Abstract
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease primarily affecting apocrine gland-rich areas of the body and presenting with painful nodules, abscesses, sinus tracts, and scarring. HS is a multifactorial disease in which genetic and environmental factors play a key role. The primary defect in HS pathophysiology involves follicular occlusion of the folliculopilosebaceous unit, followed by follicular rupture, and immune responses (perifollicular lympho-histiocytic inflammation), finally leading to the development of clinical HS lesions. HS has a destructive impact on the patient’s quality of life, being a very challenging disease. Available treatments are limited, mostly off-label and with high variability in the reported efficacy. Fortunately, a monoclonal antibody against tumor necrosis factor alpha has been recently approved for treatment of moderate to severe HS, offering patients a promising new option. This review focuses on the main features of HS, including epidemiology, clinical aspects, pathogenesis, severity classifications, comorbidities, and currently available treatments.
Introduction
Hidradenitis suppurativa (HS) is a chronic, inflammatory, debilitating skin disease characterized by recurrent, painful, nodules and abscesses that rupture, leading to the formation of sinus tracts and scarring.Citation1 Lesions usually affect apocrine gland-bearing anatomical areas of the body. HS typically occurs after puberty, with the average age of onset in the second or third decades of life and with a female predominance.Citation2 Onset after menopause is rare, but isolated case reports have described HS development in prepubescent subjects experiencing premature adrenarche.Citation3 Due to its chronic nature and frequently occurring relapses, HS has a great impact on the patient’s quality of life, deeply affecting social, working, and psychological aspects.Citation3 The exact prevalence of HS remains unknown: in Europe, several studies have estimated a prevalence of 1% in the general populationCitation4,Citation5 and of 4% in young adult women,Citation6 whereas epidemiological data from American surveys reported a prevalence between 0.05% and 0.20%.Citation7,Citation8 As there is no biological or pathological test to facilitate diagnosis, HS is defined only by its clinical features and its chronicity.
Early diagnosis is very important for HS patients in order to ensure the best possible course of this stigmatizing and painful disease and to reduce the number of working days lost through sickness and HS-related healthcare issues. However, HS diagnosis generally occurs after an average 7-year delay.Citation9 Due to the peculiar clinical aspects of HS, non-dermatologists such as primary care physicians and surgeons are often the first providers to see HS patients and may have difficulty identifying the condition, even if the clinical presentation is typical and a reliable diagnosis can be made based on simple questions.Citation10
This review highlights the main features of HS in order to increase the awareness of this disease, avoid delay in diagnosis, and ensure prompt disease management.
Etiology and pathogenesis
The exact etiology of HS is still unproven. In the last few years, numerous studies hypothesized that the disease is triggered by genetic and environmental factors ().Citation1,Citation7,Citation11–Citation13
Figure 1 Hidradenitis suppurativa pathophysiology: a schematic overview.
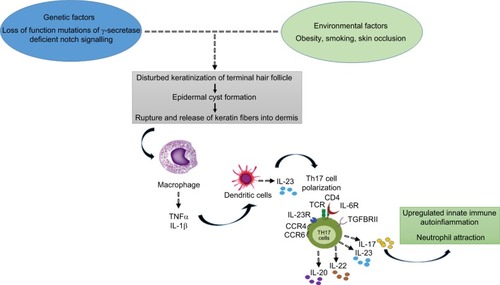
Cigarette smoking, obesity, and overweight are eminent environmental risk/trigger factors in HS development.Citation5 At the same time, the importance of genetic factors is highlighted by studies showing that 30%–40% of HS patients reported a family history of HS.Citation5,Citation14,Citation15 Moreover, familial forms of HS following an autosomal dominant pattern of inheritance with 100% penetrance have been described in different populations, being linked to mutations in subunits of the gamma-secretase proteins (up to 5% of HS cases).Citation16–Citation18
The primary defect in HS pathophysiology involves occlusion and subsequent inflammation of the hair follicle; these conditions, together with both innate and adaptive immune dysregulation, are necessary to initiate the development of clinical HS.Citation19 Bacterial infection and colonization are considered a secondary pathogenic factor that can worsen HS. Follicular occlusion leads to dilatation followed by rupture, resulting in the follicular contents, including keratin and bacteria, spilling into the surrounding dermis and inducing a vigorous chemotactic response from neutrophils and lymphocytes. The inflammatory cellular infiltrate causes abscess formation, leading to the destruction of the pilosebaceous unit and eventually of other adjacent adnexal structures.Citation1,Citation11,Citation20–Citation23 Other factors that may contribute to HS include altered expression of antimicrobial peptides’ abnormal secretion of apocrine glands, abnormal invaginations of the epidermis leading to sinus tract formation, and deficient numbers of sebaceous glands.Citation24–Citation30
