitors: a case seriesAnn Emerg Med201056327828220447725
- Vanderbilt UniversityEffect of Bradykinin Receptor Antagonism on ACE Inhibitor-associated AngioedemaClinical Trials gov [website on the Internet]Bethesda, MDUS National Library of Medicine122012 Available from: http://clinicaltrials.gov/ct2/show/NCT01574248. NLM identifier: NCT01574248Accessed April 4, 2013