Abstract
Actinic keratoses (AKs), especially on areas of the face, have a negative impact on a patient’s quality of life (QoL). These lesions manifest on sun-damaged skin and have the potential to progress to squamous cell carcinoma. Field-directed therapy alone and in combination with lesion-directed treatment is effective in clearing both visible and nonvisible AK lesions. Topical treatments of AKs thus have the potential to improve a patient’s well-being. However, evidence demonstrating improvements in patient QoL is limited, and is mostly based on observational or retrospective studies. Some prospective studies have reported unchanged or even worsening QoL despite excellent treatment outcomes. Our prospective, pilot study demonstrated a significant increase in QoL in 28 subjects with AKs of the face treated with ingenol mebutate gel 0.015%. QoL was assessed at days 0 and 60 using the Skindex-16 survey. Mean overall scores improved from 24.5% at baseline to 15.5% at day 60 (P=0.031). Improvements in QoL were consistent with an 80% reduction in AK lesion number at day 60. These improved QoL findings are in line with those from a recent retrospective study using ingenol mebutate 0.015% gel. This study therefore further demonstrates the potential for field therapy to improve both treatment outcomes and patient satisfaction.
Introduction
Actinic keratosis (AK) results primarily from the damaging effects of long-term ultraviolet radiation on the skin.Citation1–Citation3 Accordingly, the majority of lesions appear on the head, neck, and upper limbs.Citation4,Citation5 Considered a field disease, AK typically involves the presence of subclinical and therefore nonvisible lesions within an area of damaged skin, in close proximity to the clinically detectable AK lesion.Citation6,Citation7 In the United States, clinically apparent lesions are generally treated individually with cryotherapy.Citation8 However, topical therapies can potentially treat an entire field of damaged skin, including not only the visible lesions but also the subclinical lesions as well. Recommended topical therapies for field-directed treatment of AKs include diclofenac gel, 5-fluorouracil (5-FU) cream, imiquimod cream, and ingenol mebutate gel.Citation9 Photodynamic therapy with red, blue, or daylight illumination can also be used as a field-directed or lesion-directed therapeutic approach for AK.Citation10
One difficulty in assessing AK treatment success is that the criteria for success in dermatologic disease are not easily quantifiable. Factors such as the patient’s quality of life (QoL) as well as the clinical outcome determine treatment success.Citation11 In dermatologic conditions, variables that impact QoL are often highly context dependent, such as the location of the damaged skin or the age and sex of the patient.Citation12–Citation14 In addition, characteristics of the treatment regimen itself, such as its length, complexity, or association with adverse events, are likely to affect patient QoL. Taken together, these factors make it difficult to predict how a particular treatment regimen might affect a patient’s QoL, even with demonstration of clinical improvement.
As such, QoL may be based more on a balance between treatment tolerance and treatment outcomes. Some prior studies have demonstrated unchanged or even decreased QoL despite excellent treatment outcomes.Citation15,Citation16 Recently, a post hoc, exploratory analysis of AK subjects enrolled in Phase 3 clinical trials involving ingenol mebutate gel found that this therapy was associated with significant improvements in QoL.Citation17 Here, we report the results of QoL assessments from a prospective clinical study in subjects with AKs of the face who were treated with ingenol mebutate gel 0.015% for three consecutive days.
Methods
This study was part of a clinical trial that examined the effects of ingenol mebutate gel 0.015% applied once daily for three consecutive days to 28 subjects with AKs of the face. Treatment effects were assessed by clinical assessment, noninvasive imaging, and a QoL questionnaire. Subjects were recruited from the Brooklyn Campus of the Veterans Affairs New York Harbor Healthcare System, all were white males, ≥65 years of age, who had at least seven AKs on the face. Patients provided written informed consent and allowed photographs of the selected treatment area to be taken. This study received continuing Institutional Review Board approval on March 13, 2015 from the New York Harbor Healthcare System Department of Veterans Affairs’ Subcommittee for Human Studies.