The basis for follicular occlusion in HS is yet to be fully defined. Melnik and Plewig recently proposed the concept of HS as an auto-inflammatory disease characterized by dysregulation of the gamma-secretase/Notch pathway.Citation31,Citation32 Appropriate Notch signaling is of pivotal importance for maintaining the inner and outer root sheath of the hair follicle and skin appendages. Deficiency in the Notch signaling pathway results in conversion of hair follicles to keratin-enriched epidermal cysts, compromises apocrine gland homoeostasis, and leads to the stimulation of toll-like receptor (TLR)-mediated innate immunity, supporting and maintaining chronic inflammation.Citation33,Citation34 In support of this hypothesis, elevated levels of several pro-inflammatory cytokines, most notably tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-17, have been observed in HS lesions.Citation35 Altered TLRs signaling on macrophages and dendritic cells (DCs), the most abundant cells in HS lesions, produces increased amounts of these cytokines, leading to activation of DCs, which secrete IL-23 promoting Th17 cell polarization (IL-17-producing T helper cells were found to infiltrate the dermis in chronic HS lesions).Citation35–Citation40 In particular, one of the major actors in HS pathogenesis is TNF-α, whose overexpression has been observed in lesional and perilesional skin of HS, together with a positive correlation with disease severity.Citation41 Hence, it is clear that HS is a follicular disease showing some defect in keratin clearance, with resultant follicular occlusion, where defective innate cellular immunity plays an important role.Citation42
Although HS is not primarily an infectious disease, the role of bacteria seems to be very important in HS pathophysiology. Follicular hyperkeratinization and occlusion result in the rupture of pilosebaceous units, releasing bacteria within the dermis and triggering a local inflammatory response and thereby sustaining chronic inflammation. In addition, colonies of specialized bacteria that are difficult to eradicate form bacterial biofilms that bind irreversibly to sinus tract epithelium and hair follicles, further sustaining chronic inflammation.Citation43,Citation44 A microbiological study of 102 HS lesions from 82 patients showed Staphylococcus lugdunensis to be the most prevalent species, found as a unique or predominant isolate in 58% of HS nodules and abscesses.Citation26 Other common species dominating HS lesions included polymicrobial anaerobic microflora consisting of strict anaerobes, milleri group streptococci, and actinomycetes (found in 24% of abscesses or nodules and in 87% of chronic suppurating lesions).Citation26
Clinical aspects
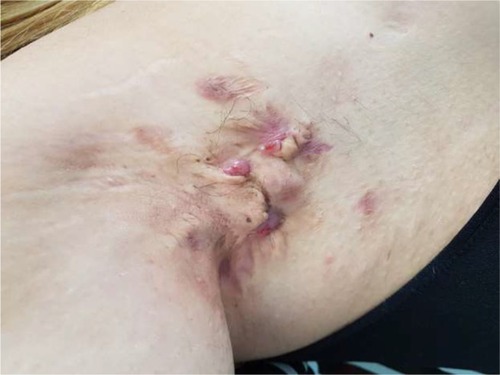
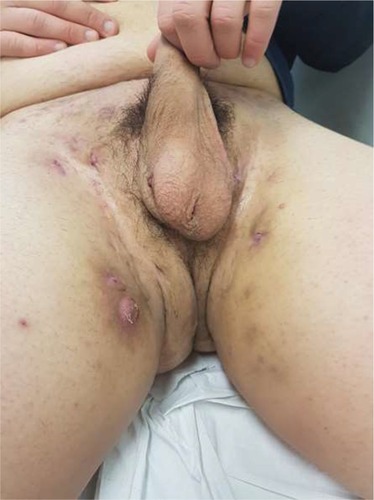
In general, HS is localized in the apocrine gland-bearing areas of the body such as axillae, inguinal and anogenital regions, perineum, and inframammary area of female patients (although aberrant lesions may occur in the waist, abdomen, specially periumbilical region, and thorax).Citation1 The sites affected by HS correspond not only to the location of apocrine glands but also to the location of terminal hair follicles dependent on low androgen concentrations.Citation45 HS is initially characterized by the presence of tender subcutaneous nodules (commonly indicated as “boils” or “pimples”) ( and ). Up to 50% of patients report a burning or stinging sensation, pain, pruritus, warmth, and/or hyperhidrosis, 12–48 hours before an overt nodule occurs.Citation5 Mean duration of a single painful nodule is 7–15 days. With time, the nodules may rupture, resulting in painful, deep dermal abscesses. After rupture, the lesions often extrude a purulent, foul-smelling discharge. With disease progression, draining sinus tracts, fibrosis, and scarring can be observed.Citation46
Primary HS lesions may also present as painful and/or tender erythematous papules <1 cm in diameter, as dermal contractures and rope-like elevation of the skin, or as double-ended comedones.Citation47 Follicular papules and pustules may be seen in areas associated with HS as well as in other areas; they do not constitute a diagnostic clue for HS. On the but tocks, folliculitis can leave round, slightly depressed scars. The sites affected by HS include, in order of decreasing frequency, axillary, inguinal, perineal and perianal, mammary and inframammary, buttocks, pubic region, chest, scalp, retroauricular, and eyelid.Citation48 Patients with anogenital HS may also have disease in the anal canal corresponding to the distribution of apocrine glands and hair follicles in that region.
Chronicity is the hallmark of HS. The disease course is characterized by recurrent flares, as well as pain causing significant quality-of-life impairment.Citation49 Patients reported changes in self-perception, daily living activities, mood state, social functioning, and physical discomfort. HS patients experience a quality of life worse than that of patients with alopecia, mild to moderate psoriasis, and several other dermatologic conditions.Citation3
Diagnosis of HS is made by clinical observation, and a biopsy is rarely needed. HS can easily be differentiated from other diseases by the age of onset and by the characteristic locations of the lesions.Citation50 presents the primary differential diagnoses of HS.
Table 1 Differential diagnosis of hidradenitis suppurativa
Classification and severity assessment
A clinically relevant staging and disease severity assessment are essential for the development of evidence-based treatments. There are several scoring systems for the assessment of disease severity of HS, including Hurley staging, HS Physician’s Global Assessment (PGA), the modified Sartorius score (MSS), and HS Severity Index (HSSI).Citation51–Citation54 Each of these assessments has both advantages and limitations in daily practice; to date, there is no gold standard.
Hurley staging system
This is the simplest and most widely used instrument for HS classification in routine clinical practice. It classifies HS into three stages:Citation55 1) stage I: abscess formation, single or multiple, without sinus tracts and cicatrization; 2) stage II: recurrent abscesses with tract formation and cicatrization, single or multiple, widely separated lesions; and 3) stage III: diffuse or near-diffuse involvement, or multiple interconnected tracts and abscesses across the entire area. Although the system is fast and easy, Hurley classification is not suitable for monitoring the efficacy of therapeutic interventions in clinical trials, since the classification is not quantitative.
