Abstract
Background
Application of topical moisturizers is an essential part of the management of atopic dermatitis (AD). Linoleic acid (LA), the most abundant fatty acid in the epidermis, and its derivatives have an essential role in the structure and function of the epidermal barrier, and their defects are prominent in AD. The aim of this study was to compare the efficacy and safety of two cosmetic products containing either LA or urea in patients with AD.
Patients and methods
A total of 20 patients with AD who met the eligibility criteria and provided written informed consents were enrolled in this randomized, intra-individual split-body, single-center trial. Symmetrical lesions of patients were randomized for treatment with LA- or urea-containing water-in-oil (w/o) emulsions applied two to three times daily for 4 weeks. The efficacy of the two products was evaluated by local Scoring Atopic Dermatitis (SCORAD) of both lesions and also patient (or guardian) satisfaction. In addition, trans-epidermal water loss (TEWL), stratum corneum (SC) hydration, pH, sebum, temperature, erythema, melanin content, and ultrasonographic thickness and echo density of epidermis and dermis were measured before, and 2 and 4 weeks after, treatment.
Results
Four weeks of treatment with the LA-containing product resulted in a significant decrease in local SCORAD, TEWL, erythema, and echo density of dermis, as well as an increase in SC hydration compared to baseline. The urea-containing product also reduced the local SCO-RAD and echo density of dermis and increased SC hydration. In contrast to the LA-containing product, changes in TEWL and erythema were not significant. Moreover, the reduction of erythema was significantly higher in the LA-containing product-treated side compared to the urea-containing product-treated side (p = 0.006).
Conclusion
Both LA- or urea-containing w/o emulsions can significantly improve barrier dysfunction and clinical severity of AD. In agreement with literature, it was confirmed that an LA-containing w/o emulsion exhibited erythema-reducing effects. Since emollients should be used on a regular basis, patients should choose a product by individual preference following recommendation by their dermatologists.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory pruritic skin disorder characterized by increased trans-epidermal water loss (TEWL) which requires appropriate skin care on a regular basis.Citation1–Citation3
The first line of skin protection against the environmental hazardous effects, water loss, and conservation of electrolyte balance is the epidermal barrier (EB). The stratum corneum (SC), a unique differentiation end product of the epidermis, produces a set of protective/defensive functions.Citation4
Inherited barrier abnormalities, exogenous and endogenous stressors with additional exacerbation of barrier dysfunction, and compromised antimicrobial defense with further impairment of barrier function are the main causes of barrier dysfunction in AD.Citation4–Citation6
One of the most important clinical features of AD which results from a dysfunctional EB is very dry skin (xerosis). Moisturizers with different agents containing varying amounts of emollients, occlusives, and/or humectants are used to reduce TEWL, increase skin hydration, and thus improve xerosis in AD patients.Citation7 Emollients soften and smooth the skin by filling the spaces between desquamating corneocytes and provide increased cohesion leading to a smoother surface with less friction.Citation8
Occlusive agents (such as petrolatum, mineral oil, and lanolin) retard evaporation of water by coating the SC. By decreasing TEWL, these agents are one of the best choices for treating xerosis.Citation9
Humectants (such as glycerol, lactic acid, and urea) are water-soluble agents with the capacity to complex and hold water.Citation10 Urea, as a humectant, decreases TEWL in normal skin and dry skin of patients with AD.Citation11–Citation14
Linoleic acid (LA), the most abundant fatty acid in the epidermis, and its derivatives have an essential role in the structure and function of the SC permeability barrier, and their defects are most prominent in AD.Citation15,Citation16 Moreover, it has also been shown that LA has anti-inflammatory effects.Citation17,Citation18
In this study, we compared the efficacy and safety of a water-in-oil (w/o) emulsion containing 1.5% LA with a different w/o emulsion containing 5% urea in patients with AD.