Subject treatment and assessment
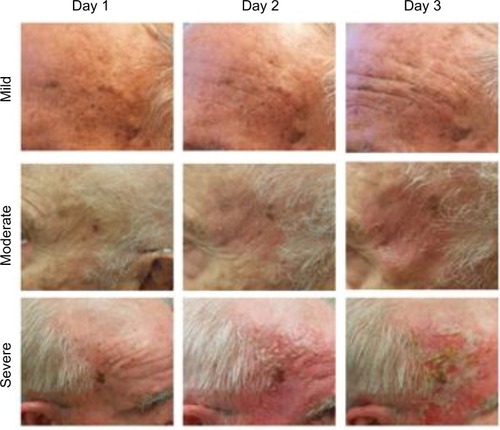
Ingenol mebutate gel 0.015% was applied by the clinical staff once daily for three consecutive days to a 25 cm2 contiguous area of the face that contained at least three clinical AKs. Local skin reactions (LSRs) were recorded with clinical photographs taken on each day of treatment. The efficacy of ingenol mebutate gel 0.015% was assessed at day 60 by clinical assessment and by the Skindex-16 dermatologic questionnaire, a single-page, 16-item patient questionnaire that measures the effects of skin disease on the subject’s QoL ().Citation18 The 16 questions cover three QoL subscales: symptoms (Q1–Q4), emotions (Q5–Q11), and functioning (Q12–Q16). Responses are recorded using a 0–6 numerical analog scale to measure bothersomeness, where 0 indicates “never bothered” and 6 indicates “always bothered.”
Table 1 Summary of Skindex-16 survey questions
Statistical methods
Overall QoL scores and individual subscale QoL scores were computed as a standardized percentage by adding the scores from all questions, dividing this number by the maximum possible score, and then multiplying by 100. Means and standard deviations were computed for QoL scores, and a paired t-test was performed to test the null hypothesis that the mean QoL score did not change from days 0 to 60. Statistical significance was determined by alpha =0.05, with no multiple-comparison correction in order to maximize statistical power. For analysis of the potential relationship between the subject’s LSRs and QoL improvement, only subjects who completed the Skindex-16 survey on both days 0 and 60 were included in the analysis. The value of Cohen’s d was computed as a measure of effect size. The practical significance of the effect size was based on the value of Cohen’s d, using the guidelines of d=0.2 (small), d=0.5 (medium), and d=0.8 (large).
Results
Relationship between clinical assessment and subjects’ QoL
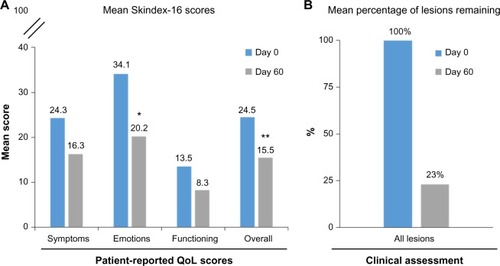
Of the 28 subjects enrolled in the study, 25 subjects completed the Skindex-16 survey on both days 0 and 60 and were included in the analysis. The mean (standard deviation [SD]) overall Skindex-16 score in the 25 subjects was 24.5 (20.9) at day 0 and 15.5 (19.3) at day 60 of the study (). The reduction in Skindex-16 scores indicated a statistically significant improvement in QoL (P=0.031). In addition, there were numeric reductions in scores in all three subscales (symptoms, emotions, and functioning), with symptoms reducing from 24.3 (20.6) to 16.3 (18.7), emotions reducing from 34.1 (26.6) to 20.2 (23.8), and functioning reducing from 13.5 (22.1) to 8.3 (18.4). However, statistical significance was achieved in the emotions subscale only (P=0.011). Improvements in QoL were consistent with the clinical assessment, in which a 77% reduction in lesions (95% CI, 68%–86%) was recorded. Specifically, in areas of the skin treated with ingenol mebutate gel 0.015%, 83 lesions (sum of clinical and subclinical) were identified at baseline, and 19 lesions were identified at day 60 ().