MSS
This is a more detailed and dynamic classification system, based on the counting of individual nodules and fistulas within seven anatomical regions. The system, which was developed by Sartorius et al and later modified, is the first disease-specific instrument for dynamically measuring clinical severity of HS.Citation56,Citation57 Calculating MSS requires measuring the longest distance between two lesions of the same type within each anatomical region and applying predetermined weights to specific types of lesion characteristics. Disadvantages of the MSS are that the system is time consuming and sometimes difficult to interpret; consequently, MSS is not optimal for evaluating inflammatory manifestations in clinical practice or trials.Citation42
HS-PGA
HS-PGA is relatively easy to use and is frequently used to measure clinical improvement in clinical trials of medical treatments.Citation51 It classifies HS severity by counting the number of abscesses, fistulas, and inflammatory and noninflammatory nodules in all skin areas. The system describes six disease stages, increasing in severity on a scale from 1 to 6 (from stage 1: clear, no inflammatory or noninflammatory nodules to stage 6: severe, >5 abscesses or draining fistulas).Citation11,Citation51 However, a serious limitation of HS-PGA is that patients could experience clinically important improvement but not gain a meaningful reduction in their HS-PGA score, as patients in the most severe category may show marked heterogeneity.
HSSI
HSSI is another HS-specific severity index.Citation53,Citation58 This score incorporates categorical objective parameters with categorical subjective parameters: body surface area involved, number of skin lesions, pain severity (determined through a visual analog scale), and drainage (determined by the number of dressing changes/working hours). HSSI scores ≥13 indicate severe disease, scores between 8 and 12, moderate disease, and scores between 0 and 7, mild disease.
Comorbidities
Recent studies have proposed defining HS as a systemic disease linked to several comorbidities.Citation59–Citation62 Observed comorbidities fall into different classes: obesity and metabolic syndrome (MetS), depression, and inflammatory bowel disease (IBD).Citation63 Rates of obesity in HS range from 12% to 88%, depending on the population. HS disease severity is associated with an elevated body mass index (BMI).Citation64–Citation66 In recent studies, obese patients with HS showed dysregulated adipokine levels;Citation18,Citation67 in these patients, macrophages in visceral fat could secrete increased levels of pro-inflammatory cytokines, such as TNF-α and IL-1β, which could then exacerbate HS disease activity.Citation3
HS patients are at higher risk of MetS and its components. Sabat et al reported an odds ratio (OR) of 4.46 for MetS comparing HS patients with healthy controls; similarly, in this patient population, OR values for central obesity, hypertriglyceridemia, hypo-HDL-cholesterolemia, and hyperglycemia were 5.88, 2.24, 4.56, and 4.09, respectively.Citation68 Hence, it is not surprising that HS patients have also shown an increased cardiovascular risk and adverse cardiovascular outcomes in both controls and patients with severe psoriasis.Citation69,Citation70 Therefore, clinicians should take into account that HS patients may have ≥1 undiagnosed components of MetS, despite their youth, and initiate appropriate targeted screening. Patients affected by HS may benefit from early dietary interventions aimed at the metabolic comorbidities that may predispose the patient to HS itself, with weight reduction being a secondary benefit.
Patients with HS have a higher risk of developing depression. A cross-sectional study involving 9,619 patients found a higher prevalence of depression (5.9%) in HS patients relative to controls.Citation71 In another study, the Dermatology Life Quality Index value was significantly higher for HS patients than for the control patients (8.4±7.5 vs 4.3±5.6; P<0.0001).Citation72
Patients with HS also have a higher prevalence of gastrointestinal disease. The prevalence of IBD is 4–8 times higher in HS patients than in the general population, although there is no association with any distinct HS subtype.Citation63 Moreover, compared to the general population, patients with IBD are 9 times more likely to develop HS (incidence rate ratio of 8.9).Citation73 Perianal disease is the most common feature in patients with both HS and Crohn disease, while the majority of ulcerative colitis patients who developed HS did so after colectomy, in the setting of pouchitis.Citation74 HS has also been reported to be associated with pyoderma gangrenosum (PG) which is also frequently linked to IBD.Citation75 A multicenter retrospective study conducted in 2010 reported 11 cases of patients with HS and PG: HS usually predated PG with a mean time of 2.5 years prior to PG often having a severe, refractory course.Citation75 Moreover, three syndromes with PG and HS are reported in the literature. Each of these syndromes consists of the triad of PG, acne conglobata, and HS, and are differentiated clinically by the presence/absence and/or type of articular involvement: PAPASH (PG, acne, HS, and pyogenic arthritis), PASS (PG, acne conglobata, HS, and axial spondyloarthropathy), and PASH (PG, acne conglobata, and HS).Citation70 Other rheumatologic conditions may also be linked to HS.Citation76,Citation77 A multicenter, observational study of 640 patients with HS showed that 184 (28.8%) had musculoskeletal symptoms, 43 (6.9%) had evidence of arthritis, enthesitis, or inflammatory back pain, and 24 (3.7%) were diagnosed with spondyloarthropathy after evaluation by X-ray, magnetic resonance, and a rheumatologist.Citation76 Usually, HS preceded joint pain in 90% of cases by a mean of 3.6 years. In a retrospective study of 29 cases of HS and spondyloarthropathy, involvement of axial (69%) and peripheral (86%) joints was common with the knee being reported as the most frequently affected peripheral joint (59%).Citation77 An association between rheumatologic joint conditions, namely spondyloarthropathies, and synovitis, arthritis, pustulosis, hyperostosis, osteitis syndrome has also been seen in conjunction with HS.Citation70,Citation76 Moreover, due to common shared etiopathogenetic factors such as follicular occlusion from infundibular hyperkeratosis and follicular epithelium hyperplasia, HS has also been possibly reported in conjunction with other follicular occlusion disorders like acne conglobata, dissecting cellulitis of the scalp, and sinus pilonidalis,Citation70 creating the follicular occlusion tetrad: HS, acne conglobata, dissecting cellulitis, and pilonidal cysts.Citation78 Finally, as regards HS-related malignancies risk, squamous cell carcinoma (SCC) has been reported to possibly arise within or neighboring HS lesions (from 1% to 3.1% of evaluated HS patients).Citation79 The risk seems to be more frequent in gluteal, perianal, and perineal areas due to chronic inflammation of HS, impaired cellular immunity, and the presence of the human papillomavirus.Citation80 Therefore, clinicians should raise their index of suspicion for this malignancy and lower their biopsy threshold in HS patients to prevent or minimize SCC metastasis especially when ulcerative lesions, chronic wounds, nodules, or ulcerative nodules arise and do not show response to common antibiotic or immunosuppressive treatments used in HS patients.