Patients and methods
This study was an open, randomized, intra-individual split-body, single-center trial. The study protocol as well as other essential documents was approved by the Ethics Committee of Tehran University of Medical Sciences and was registered in Iran Randomized Controlled Trial (IRCT; IR.TUMS.REC.1394.32) Registry with registration number IRCT2015062017994N1.
A total of 20 patients with AD who were referred to the outpatient skin clinic of the Center for Research and Training in Skin Diseases and Leprosy, met the eligibility criteria, and provided written informed consent were enrolled in this study. The inclusion criteria were female or male patients at least 2 years old and with mild-to-moderate AD (defined as Scoring Atopic Dermatitis [SCORAD] between 4 and 12, erythema and pruritus score of at least 1) without any signs of infection having at least two symmetrical lesions on arms or legs with similar local SCORAD.
Patients with severe AD (SCORAD > 12), pregnancy or lactation, drug addiction and alcoholism, AIDS or other infectious diseases (such as hepatitis), active skin disease (other than AD) at test area, documented allergies to any ingredients of study products, use of other skin care products (e.g., creams, lotions, and sunscreens) at the treatment areas throughout the course of the study, poor compliance, or enrollment in any clinical trial within the past 3 months were excluded from our study.
Symmetrical lesions of patients were randomized using a software generated randomization list for treatment with a 1.5% LA-containing w/o emulsion (aluminum stearate, aqua, beta-carotene, canola oil, cera alba, cetearyl alcohol, decyl oleate, Helianthus annuus, hydrogenated coco-glycerides, lanolin, lanolin alcohol, isomerized safflower acid, magnesium stearate, paraffin, paraffinum liquidum, petrolatum, sorbitan stearate; “Linola-F” cream; Dr. August Wolff GmbH & Co. KG Arzneimittel, Bielefeld, Germany) or a 5% urea-containing eucerin (cetylstearyl alcohol, white vaseline, and wool wax alcohols), purified water, and urea in w/o emulsion system (Samin Co., Tehran, Iran) applied two to three times daily for 4 weeks.
The efficacy of the products was evaluated by local SCORAD of both lesions and, in addition, by patient (or guardian) satisfaction. Moreover, TEWL, SC hydration, pH, sebum, temperature, erythema, melanin content, and ultrasonographic characteristics of skin (thickness and echo density of epidermis and dermis) were measured using the corresponding probes of Tewameter, Corneometer, pH meter, Sebumeter, Thermometer, and Mexameter of Cutometer® MPA 580 (CK Company, Cologne, Germany) and 22 MHz probe of high-frequency skin ultrasonography (DUB Skin Scanner; TPM, Luneburg, Germany) in standardized conditions of temperature and humidity. All the assessments were performed at baseline and 2 and 4 weeks after beginning the treatment.
Furthermore, any local adverse events at the site of applications were recorde, and the participants answered a questionnaire regarding tolerability and acceptance of each product (Figure S1).
Each subject was asked to rate the severity of itching and burning in both treatment areas from 0 (none) to 5 (severe). Patients were also asked to rate the level of satisfaction from treatment, on a 5-grade scale (5 = very satisfied, 4 = somewhat satisfied, 3 = indifferent, 2 = somewhat dissatisfied, 1 = very dissatisfied). In case of younger children and infants who were not capable of answering/filling the questionnaire, patients’ parents were asked to fill it.
Results were presented as median (quartile 1 – quartile 3), and differences were compared between two treatment groups using Wilcoxon signed-rank test.
The SPSS software version 18 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Data are presented as mean ± SD or median (quartile 1 – quartile 3) unless stated otherwise and analyzed by the Wilcoxon signed-rank test. The statistical significance level was defined as p < 0.05.
Results
In this pilot study, 20 patients (12 females and 8 males) with a mean age of 16.75 years (SD = 13.67, range 2–45 years) and a mean SCORAD of 10.46 (SD = 1.02, range 8.7–11.9) were included, and 16 patients completed the study. The local SCORAD and the biophysical measurements at baseline and after 2 and 4 weeks of treatment on both sides are shown in .