Figure 1 QoL and clinical assessments of ingenol mebutate treatment effect
Abbreviations: CI, confidence interval; QoL, quality of life.
Impact of LSRs on subjects’ QoL
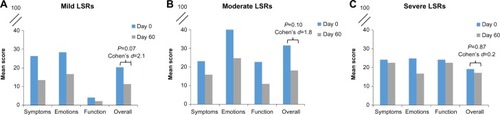
Following application of ingenol mebutate gel 0.015%, LSRs peaked at day 3 in the 3-day treatment regimen (). Responses were classified as mild, moderate, or severe, with most subjects (75%) having a mild-to-moderate response. Skindex-16 scores were then grouped by LSR severity for subjects with mild (n=9), moderate (n=11), and severe (n=5) reactions. Numeric improvements were seen in overall QoL scores regardless of LSR severity, although the differences did not reach statistical significance (). The potential impact of ingenol mebutate treatment on QoL was further examined by calculating the effect size using Cohen’s d. For overall QoL scores, the absolute Cohen’s d values were 2.1, 1.8, and 0.2 for subjects with mild, moderate, and severe LSRs, respectively (). Thus, ingenol mebutate gel 0.015% had a large positive impact on QoL for subjects with mild and moderate LSRs and little impact on subjects with severe LSRs.
Discussion
Studies on the relationship between the use of AK topical therapies and QoL have yielded inconsistent results despite the demonstrated clinical effectiveness of these treatments. In the VA Topical Tretinoin Chemoprevention (VATTC) Trial, past use of 5-FU was associated with a worse QoL.Citation16 However, this study did not track a single population of subjects before and after 5-FU use, thus limiting the interpretation of this result. Health-related QoL was investigated in a study of 118 AK subjects treated with imiquimod 0.05% cream for 8 weeks, in which QoL was assessed using a Skindex-16 survey and the Skin Cancer Index.Citation15 The small improvements in QoL seen at week 8 were judged to be lacking in clinical relevance. Low baseline impairments in QoL may have limited the ability to detect more robust improvements. A study of AK subjects treated with 3% diclofenac in 2.5% hyaluronic acid gel for up to 6 months demonstrated improvements in both clinical outcomes and QoL, as assessed by the Dermatology Quality of Life Index, despite the fact that skin reactions led to interruption or discontinuation of treatment in 13.6% of the subjects.Citation19 Finally, a post hoc analysis of 1,005 subjects enrolled in Phase 3 ingenol mebutate clinical trials for the treatment of AK revealed improved QoL, as assessed by the Treatment Satisfaction Questionnaire for Medication and Skindex-16 surveys.Citation17 For the Skindex-16 survey of subjects treated with ingenol mebutate on the face and scalp, significant improvements from baseline versus vehicle were seen in the overall score as well as in the scores for the three subscales, with the emotions subscale showing greatest improvement in mean score, followed second by symptoms and then functioning.
In contrast to the post hoc analysis of the Phase 3 ingenol mebutate study, our present study was a prospective analysis of 28 subjects at a single center. Despite this relatively small sample size, we saw a significant improvement in overall QoL as measured by the Skindex-16 survey. Interestingly, and consistent with the findings of the post hoc analysis, the greatest numeric improvement was seen in the emotions subscale, followed by the symptoms and then the functioning subscales.
As with other topical AK treatments, LSRs occur with the use of ingenol mebutate gel 0.015% and have the potential to negatively affect QoL. When the subjects in our study were grouped by the severity of their LSRs, each of the groups attained numeric improvements in overall QoL. The small cohort sizes may have precluded the attainment of statistical significance. Calculation of effect size using Cohen’s d revealed that subjects with mild and moderate LSRs achieved a large improvement in overall QoL scores, while subjects with severe LSRs did not have an improvement in QoL. Nevertheless, the AK clearance rates in subjects who experienced severe LSRs were similar to those in subjects with mild and moderate LSRs. These observations suggest that patients who experience severe LSRs early in the treatment course might have limited improvements in QoL even if they show significant clearance of AK lesions. These patients might benefit from additional counseling that emphasizes not only their achievement of clinical improvement but also the importance of detecting and treating new lesions that might arise in the future.