Treatment
HS treatment choices should be determined by disease severity and its individual subjective impact. The degree of HS clinical involvement is usually ascertained according to the three-stage Hurley system described above.Citation55
Topical antibiotics
Clindamycin is the only antibiotic that has been studied as a topical agent for HS.Citation81,Citation82 The most significant effect was seen with superficial lesions (folliculitis, papules, and pustules); in contrast, the treatment efficacy was poor with deep lesions (nodules and abscesses). As a topical treatment, clindamycin seems to be indicated only in localized Hurley stage I or mild stage II disease.Citation11
Systemic treatments
Systemic treatment is indicated when more severe or widely spread lesions are present (moderate to severe disease).Citation11
Clindamycin–rifampicin
In widespread Hurley stage I or mild stage II disease, the combined use of systemic clindamycin and systemic rifampicin (300 mg of clindamycin b.i.d. given in combination with rifampicin [600 mg daily given as either 1 or 2 doses] for 10 weeks) has proved beneficial, with variable results.Citation83–Citation87 In a study conducted by van der Zee et al, 47% of HS patients receiving systemic clindamycin/rifampicin treatment reported total remission after 10 weeks, while an additional 35% of subjects experienced at least some improvement, whereas another survey reported that 70/116 patients (~70%) had significant improvement after 10 weeks of treatment.Citation83,Citation85 A very recent, prospective, hospital-based study reported clinical response in 19 patients (73%) after 12 weeks of treatment, supporting the efficacy and tolerability of this combination therapy. Response was associated only with female sex and not with BMI, Hurley stage, or lesion location. The authors also reported that there was sustained efficacy in 7 (41%) patients at the 1-year follow-up, whereas 10 subjects (59%) had disease relapse after a mean time of 4.2 months.Citation87
Tetracycline
Tetracycline (500 mg b.i.d.) has not proven effective compared to topical clindamycin in widespread Hurley stage I or mild stage II disease.Citation82
Rifampicin–moxifloxacin–metronidazole
Rifampicin 10 mg/kg once daily, moxifloxacin 400 mg daily, and metronidazole 500 mg t.i.d. for 6 weeks followed by rifampicin–moxifloxacin therapy has been shown to be effective, with 16 of 28 patients (57.1%) with long-lasting refractory HS achieving complete remission and 14 of 16 patients (87.5%) with Hurley stage I or II disease achieving complete remission.Citation88
Ertapenem
A study showed that ertapenem 1 g daily intravenously for 6 weeks was able to decrease the median Sartorius score from 49.5 to 19.0, reflecting a significant decrease in the number and clinical severity of active HS areas in a group of 30 patients with severe HS. Altogether, 67% (29/43) and 26% (13/50) of Hurley stage I and II patients’ body areas reached clinical remission after ertapenem, respectively.Citation89
Acitretin
Acitretin is indicated for early HS stages (Hurley I or mild II); however, it seems reasonable that this medication could also be advocated in the chronic stages of HS with recurrent abscesses with sinus tracts (even interconnected) and/or scarring.Citation90–Citation93 Daily doses of 0.25–0.88 mg/kg for 3–12 months have been used in studies concerning acitretin therapy in HS patients.Citation90,Citation91 Studies involving acitretin treatment comprised in total 46 patients, reporting a moderate to high response rate with significant improvements in 28 of 46 subjects (60.9%) after therapy.Citation92–Citation97 However, a recent 5-year retrospective study conducted in a tertiary medical center (14 patients) showed that acitretin monotherapy was ineffective for the treatment of Hurley stage II–III HS with acitretin being more effective when used as an adjuvant to other systemic medications.Citation92
Cyclosporine A
Beneficial effects of cyclosporine are reported in limited cases.Citation98–Citation100 A recent exploratory 4-year retrospective review performed at three departments of dermatology with a special interest in HS showed that 9/18 (50%) patients treated with cyclosporine reported some benefit (mainly slight improvement).Citation101 Therefore, use of cyclosporine should be reserved for cases where failure of response to standard first-, second-, and third-line therapies occurs until further evidence is available.Citation11
Dapsone
Several studies involving oral dapsone have been published with mixed results.Citation102–Citation104 The most important study was conducted in 2011. It involved 24 HS patients with reported improvement in only 38% of cases, and none of the 4 cases with severe disease experiencing any kind of improvement. Rapid recurrence of disease at the cessation of treatment occurred.Citation102 Thus, it seems that dapsone may be best used in stage I or sometimes in mild stage II patients, preferably in combination with other agents.