Figure 1 The local SCORAD (A), hydration (B), melanin content (C), erythema level (D), TEWL (E), temperature (F), pH (G), and sebum content (H) at baseline and after 2 and 4 weeks in LA- and urea-containing products. *p<0.05.
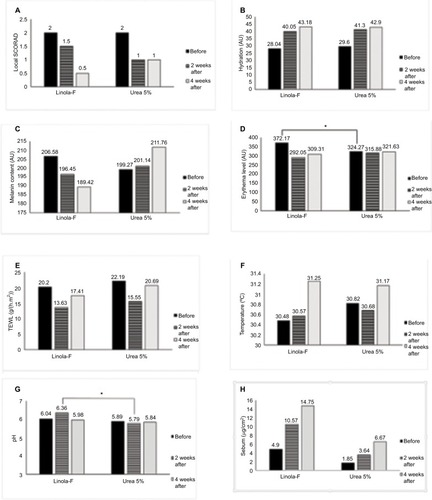
At baseline, there was no significant difference in local SCORAD and in any of the measured variables, except for the mean erythema level which was significantly higher in site designated to be treated by LA-containing product.
There was no significant differences between the two treated sides in any parameter, except for a higher pH in LA-containing product-treated side after 2 weeks of treatment (p-value = 0.003).
Four weeks of treatment with a LA-containing product resulted in a significant decrease in local SCORAD, TEWL, and erythema as well as an increase in SC hydration compared to baseline ().
Treatment with a urea-containing product cream resulted in a significant decrease in local SCORAD and an increase in SC hydration. TEWL and erythema reduction after application of the urea-containing product, however, was not significant. The erythema was significantly reduced after the application of the LA-containing product-treated side compared to the side treated with the urea-containing product (p = 0.006). There was a clear, but not statistically significant trend (p = 0.098) regarding the reduction in skin melanin content in the LA-containing product-treated side, whereas skin melanin content even increased in the urea-containing product-treated side ().
The ultrasonographic measurements showed a decrease at week 4 for both products; however, there was no statistically significant difference ().
Table 1 Comparison of high-frequency ultrasonography (22 MHz) parameters at visit 0 (day 0), visit 1 (day 14 + 2), and visit 2 (day 28 + 4) between treatment groups
Moreover, patients’ satisfaction with treatment was higher for the LA-containing product, and this difference was statistically significant (p = 0.046).
No statistically significant differences were found in the severity of itching and burning between two groups (p-value=0.912 and 0.961, respectively). No other adverse reactions were reported or observed in any of the two treatment groups.
Discussion
It is well known to dermatologists and just recently confirmed by a Cochrane Review that topical application of emulsions prolongs the time to flare, decrease the number of flares, and reduce the amounts of topical corticosteroids needed.Citation19 Therefore, the current available guidelines recommend the use of emulsions as a key and basic step in the treatment of AD,Citation20–Citation23 in particular, since several recent studies showed that preventing degradation and repairing the barrier dysfunctions are critical strategies for reducing the risk of relapse in AD.Citation4,Citation24,Citation25
In this study, we assessed the effects of two w/o emulsions in AD lesions in a randomized, intra-individual, controlled clinical trial. One of the products contained 1.5% LA as cosmetic active ingredient, whereas the other one contained 5% urea. After 4 weeks of treatment, both products significantly decreased local SCORAD and significantly increased SC hydration, although reduction in skin erythema and TEWL was only significant in the lesions treated with the LA-containing product. An impaired skin barrier function and clinical signs of dryness are usually expected to be improved after use of the appropriate topical emulsion. Unfortunately, clinical improvement in xerosis and eczematous lesions may not necessarily induce normalization of TEWL.Citation26
The beneficial effects of the two products, both w/o emulsions (appropriate pharmaceutical formulation for xerosis), can be explained by the measured increase in skin hydration and through possible effects on the barrier function, although only for the LA-containing product, a statistically significant change was observed for the TEWL. Components of the LA-containing product, such as complex mixture of esters, diesters and hydroxy esters of high molecular weight, lanolin alcohols, and lanolin acids, form an inert layer on the skin and can also penetrate the damaged skin and repair the EB leading to a reduction in TEWL.Citation27–Citation31 Thus, occlusion is the most predictable mechanism by which water loss is reduced from the skin.Citation32 However, since the two products tested in this clinical study are not identical in their composition, they exhibit different effects on the skin (as shown).