Limitations of this study include the relatively small cohort size, the homogeneity of the subjects with respect to race, age, and sex, and the fact that the study was performed at a single center.
In summary, our prospective clinical study demonstrated improved QoL in subjects who were treated with ingenol mebutate gel 0.015% for AKs. Improvements in QoL were consistent with the clinical improvements seen in these subjects. These results confirm reports of treatment-related QoL improvements in AK patients.
Acknowledgments
Editorial support was provided by p-value communications, and funded by LEO Pharma Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
- WhellerLSoyerHPClinical features of actinic keratoses and early squamous cell carcinomaCurr Probl Dermatol201546586325561207
- BermanBCockerellCJPathobiology of actinic keratosis: ultraviolet-dependent keratinocyte proliferationJ Am Acad Dermatol2013681 Suppl 1S10S1923228301
- DoddsAChiaAShumackSActinic keratosis: rationale and managementDermatol Ther (Heidelb)201441113124627245
- FrostCAGreenACEpidemiology of solar keratosesBr J Dermatol199413144554647947197
- SalascheSJEpidemiology of actinic keratoses and squamous cell carcinomaJ Am Acad Dermatol2000421 Pt 24710607349
- SlaughterDPSouthwickHWSmejkalWField cancerization in oral stratified squamous epithelium: clinical implications of multicentric originCancer19536596396813094644
- GoldenbergGPerlMActinic keratosis: update on field therapyJ Clin Aesthet Dermatol2014710283125371768
- HageleTJLevenderMMDavisSAWillifordPMFeldmanSRPractice trends in the treatment of actinic keratosis in the United States: 0.5% fluorouracil and combination cryotherapy plus fluorouracil are underused despite evidence of benefitJ Cutan Med Surg20121610711422513063
- StockflethEThe paradigm shift in treating actinic keratosis: a comprehensive strategyJ Drugs Dermatol201211121462146723377517
- WiegellSRUpdate on photodynamic treatment for actinic keratosisCurr Probl Dermatol20154612212825561216
- ChrenMMThe Skindex instruments to measure the effects of skin disease on quality of lifeDermatol Clin201230223123622284137
- EsmannSVindingGRChristensenKBJemecGBAssessing the influence of actinic keratosis on patients’ quality of life: the AKQoL questionnaireBr J Dermatol2013168227728322962980
- de JagerMEde JongEMvan de KerkhofPCEversAWSeygerMMAn intrapatient comparison of quality of life in psoriasis in childhood and adulthoodJ Eur Acad Dermatol Venereol201125782883121039918
- MabuchiTYamaokaHKojimaTIkomaNAkasakaEOzawaAPsoriasis affects patient’s quality of life more seriously in female than in male in JapanTokai J Exp Clin Med2012373848823032250
- Waalboer-SpuijRHolterhuesCvan HattemSPatient perception of imiquimod treatment for actinic keratosis and superficial basal cell carcinoma in 202 patientsDermatology2015231566225925162
- WeinstockMALeeKCChrenMMMarcolivioKVATTC Trial GroupQuality of life in the actinic neoplasia syndrome: the VA Topical Tretinoin Chemoprevention (VATTC) TrialJ Am Acad Dermatol200961220721519398145
- AugustinMTuJHKnudsenKMErntoftSLarssonTHankeCWIngenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomesJ Am Acad Dermatol201572581682125770879
- ChrenMMLasekRJSahayAPSandsLPMeasurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseasesJ Cutan Med Surg20015210511011443481
- PflugfelderAWelterAKLeiterUOpen label randomized study comparing 3 months vs. 6 months treatment of actinic keratoses with 3% diclofenac in 2.5% hyaluronic acid gel: a trial of the German Dermatologic Cooperative Oncology GroupJ Eur Acad Dermatol Venereol2012261485321414035