Isotretinoin
The results of isotretinoin therapy in HS have not been encouraging. Several studies showed that oral isotretinoin has only little effect on HS (eg, in a retrospective study on 358 patients, Soria et al showed that only 16.1% of patients experienced any improvement).Citation105–Citation107 Isotretinoin has maximal effect on sebaceous gland activity; however, sebum production is normal in HS. Consequently, it is unsurprising that isotretinoin therapy is ineffective.Citation108
Biologics
Recent studies have shown that adalimumab and infliximab, two different monoclonal antibodies against TNF-α, are effective in the treatment of moderate to severe HS (Hurley II–III), with improvement in the patient’s quality of life, with adalimumab being more tolerable.Citation109–Citation111 As regards infliximab, a cumulative response rate of 58% (improvement ≥50% in 42 patients) has been reported in case reports with 73 patients with moderate to severe HS.Citation112–Citation121 Only a single randomized controlled trial (RCT), Phase II crossover study on 38 HS patients treated with infliximab was published in 2010 and no additional Phase III RCT studies have been published so far.Citation58
Infliximab (5 mg/kg body weight) is administered intravenously over a period of 2 hours on day 0, 2, 6, and then regularly every 8 weeks.Citation11,Citation51 There are different rates of response to adalimumab reported in case series and in a current, prospective controlled study. Adalimumab, a human monoclonal antibody that binds to and neutralizes TNF-α, is the first Food and Drug Administration (FDA)-approved treatment for moderate to severe HS in adults.Citation110 Administration of adalimumab with a cumulative response rate of 58% (improvement ≥50% in 23 patients) has been reported in case reports with 42 patients with moderate to severe HS.Citation53,Citation122–Citation125 Two Phase III, randomized, double-blind clinical trials (PIONEER I and PIONEER II) to assess the safety and efficacy of adalimumab in the treatment of patients with moderate–severe HS were recently completed.Citation126–Citation128 Both the studies were multicentered, 36-week trials with two double-blind, placebo-controlled periods (12-week period 1 and 24-week period 2). In period 1, patients were randomly assigned in a 1:1 ratio to 40 mg of adalimumab weekly or matching placebo for 12 weeks. In period 2, patients were reassigned to adalimumab at a weekly or every-other-week dose or to placebo for 24 weeks. Particularly, 307 and 326 patients were enrolled in PIONEER I and PIONEER II studies, respectively. Inclusion criteria included adults (between 18 and 99 years of age) with a diagnosis of HS for at least 1 year and the presence of at least two areas exhibiting HS lesions with at least one categorized as Hurley stage II or stage III, stable HS for at least 60 days prior to screening and baseline visits, previous inadequate response to other HS treatments, and total abscess and inflammatory nodule count of ≥3 at baseline. Primary efficacy outcome of 50% reduction in abscess and inflammatory nodule count was seen in 41.8% and 58.9% of participants receiving adalimumab in PIONEER I and PIONEER II studies, respectively, showing substantial improvement compared with placebo groups in both the trials (26.0% and 27.6%, respectively). Serious adverse events in period 1 (excluding worsening of underlying disease) occurred in 1.3% of patients receiving adalimumab and 1.3% of those receiving placebo in PIONEER I and in 1.8% and 3.7% of patients in PIONEER II. In period 2, the rates of serious adverse events were ≤4.6% in all the groups in both studies, with no significant differences between placebo and treatment groups. On the other hand, another biologic drug, ustekinumab, a monoclonal antibody directed against IL-12 and IL-23, has been shown to reduce the mean MSS with a 46.3% improvement in 12 patients with moderate to severe (Hurley stage II–III) disease treated with 45 or 90 mg ustekinumab (depending on body weight) at weeks 0, 4, 16, and 28.Citation129 However, further studies are needed to evaluate its efficacy in HS patients because, apart from the study of Blok et al, its use in HS has been described only in case reports or very small case series.Citation130–Citation133
Conclusion
HS is a chronic inflammatory skin disease that can have a debilitating effect on a patient’s social activities, work activities, and overall quality of life. The disease is multifactorial, with interplay between multiple genetic, immunological, behavioral, and endocrine factors playing a key role in its development. HS can greatly impact patients’ quality of life and social and work activities due to frequent disease relapses with painful and foul-smelling lesions. Therefore, prompt treatment is required to reduce and limit HS burden. Nevertheless, therapeutic weapons against HS include several treatments; most of them are used off-label, with adalimumab being the only FDA-approved drug for moderate to severe HS. HS patients often need a multidisciplinary approach, incorporating both medical and surgical treatments in addition to lifestyle modification. HS remains a challenging disease that is difficult to treat. Further studies are needed to ascertain whether certain genetic, clinical, or phenotypic factors may predict or guide treatments outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
- KurzenHKurokawaIJemecGBWhat causes hidradenitis suppurativa?Exp Dermatol20081745547218400064
- NaldiLEpidemiologyJemecGRevuzJLeydenJJHidradenitis SuppurativeBerlinSpringer20065864
- AlikhanALynchPJEisenDBHidradenitis suppurativa: a comprehensive reviewJ Am Acad Dermatol200960453956119293006
- RevuzJECanoui-PoitrineFWolkensteinPPrevalence and factors associated with hidradenitis suppurativa: results from two case-control studiesJ Am Acad Dermatol20085959660118674845
- JemecGBHeidenheimMNielsenNHThe prevalence of hidradenitis suppurativa and its potential precursor lesionsJ Am Acad Dermatol1996351911948708018
- JemecGBThe symptomatology of hidradenitis suppurativa in womenBr J Dermatol19881193453503179207