Moreover, different effects of products used for the treatment of xerosis in AD patients are well described in the literature.Citation12,Citation33–Citation35 Patients with similar disease characteristics have been considered to benefit from different topically applied emulsions with different ingredients.Citation36 Unfortunately, up to now, there is no unifying clinical classification system available to decide which type of products are best suited for different degrees of xerosis due to different AD phenotypes.Citation36 Humectants such as urea in emulsions are absorbed into the SC and can increase skin hydration by attracting water. Although, in our study, the TEWL following application of urea-containing product decreased from baseline, the difference was not statistically significant, whereas skin hydration increased, which can be explained by the same mechanism mentioned earlier.
Essential fatty acids (EFAs) such as LA are necessary for the synthesis of ceramides, e.g., CER[EOH], CER[EOS] and CER[EOP], which play a critical role in barrier function of the epidermis.Citation14,Citation15 In contrast to the urea-containing product, the LA-containing product significantly decreased erythema (one of the signs of inflammation) in this clinical study. According to the experimental literature, this is not surprising knowing that LA has anti-inflammatory effects.Citation17,Citation18,Citation37 Since LA is a potent naturally occurring ligand and activator of PPRAα,Citation38 skin inflammation of irritant contact dermatitis is reduced by the topical application of LA in a mice model.Citation39 Moreover, an erythema-reducing effect was already reported in a previous clinical study in an irritative contact dermatitis model using a different LA-containing product. The effect of LA-containing product in that study was comparable to a 0.25% hydrocortisone-containing formulation.Citation40
Although skin pigmentation was not in the focus of our study, a clear trend toward a reduction in the melanin content in the LA-containing product-treated side was observed. This effect of the LA-containing product on melanin content may be explained via the abovementioned anti-inflammatory properties of LA leading to a reduction in post-inflammatory hyperpigmentation.Citation41 Of course, anti-pigmentary effect of other components in the LA-containing product cannot be ruled out.
Furthermore, unsaturated fatty acids such as oleic acid and LA can decrease tyrosinase activity (via posttranscriptional events and acceleration of the proteolysis of tyrosinase) and thus subsequently reduce melanin synthesis.Citation42
In this study, we also used high-frequency ultrasonography to assess dermal changes after treatment with the w/o emulsions. Both creams decreased dermal echo density significantly, which might be the result of a decrease in inflammation in the dermis. In contrast to another study that compared the results of ultrasound images with pathologic findings in AD, the echogenicity of dermis had a strong negative correlation with the intensity of inflammation.Citation43
It is worth to mention that, in this study, patients were more satisfied using the LA-containing w/o emulsion compared to the urea-containing product. This may be explained by the stinging and burning sensation of urea immediately after its application, which has been reported in previous studies.Citation14,Citation44
Finally, we are aware of limitations of our study. First, this study was a small pilot study and exploratory in nature with low number of patients (n = 20). Second, we used split-body design to compare two products. Third, although we focused on urea and LA, we cannot and are not excluding the effect of whole product on seen clinical improvements. On the other hand, strengths of this study are direct comparison of products with objective as well as subjective assessment of efficacy.
Conclusion
Both products, the LA- and the urea-containing w/o emulsion, increased skin hydration and thus improved the clinical severity of AD in this clinical study. However, only the LA-containing product due to the known anti-inflammatory effects of LA reduced erythema significantly after treatment. In general, the choice of moisturizers can be determined by individual preference, safety, and efficacy, and the absence of fragrances, additives, or other sensitizing agents.Citation45
Acknowledgments
This study was supported by research grant from Sina Tejarat Pishgam Co., Tehran, Iran.