- VazquezBGAlikhanAWeaverALWetterDADavisMDIncidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, MinnesotaJ Invest Dermatol20131339710322931916
- CosmatosIMatchoAWeinsteinRMontgomeryMOStangPAnalysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United StatesJ Am Acad Dermatol20136841241922921795
- MargessonLJDanbyFWHidradenitis suppurativaBest Pract Res Clin Obstet Gynaecol2014281013102725214437
- EsmannSDufourDNJemecGBQuestionnaire-based diagnosis of hidradenitis suppurativa: specificity, sensitivity and positive predictive value of specific diagnostic questionsBr J Dermatol201016310210620331444
- ZouboulisCCDesaiNEmtestamLEuropean S1 guideline for the treatment of hidradenitis suppurativa/acne inversaJ Eur Acad Dermatol Venereol20152961964425640693
- de WinterKvan der ZeeHHPrensEPIs mechanical stress an important pathogenic factor in hidradenitis suppurativa?Exp Dermatol20122117617722379963
- SchraderAMDeckersIEvan der ZeeHHBoerJPrensEPHidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severityJ Am Acad Dermatol20147146046724880664
- FitzsimmonsJSGuilbertPRA family study of hidradenitis suppurativaJ Med Genet1985223673732934550
- PinkAESimpsonMADesaiNMutations in the gamma-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa)J Invest Dermatol20121322459246122622421
- WangBYangWWenWGamma-secretase gene mutations in familial acne inversaScience2010330106520929727
- PinkAESimpsonMADesaiNTrembathRCBarkerJNGamma-secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesisJ Invest Dermatol201313360160723096707
- ScheinfeldNHidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patientsDermatol Online J2013191
- PrensEDeckersIPathophysiology of hidradenitis suppurativa: an updateJ Am Acad Dermatol2015735 Suppl 1S8S1126470623
- JemecGBThomsenBMHansenUThe homogeneity of hidradenitis suppurativa lesions. A histological study of intra-individual variationAPMIS19971053783839201239
- JemecGBHansenUHistology of hidradenitis suppurativaJ Am Acad Dermatol1996349949998647993
- von LaffertMHelmboldPWohlrabJFiedlerEStadieVMarschWCHidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermisExp Dermatol20101953353719659829
- BoerJWeltevredenEFHidradenitis suppurativa or acne inversa. A clinicopathological study of early lesionsBr J Dermatol19961357275
- KampSFiehnAMStenderupKHidradenitis suppurativa: a disease of the absent sebaceous gland? Sebaceous gland number and volume are significantly reduced in uninvolved hair follicles from patients with hidradenitis suppurativaBr J Dermatol201116451017102221250966
- KathjuSLaskoLAStoodleyPConsidering hidradenitis suppurativa as a bacterial biofilm diseaseFEMS Immunol Med Microbiol201265238538922353357
- Guet-RevilletHCoignard-BiehlerHJaisJPBacterial pathogens associated with hidradenitis suppurativa, FranceEmerg Infect Dis201420121990199825418454
- MatusiakLBieniekASzepietowskiJCBacteriology of hidradenitis suppurativa – which antibiotics are the treatment of choice?Acta Derm Venereol201494669970224604152
- HofmannSCSaborowskiVLangeSKernWVBruckner-TudermanLRiegSExpression of innate defense antimicrobial peptides in hidradenitis suppurativaJ Am Acad Dermatol20126696697421982063
- EmelianovVUBecharaFGGläserRImmunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversaBr J Dermatol20121661023103422136668
- BecharaFGSandMSkryganMKreuterAAltmeyerPGambichlerTAcne inversa: evaluating antimicrobial peptides and proteinsAnn Dermatol20122439339723197903
- MammucariCTommasi di VignanoASharovAAIntegration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation controlDev Cell2005866567615866158
- MelnikBCPlewigGImpaired Notch-MKP-1 signalling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biologyExp Dermatol20132217217723489419
- RangarajanATaloraCOkuyamaRNotch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiationEMBO J2001203427343611432830
- PanYLinMHTianXGamma-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesisDev Cell2004773174315525534
- van der ZeeHHLamanJDde RuiterLDikWAPrensEPAdalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo studyBr J Dermatol201216629830522013960
- HungerRESurovyAMHassanASBraathenLRYawalkarNToll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptorBr J Dermatol200815869169718241264
- ReFStromingerJLToll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cellsJ Biol Chem2001276376923769911477091
- SchlapbachCHänniTYawalkarNHungerREExpression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativaJ Am Acad Dermatol20116579079821641076
- Giamarellos-BourboulisEJAntonopoulouAPetropoulouCAltered innate and adaptive immune responses in patients with hidrad-enitis suppurativaBr J Dermatol2007156515617199566
- ZhangQWangCLiuZNotch signal suppresses Toll-like receptor-triggered inflammatory responses in macrophages by inhibiting extracellular signal-regulated kinase 1/2-mediated nuclear factor κB activationJ Biol Chem20122876208621722205705
- MozeikaEPilmaneMNürnbergBMJemecGBTumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativaActa Derm Venereol20139330130423096596
- van der ZeeHHLamanJDBoerJPrensEPHidradenitis suppurativa: viewpoint on clinical phenotyping, pathogenesis and novel treatmentsExp Dermatol20122173573922882284
- KathjuSLaskoLStoodleyPConsidering hidradenitis suppurativa as a bacterial biofilm diseaseFEMS Immunol Med Microbiol20126538538922353357
- JahnsACKillasliHNosekDMicrobiology of hidradenitis suppurativa (acne inversa): a histological study of 27 patientsAPMIS201412280480924475943
- YuCCCookMGHidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glandsBr J Dermatol19901227637692369556
- EsmannSJemecGBPsychosocial impact of hidradenitis suppurativa: a qualitative studyActa Derm Venereol20119132833221394419
- BrownTJRosenTOrengoIFHidradenitis suppurativaSouth Med J199891110711149853721
- SladeDEMPowellBWMortimerPSHidradenitis suppurativa: pathogenesis and managementBr J Plast Surg20035645146112890458
- ShalomGFreudTHarman-BoehmIPolishchukICohenADHidradenitis suppurativa and metabolic syndrome: a comparative cross-sectional study of 3207 patientsBr J Dermatol2015173246447025760289
- RevuzJHidradenitis suppurativaJ Eur Acad Dermatol Venereol200923998599819682181
- KimballABKerdelFAdamsDAdalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trialAnn Intern Med201215784685523247938
- SartoriusKKillasliHHeilbornJJemecGBLapinsJEmtestamLInterobserver variability of clinical scores in hidradenitis suppurativa is lowBr J Dermatol20101621261126820184581
- AmanoMGrantAKerdelFAA prospective open-label clinical trial of adalimumab for the treatment of hidradenitis suppurativaInt J Dermatol20104995095521128923
- JemecGBBiomarkers in hidradenitis suppurativaBr J Dermatol201316811511153
- HurleyHJAxillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approachRoenigkRKRoenigkHHDermatologic SurgeryNew YorkMarcel Dekker1996623645
- SartoriusKEmtestamLJemecGBLapinsJObjective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesityBr J Dermatol200916183183919438453
- SartoriusKLapinsJEmtestamLJemecGBSuggestions for uniform outcome variables when reporting treatment effects in hidradenitis suppurativaBr J Dermatol200314921121312890229
- GrantAGonzalezTMontgomeryMOCardenasVKerdelFAInfliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trialJ Am Acad Dermatol20106220521720115947
- PascoeVLKimballABHidradenitis suppurativa: current progress and future questionsJAMA Dermatol20141501263126425229429
- GoldDAReederVJMahanMGHamzaviIHThe prevalence of metabolic syndrome in patients with hidradenitis suppurativaJ Am Acad Dermatol20147069970324433875
- ShlyankevichJChenAJKimGEKimballABHidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysisJ Am Acad Dermatol20147161144115025440440
- MillerIMEllervikCVindingGRAssociation of metabolic syndrome and hidradenitis suppurativaJAMA Dermatol20141501273128025229996
- DeckersIEBenhadouFKoldijkMJInflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional studyJ Am Acad Dermatol2017761495327793450
- NapolitanoMMegnaMMonfrecolaGInsulin resistance and skin diseasesScientific World J20152015479354
- BettoliVNaldiLCazzanigaSOverweight, diabetes and disease duration influence clinical severity in hidradenitis suppurativa–acne inversa. Evidence from the national Italian registryBr J Dermatol2016174119519725913460
- MonfrecolaGBalatoACaiazzoGMammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loopJ Eur Acad Dermatol Venereol20163091631163326299257
- TianGLiangJNWangZYZhouDEmerging role of leptin in rheumatoid arthritisClin Exp Immunol201417755757024802245
- SabatRChanwangpongASchneider-BurrusSIncreased prevalence of metabolic syndrome in patients with acne inversaPLoS One20127e3181022359634
- EgebergAGislasonGHHansenPRRisk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativaJAMA Dermatol2016152442943426885728
- MillerIMMcAndrewRJHamzaviIPrevalence, risk factors, and comorbidities of hidradenitis suppurativaDermatol Clin201634171626617352
- ShavitEDreiherJFreudTHalevySVinkerSCohenADPsychiatric comorbidities in 3207 patients with hidradenitis suppurativaJ Eur Acad Dermatol Venereol20152937137624909646
- OnderdijkAJvan der ZeeHHEsmannSDepression in patients with hidradenitis suppurativaJ Eur Acad Dermatol Venereol201327447347822339940
- YadavSSinghSEdakkanambeth VarayilJHidradenitis suppurativa in patients with inflammatory bowel disease: a population-based cohort study in Olmsted County, MinnesotaClin Gastroenterol Hepatol2016141657025952308
- KamalNCohenBLBucheSDelaporteEColombelJFFeatures of patients with Crohn’s disease and hidradenitis suppurativaClin Gastroenterol Hepatol2016141717925956836
- HsiaoJLAntayaRJBergerTMaurerTShinkaiKLeslieKSHidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature reviewArch Dermatol2010146111265127021079064
- RichettePMoltoAViguierMHidradenitis suppurativa associated with spondyloarthritis – results from a multicenter national prospective studyJ Rheumatol20144149049424429166
- BhallaRSequeiraWArthritis associated with hidradenitis suppurativaAnn Rheum Dis19945364668311560
- VasanthVChandrashekarBSFollicular occlusion tetradIndian Dermatol Online J2014549149325396138
- ConstantinouCWidomKDesantisJObmannMHidradenitis suppurativa complicated by squamous cell carcinomaAm Surg200874121177118119097532
- ScheinfeldNA case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinomaDermatol Online J2014203
- ClemmensenOJTopical treatment of hidradenitis suppurativa with clindamycinInt J Dermatol1983223253286347922
- JemecGBEWendelboePTopical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativaJ Am Acad Dermatol1998399719749843011
- GenerGCanoui-PoitrineFRevuzJECombination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patientsDermatology200921914815419590173
- MendoncaCOGriffithsCEClindamycin and rifampicin combination therapy for hidradenitis suppurativaBr J Dermatol200615497797816634904
- van der ZeeHHBoerJPrensEPJemevJBThe effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativaDermatology200921914314719590174
- BettoliVZauliSBorghiAOral clindamycin and rifampicin in the treatment of hidradenitis suppurativa–acne inversa: a prospective study on 23 patientsJ Eur Acad Dermatol Venereol20142812512623451831
- DessiniotiCZisimouCTzanetakouVStratigosAAntoniouCOral clindamycin and rifampicin combination therapy for hidradenitis suppurativa: a prospective study and 1-year follow-upClin Exp Dermatol201641885285727753139
- Join-LambertOCoignardHJaisJPEfficacy of rifampin–moxifloxacin–metronidazole combination therapy in hidradenitis suppurativaDermatology20112221495821109728
- Join-LambertOCoignard-BiehlerHJaisJPEfficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patientsJ Antimicrob Chemother201671251352026565016
- MeixnerDSchneiderSKrauseMSterryWAcne inversaJ Dtsch Dermatol Ges2008618919618093218
- BoerJNazaryMLong-term results of acitretin therapy for hidradenitis suppurativa. Is acne inversa also a misnomer?Br J Dermatol201116417017520874789
- MatusiakLBieniekASzepietowskiJCAcitretin in hidradenitis suppurativa treatment – own experiencesPrzegl Dermatol201299356357
- TanMGShearNHWalshSAlhusayenRAcitretin: monotherapy or combined therapy for hidradenitis suppurativa?J Cutan Med Surg2016211485327432818