Disclosure
Christoph Abels, MD, PhD, a board-certified dermatologist, is an employee of Dr. August Wolff GmbH & Co. KG Arzneimittel, Bielefeld, Germany. The other authors report no conflicts of interest in this work.
References
- BieberTAtopic dermatitisAnn Dermatol201022212513720548901
- FiroozAGorouhiFDavariPComparison of hydration, sebum and pH values in clinically normal skin of patients with atopic dermatitis and healthy controlsClin Exp Dermatol200732332132217335552
- WollenbergAEhmannLMLong term treatment concepts and proactive therapy for atopic eczemaAnn Dermatol201224325326022879707
- EliasPMHatanoYWilliamsMLBasis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanismsJ Allergy Clin Immunol200812161337134318329087
- EliasPMTherapeutic implications of a barrier-based pathogenesis of atopic dermatitisAnn Dermatol201022324525420711259
- ThyssenJPKezicSCauses of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitisJ Allergy Clin Immunol2014134479279925065719
- RawlingsAVCanestrariDADobkowskiBMoisturizer technology versus clinical performanceDermatol Ther200417Suppl 1495614728699
- DraelosZKAtlas of Cosmetic DermatologyNew YorkChurchill Livingstone2000
- SpruitDThe interference of some substances with the water vapour loss of human skinDermatologica1971142289925575285
- IdsonBDry skin: moisturizing and emolliencyCosmet Toiletr199210710
- GriceKSattarHBakerHUrea and retinoic acid in ichthyosis and their effect on transepidermal water loss and water holding capacity of stratum corneumActa Derm Venereol19735321141184120951
- LodenMUrea-containing moisturizers influence barrier properties of normal skinArch Dermatol Res199628821031078932589
- LodenMAnderssonACLindbergMImprovement in skin barrier function in patients with atopic dermatitis after treatment with a moisturizing cream (Canoderm)Br J Dermatol1999140226426710233220
- SerupJA double-blind comparison of two creams containing urea as the active ingredient. Assessment of efficacy and side-effects by noninvasive techniques and a clinical scoring schemeActa Derm Venereol Suppl (Stockh)199217734431466184
- IshikawaJNaritaHKondoNChanges in the ceramide profile of atopic dermatitis patientsJ Invest Dermatol2010130102511251420574438
- JanssensMvan SmedenJGoorisGSLamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczemaJ Invest Dermatol2011131102136213821716325
- LiuKLBeluryMAConjugated linoleic acid reduces arachidonic acid content and PGE2 synthesis in murine keratinocytesCancer Lett19981271–215229619853
- WertzPWBiochemistry of human stratum corneum lipidsEliasPMFeingoldKRSkin BarrierNew YorkTaylor & Francis200610
- van ZuurenEJFedorowiczZArentsBWMEmollients and moisturisers for eczema: abridged Cochrane systematic review including GRADE assessmentsBr J Dermatol Epub2017422
- National Institute for Health and Care Excellence [webpage on the Internet]NICE Pathways – Mapping Our GuidanceLondonNational Institute for Health and Care Excellencec2016 [cited Jan 28, 2016]. Available from: https://pathways.nice.org.uk/pathways/eczema#path=view%3A/pathways/eczema/treating-atopic-eczema-in-children-aged-12-and-under.xml&content=view-indexAccessed November 21, 2017
- Australasian Society of Clinical Immunology and Allergy [webpage on the Internet]Atopic Dermatitis (Eczema)Brookvale, AUAustralasian Society of Clinical Immunology and Allergyc2016 [cited Jan 29, 2016]. Available from: https://www.allergy.org.au/images/pcc/ASCIA_PCC_Eczema_2015.pdfAccessed November 25, 2017
- RubelDThirumoorthyTSoebaryoRWConsensus guidelines for the management of atopic dermatitis: an Asia-Pacific perspectiveJ Dermatol201340316017123289827