- ChowETMortimerPSSuccessful treatment of hidradenitis suppurativa and retroauricular acne with etretinateBr J Dermatol1992126415
- HoganDJLightMJSuccessful treatment of hidradenitis suppurativa with acitretinJ Am Acad Dermatol1988193553562971689
- SchemanAJNodulocystic acne and hidradenitis suppurativa treated with acitretin: a case reportCutis20026928728812080949
- VahlquistAGriffithsWRetinoid therapy in hidradenitis suppurativa – a report of a caseRetinoids Today Tom1990182830
- GuptaAKEllisCNNickoloffBJOral cyclosporine in the treatment of inflammatory and noninflammatory dermatoses. A clinical and immunopathologic analysisArch Dermatol19901263393502178558
- RoseRFGoodfieldMJClarkSMTreatment of recalcitrant hidradenitis suppurativa with oral ciclosporinClin Exp Dermatol20063115415516309527
- BuckleyDARogersSCyclosporin-responsive hidradenitis suppurativaJ R Soc Med199588289290
- AndersonMDZauliSBettoliVBoerJJemecGBCyclosporine treatment of severe hidradenitis suppurativa – a case seriesJ Dermatolog Treat201627324725026406923
- YazdanyarSBoerJIngvarssonGSzepietowskiJCJemecGBDapsone therapy for hidradenitis suppurativa: a series of 24 patientsDermatology201122234234621757878
- KaurMRLewisHMHidradenitis suppurativa treated with dapsone: a case series of five patientsJ Dermatolog Treat20061721121316971313
- HoferTItinPHAcne inversa: a dapsone-sensitive dermatosisHautarzt200152989992 German11715401
- SoriaACanoui-PoitrineFWolkensteinPAbsence of efficacy of oral isotretinoin in hidradenitis suppurativa: a retrospective study based on patients’ outcome assessmentDermatology200921813413519060466
- BoerJMirjanJPGemertVLong-term results of isotretinoin in the treatment of 68 patients with hidradenitis suppurativaJ Am Acad Dermatol19994073769922015
- NorrisJFCunliffeWJFailure of treatment of familial widespread hidradenitis suppurativa with isotretinoinClin Exp Dermatol1986115795833478160
- JemecGGEGniadeckaMSebum excretion in hidradenitis suppurativaDermatology19971943253289252751
- HaslundPLeeRAJemecGBTreatment of hidradenitis suppurativa with tumour necrosis factor-alpha inhibitorsActa Derm Venereol20098959560019997689
- MegnaMBettoliVChimentiSHidradenitis suppurativa: guidelines of the Italian Society of Dermatology and Venereology (SIDeMaST) for the use of anti-TNF-α agentsG Ital Dermatol Venereol2015150673173926513043
- ShujaFChanCSRosenTBiologic drugs for the treatment of hidradenitis suppurativa: an evidence-based reviewDermatol Clin20102851152420510761
- van RappardDCMooijJEBaetenDLMekkesJRNew-onset polyarthritis during successful treatment of hidradenitis suppurativa with infliximabBr J Dermatol201116519419821428974
- BrunassoAMDelfinoCMassoneCHidradenitis suppurativa: are tumour necrosis factor-alpha blockers the ultimate alternative?Br J Dermatol200815976176318627370
- DelageMSamimiMAtlanMMachetLLoretteGMaruaniAEfficacy of infliximab for hidradenitis suppurativa: assessment of clinical and biological inflammatory markersActa Derm Venereol20119116917121384087
- FardetLDupuyAKerobDInfliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patientsJ Am Acad Dermatol20075662462817240478
- Fernandez-VozmedianoJMArmario-HitaJCInfliximab for the treatment of hidradenitis suppurativaDermatology2007215414417587838
- LasockiASinclairRFoleyPSaundersHHidradenitis suppurativa responding to treatment with infliximabAustralas J Dermatol20105118619020695857
- MekkesJRBosJDLong-term efficacy of a single course of infliximab in hidradenitis suppurativaBr J Dermatol200815837037418047504
- ParadelaSRodriguez-LojoRFernandez-TorresRArévaloPFonsecaELong-term efficacy of infliximab in hidradenitis suppurativaJ Dermatolog Treat20122327828322482700
- PedrazJDaudenEPerez-GalaSGoiriz-ValdésRFernández-PeñasPGarcía-DiezAHidradenitis suppurativa. Response to treatment with infliximabActas Dermosifiliogr200798325331 Spanish17555675
- UsmaniNClaytonTHEverettSGoodfieldMDVariable response of hidradenitis suppurativa to infliximab in four patientsClin Exp Dermatol20073220420517342798
- ArenbergerovaMGkalpakiotisSArenbergerPEffective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapyInt J Dermatol2010491445144921091684
- BlancoRMartinez-TaboadaVMVillaILong-term successful adalimumab therapy in severe hidradenitis suppurativaArch Dermatol200914558058419451504
- SotiriouEApallaZVakirlisEIoannidesDEfficacy of adalimumab in recalcitrant hidradenitis suppurativaEur J Dermatol20091918018119153066
- YamauchiPSMauNHidradenitis suppurativa managed with adalimumabJ Drugs Dermatol2009818118319213236
- AbbVieA phase 3 multicenter study of the safety and efficacy of adalimumab in subjects with moderate to severe hidradenitis suppurativa – PIONEER I ClinicalTials.gov, Identifier: NCT01468207 [last updated October 15, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01468207?term=NCT01468207&rank=1Accessed May 23, 2016
- AbbVieA phase 3 multicenter study of the safety and efficacy of adalimumab in subjects with moderate to severe hidradenitis suppurativa – PIONEER II ClinicalTials.gov, Identifier: NCT01468233 [last updated October 15, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01468233?term=NCT01468233&rank=1Accessed May 23, 2016
- KimballABOkunMMWilliamsDATwo phase 3 trials of adalimumab for hidradenitis suppurativaN Engl J Med2016375542243427518661
- BlokJLLiKBrodmerkelCHorvátovichPJonkmanMFHorváthBUstekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serumBr J Dermatol2016174483984626641739
- GulliverWPJemecGBBakerKAExperience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativaJ Eur Acad Dermatol Venereol201226791191421605174
- SharonVRGarciaMSBagheriSManagement of recalcitrant hidradenitis suppurativa with ustekinumabActa Derm Venereol201292332032122101775
- BaerveldtEMKappenJHThioHBvan LaarJAvan HagenPMPrensEPSuccessful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativaAnn Rheum Dis201372462662723148307
- Santos-PérezMIGarcía-RodicioSDel Olmo-RevueltoMAPozo-RománTUstekinumab for hidradenitis suppurativa: a case reportActas Dermosifiliogr2014105772072224308927