- SinclairWAboobakerJGreenRGuidelines on the Management of Atopic Dermatitis in South AfricaLondonDermatologyc2015 [cited Jan 28, 2016]. Available from: https://www.mm3admin.co.za/documents/docmanager/8e7be0a4-2b8d-453f-875e-cd1e5132b829/00079177.pdf
- SzczepanowskaJReichASzepietowskiJCEmollients improve treatment results with topical corticosteroids in childhood atopic dermatitis: a randomized comparative studyPediatr Allergy Immunol200819761461818208463
- SimpsonELChalmersJRHanifinJMEmollient enhancement of the skin barrier from birth offers effective atopic dermatitis preventionJ Allergy Clin Immunol2014134481882325282563
- VilaplanaJCollJTrullasCAzonAPelejeroCClinical and noninvasive evaluation of 12% ammonium lactate emulsion for the treatment of dry skin in atopic and non-atopic subjectsActa Derm Venereol199272128331350137
- FeingoldKRBrownBELearSRMoserAHEliasPMEffect of essential fatty acid deficiency on cutaneous sterol synthesisJ Invest Dermatol19868755885913772153
- LodenMThe increase in skin hydration after application of emollients with different amounts of lipidsActa Derm Venereol19927253273301361276
- Mao-QiangMBrownBEWu-PongSFeingoldKREliasPMExogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunctionArch Dermatol199513178098167611797
- RawlingsAVScottIRHardingCRBowserPAStratum corneum moisturization at the molecular levelJ Invest Dermatol199410357317417963664
- WertzPWDowningDTMetabolism of topically applied fatty acid methyl esters in BALB/C mouse epidermisJ Dermatol Sci19901133372078539
- LodenMLindbergMThe influence of a single application of different moisturizers on the skin capacitanceActa Derm Venereol199171179821676227
- HeldESveinsdottirSAgnerTEffect of long-term use of moisturizer on skin hydration, barrier function and susceptibility to irritantsActa Derm Venereol1999791495110086859
- LodenMBarrier recovery and influence of irritant stimuli in skin treated with a moisturizing creamContact Dermatitis19973652562609197961
- LodenMOlssonHSkareLAxéllTHud AbAInstrumental and sensory evaluation of the frictional response of the skin following a single application of five moisturizing creamsJ Soc Cosmet Chem1992438
- MoncrieffGCorkMLawtonSKokietSDalyCClarkCUse of emollients in dry-skin conditions: consensus statementClin Exp Dermatol2013383231238 quiz 23823517354
- RhodesLEStoreyAEssential fatty acids: biological functions and potential applications in the skinMaibachHILodenMDry Skin and MoisturizersBoca Raton, FLCRC Press2006319340
- Moya-CamarenaSYVanden HeuvelJPBlanchardSGLeesnitzerLABeluryMAConjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalphaJ Lipid Res19994081426143310428978
- SheuMYFowlerAJKaoJTopical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis modelsJ Invest Dermatol200211819410111851881
- ProkschEAbelsCEffects of linola −emulsion in comparison to a hydrocortisone-containing emulsion in the model of an irritantAkt Dermatol20073377
- Verallo-RowellVMKatalbasSSPangasinanJPNatural (mineral, vegetable, coconut, essential) oils and contact dermatitisCurr Allergy Asthma Rep20161675127373890
- AndoHWatabeHValenciaJCFatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome functionJ Biol Chem200427915154271543314739285
- WozniakAWDańczak-PazdrowskaAAPolańskaAAJanicka-JedyńskaMMMaksinKKJenerowiczDDUltrasonographic and histopathologic images of atopic dermatitis are closely relatedPathology201446124300728
- FrithzAInvestigation of Cortesal®, a hydrocortisone cream and its water-retaining cream base in the treatment of xerotic skin and dry eczemasCurr Ther Res1983335
- EichenfieldLFTomWLBergerTGGuidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapiesJ Am Acad Dermatol201471111613224813302

